Abstract
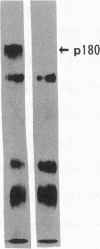
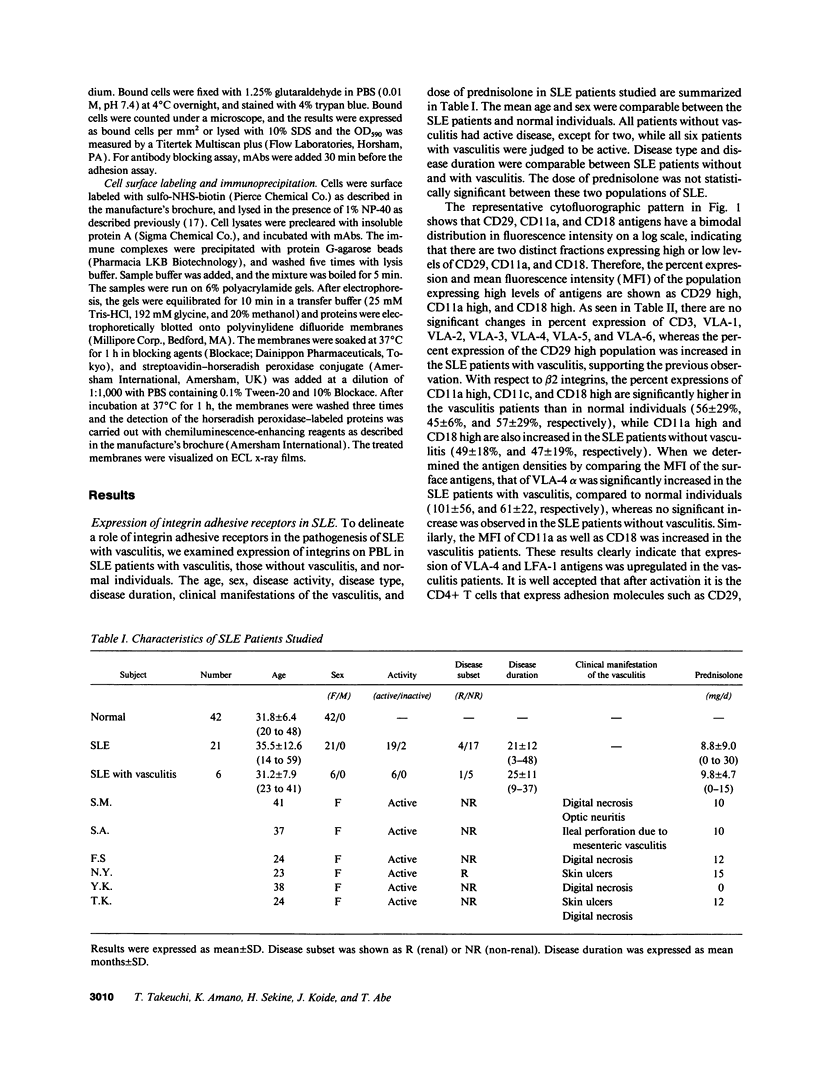
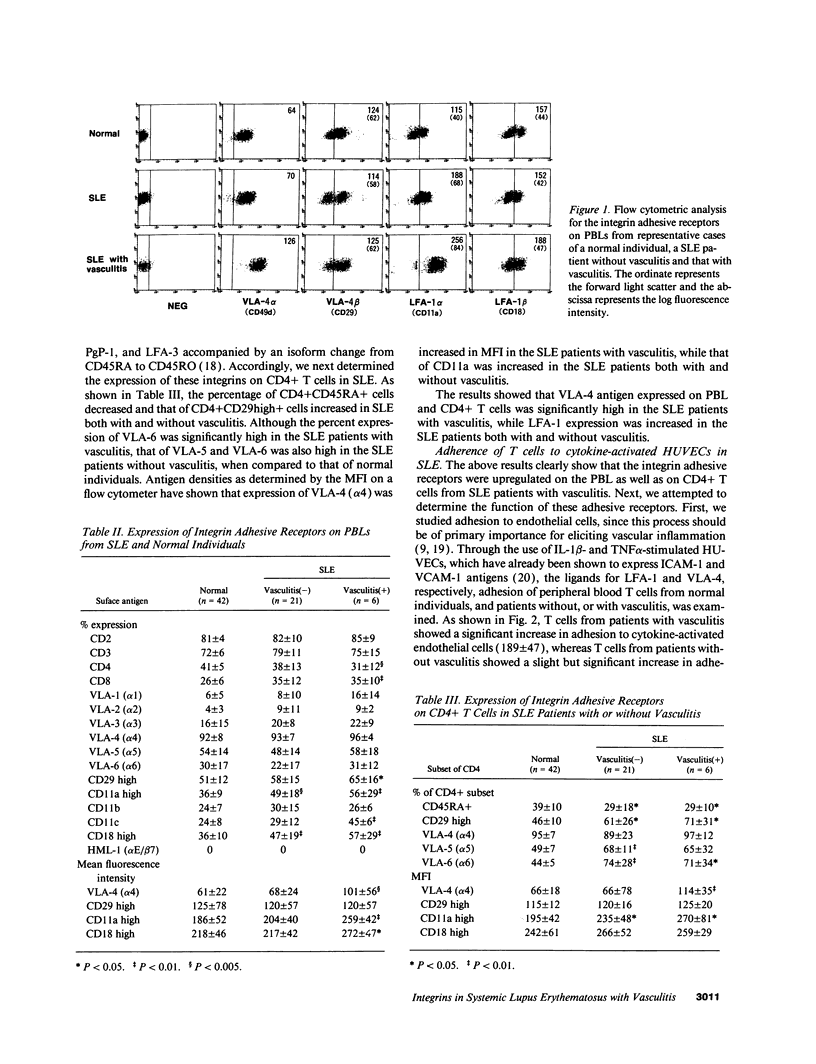
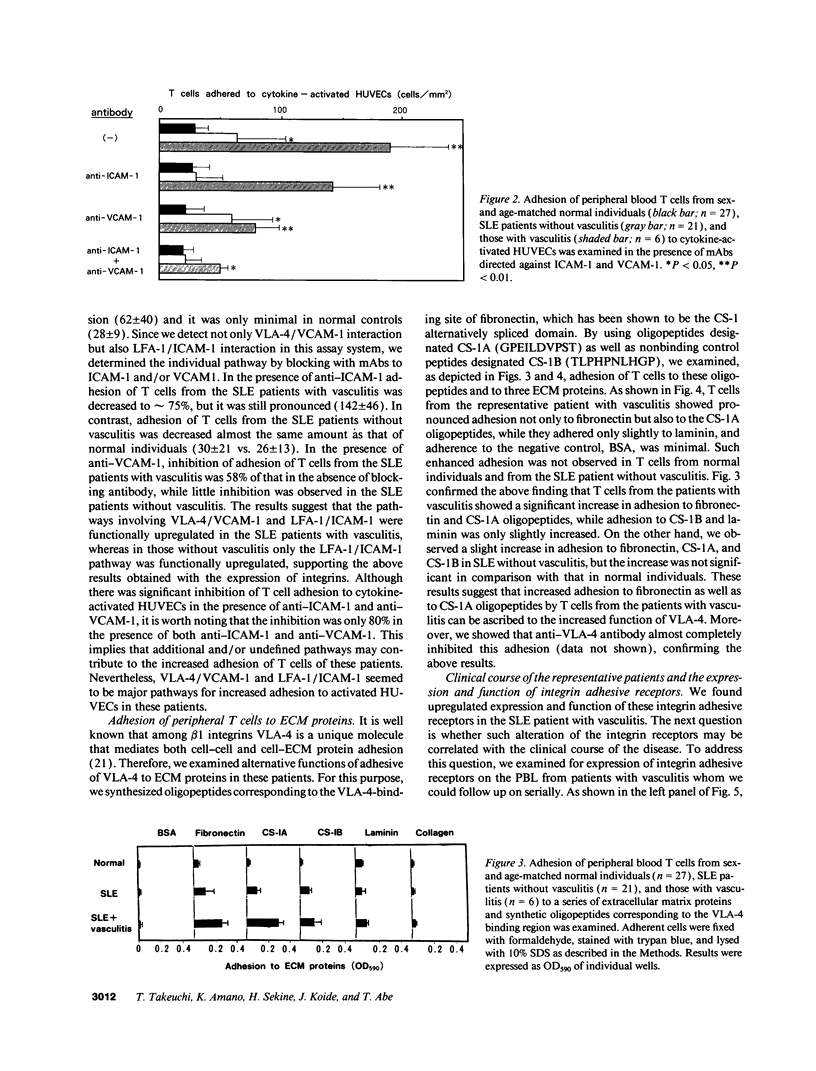
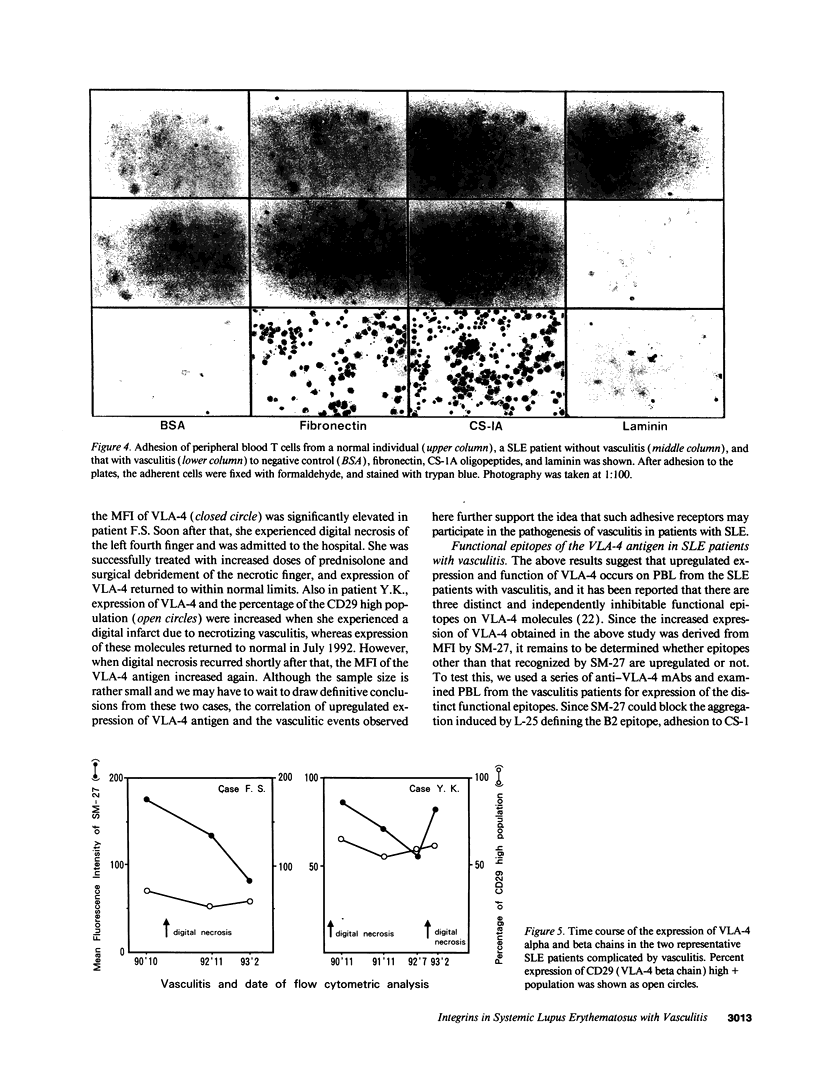
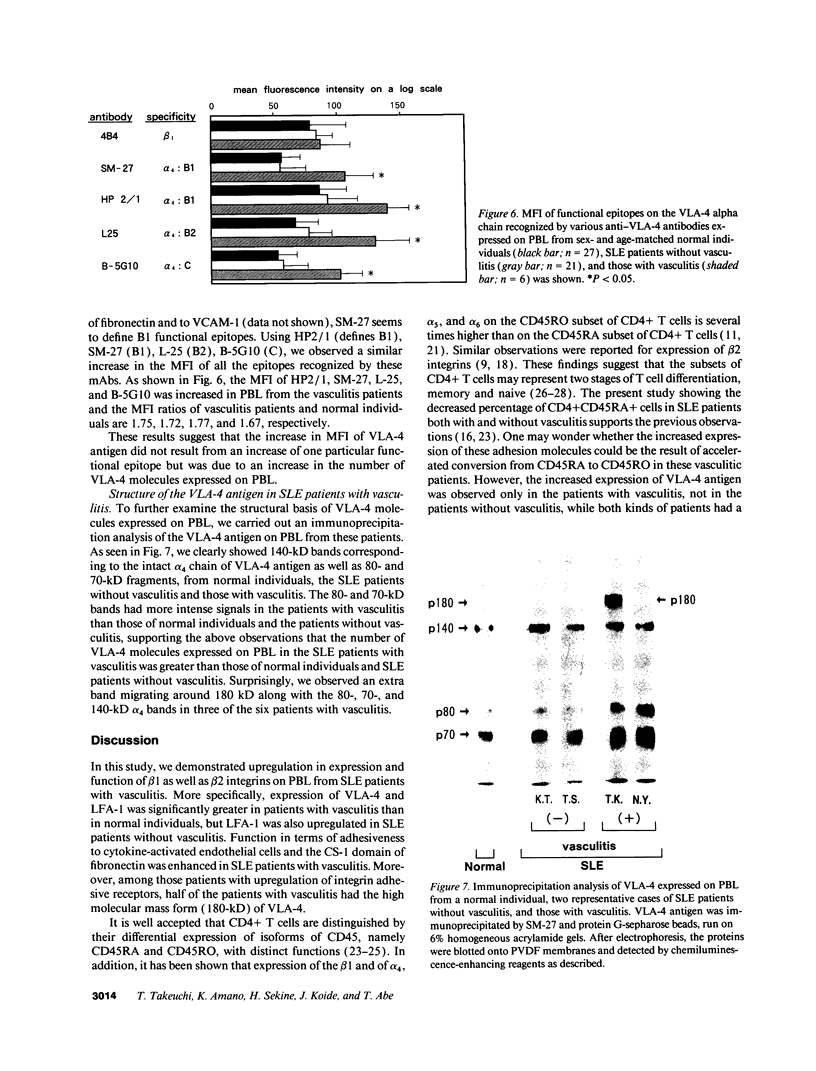
Upregulation of integrin adhesive receptors has been implicated in various pathological conditions. We examined expression and function of integrin adhesive receptors on peripheral blood lymphocytes from patients with systemic lupus erythematosus (SLE), particularly those with the complication of vasculitis, and found that VLA-4 and LFA-1 expression was increased in SLE patients with vasculitis, while LFA-1 but not VLA-4 expression was increased in those without vasculitis. These results suggested a role of VLA-4 in the pathogenesis of vasculitis in SLE. Functional studies further demonstrated that adhesion to cytokine-activated human umbilical cord vein endothelial cells and to the CS-1 alternatively spliced domain of fibronectin was significantly increased in SLE patients with vasculitis. Analysis of the functional epitopes on the alpha 4 chain demonstrated that antigen densities of all the functional epitopes were increased in those with vasculitis, indicating that the increased expression of VLA-4 resulted from the increased number of VLA-4 molecules, and was not secondary to an increase in one particular functional epitope. Immunoprecipitation studies further support these results. Interestingly, high molecular weight bands associated with VLA-4 were observed in about half of the SLE patients with vasculitis. These results introduce a possibility that upregulation of integrin adhesive receptors has a potential role in the pathogenesis of vasculitis in SLE.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Argenbright L. W., Barton R. W. Interactions of leukocyte integrins with intercellular adhesion molecule 1 in the production of inflammatory vascular injury in vivo. The Shwartzman reaction revisited. J Clin Invest. 1992 Jan;89(1):259–272. doi: 10.1172/JCI115570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bednarczyk J. L., McIntyre B. W. A monoclonal antibody to VLA-4 alpha-chain (CDw49d) induces homotypic lymphocyte aggregation. J Immunol. 1990 Feb 1;144(3):777–784. [PubMed] [Google Scholar]
- Birkenmeier T. M., McQuillan J. J., Boedeker E. D., Argraves W. S., Ruoslahti E., Dean D. C. The alpha 5 beta 1 fibronectin receptor. Characterization of the alpha 5 gene promoter. J Biol Chem. 1991 Oct 25;266(30):20544–20549. [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- DIXON F. J., FELDMAN J. D., VAZQUEZ J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med. 1961 May 1;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling F. M., Hahn B. H. Pathogenic subsets of antibodies to DNA. Int Rev Immunol. 1989;5(1):79–95. doi: 10.3109/08830188909086990. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- García-Vicuña R., Humbría A., Postigo A. A., López-Elzaurdia C., de Landázuri M. O., Sánchez-Madrid F., Laffón A. VLA family in rheumatoid arthritis: evidence for in vivo regulated adhesion of synovial fluid T cells to fibronectin through VLA-5 integrin. Clin Exp Immunol. 1992 Jun;88(3):435–441. doi: 10.1111/j.1365-2249.1992.tb06468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Elices M. J., Parker C., Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990 Apr;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iigo Y., Takashi T., Tamatani T., Miyasaka M., Higashida T., Yagita H., Okumura K., Tsukada W. ICAM-1-dependent pathway is critically involved in the pathogenesis of adjuvant arthritis in rats. J Immunol. 1991 Dec 15;147(12):4167–4171. [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Kohn F. R., Grigg M. E., Klingemann H. G. Differential regulation of fibronectin receptor subunit gene and cell surface expression in human peripheral blood T lymphocytes. J Immunol. 1991 Mar 1;146(5):1484–1489. [PubMed] [Google Scholar]
- Laffón A., García-Vicuña R., Humbría A., Postigo A. A., Corbí A. L., de Landázuri M. O., Sánchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991 Aug;88(2):546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Steinberg A. D., Letvin N. L., Hagan M., Takeuchi T., Daley J., Levine H., Schlossman S. F. A defect of immunoregulatory T cell subsets in systemic lupus erythematosus patients demonstrated with anti-2H4 antibody. J Clin Invest. 1987 Mar;79(3):762–768. doi: 10.1172/JCI112882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Parker C. M., Pujades C., Brenner M. B., Hemler M. E. Alpha 4/180, a novel form of the integrin alpha 4 subunit. J Biol Chem. 1993 Apr 5;268(10):7028–7035. [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Postigo A. A., Garcia-Vicuña R., Diaz-Gonzalez F., Arroyo A. G., De Landázuri M. O., Chi-Rosso G., Lobb R. R., Laffon A., Sánchez-Madrid F. Increased binding of synovial T lymphocytes from rheumatoid arthritis to endothelial-leukocyte adhesion molecule-1 (ELAM-1) and vascular cell adhesion molecule-1 (VCAM-1). J Clin Invest. 1992 May;89(5):1445–1452. doi: 10.1172/JCI115734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R., Elices M. J., Campanero M. R., Osborn L., Schiffer S., García-Pardo A., Lobb R., Hemler M. E., Sánchez-Madrid F. Functional evidence for three distinct and independently inhibitable adhesion activities mediated by the human integrin VLA-4. Correlation with distinct alpha 4 epitopes. J Biol Chem. 1991 Jun 5;266(16):10241–10245. [PubMed] [Google Scholar]
- Rosen G. D., Birkenmeier T. M., Dean D. C. Characterization of the alpha 4 integrin gene promoter. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4094–4098. doi: 10.1073/pnas.88.10.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Serra H. M., Krowka J. F., Ledbetter J. A., Pilarski L. M. Loss of CD45R (Lp220) represents a post-thymic T cell differentiation event. J Immunol. 1988 Mar 1;140(5):1435–1441. [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Rudd C. E., Schlossman S. F., Morimoto C. Induction of suppression following autologous mixed lymphocyte reaction; role of a novel 2H4 antigen. Eur J Immunol. 1987 Jan;17(1):97–103. doi: 10.1002/eji.1830170117. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Tanaka S., Steinberg A. D., Matsuyama T., Daley J., Schlossman S. F., Morimoto C. Defective expression of the 2H4 molecule after autologous mixed lymphocyte reaction activation in systemic lupus erythematosus patients. J Clin Invest. 1988 Oct;82(4):1288–1294. doi: 10.1172/JCI113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies in pathology and cell biology. Cell. 1991 Nov 29;67(5):841–842. doi: 10.1016/0092-8674(91)90356-4. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Lefrançois L. Differential expression of the leucocyte-common antigen family. Immunol Today. 1988 Oct;9(10):320–326. doi: 10.1016/0167-5699(88)91326-6. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Faiferman I., Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977 Jan;59(1):90–96. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]