Abstract
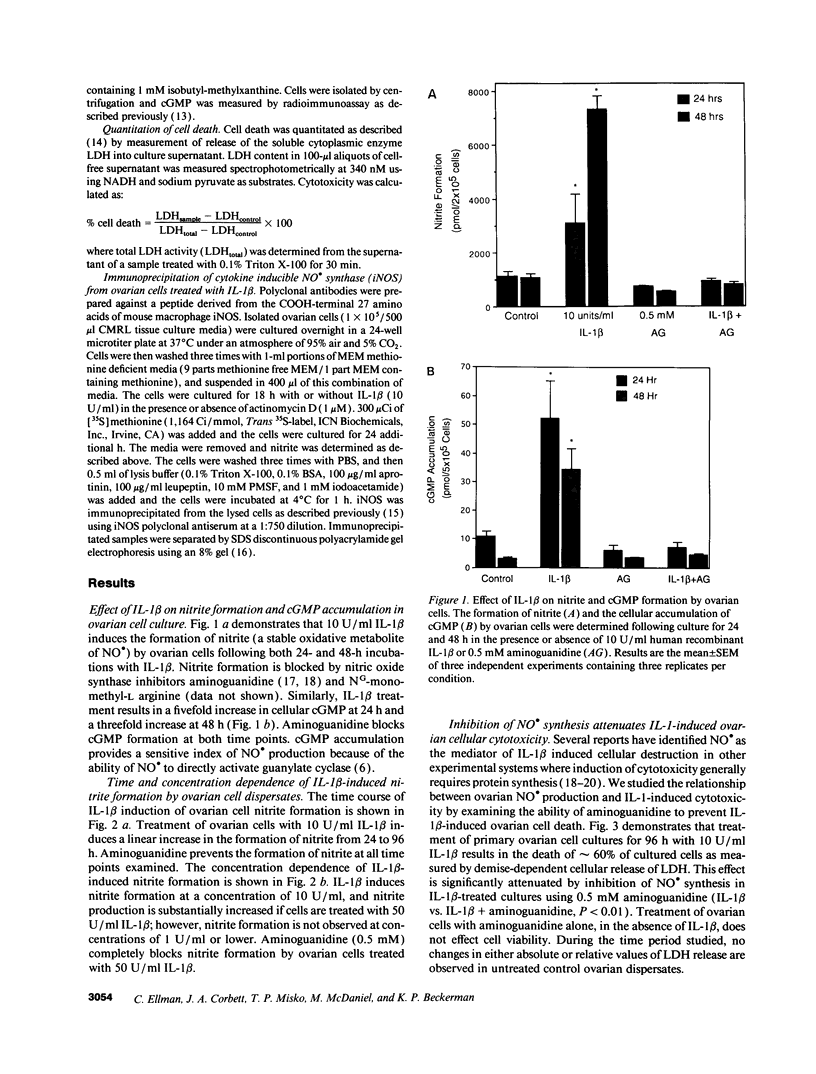
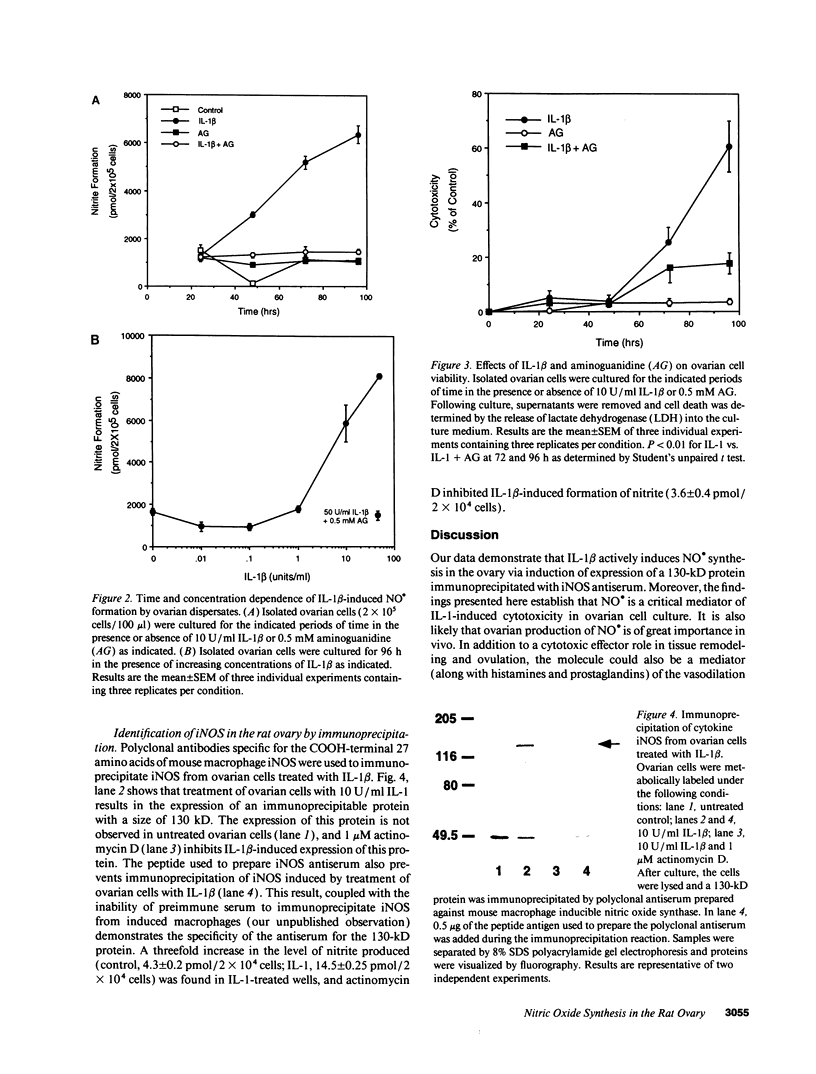
Treatment of primary cultures of rat ovarian dispersates with IL-1 beta results in morphologic and cytotoxic changes, thought to reflect tissue remodeling events associated with ovulation. We examined the role that the free radical nitric oxide plays in this process and report that IL-1 beta induces expression of the inducible isoform of nitric oxide synthase in ovarian cells as demonstrated by immunoprecipitation. We show that IL-1 beta treatment results in the formation of nitric oxide (as measured by accumulation of nitrite and cGMP) in both a time- and concentration-dependent manner that is prevented by aminoguanidine, a selective inhibitor of the inducible isoform of nitric oxide synthase. Aminoguanidine also inhibits IL-1-induced ovarian cellular cytotoxicity. These results suggest that nitric oxide is an important mediator of cell death and may act as a physiologically significant mediator of tissue remodeling events that occur in vivo during the ovulatory process.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y. Cytokine-mediated regulation of ovarian function: encounters of a third kind. Endocrinology. 1989 May;124(5):2043–2045. doi: 10.1210/endo-124-5-2043. [DOI] [PubMed] [Google Scholar]
- BULMER D. THE HISTOCHEMISTRY OF OVARIAN MACROPHAGES IN THE RAT. J Anat. 1964 Jul;98:313–319. [PMC free article] [PubMed] [Google Scholar]
- Beckerman K. P., Rogers H. W., Corbett J. A., Schreiber R. D., McDaniel M. L., Unanue E. R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993 Feb 1;150(3):888–895. [PubMed] [Google Scholar]
- Burnett A. L., Lowenstein C. J., Bredt D. S., Chang T. S., Snyder S. H. Nitric oxide: a physiologic mediator of penile erection. Science. 1992 Jul 17;257(5068):401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Cavender J. L., Murdoch W. J. Morphological studies of the microcirculatory system of periovulatory ovine follicles. Biol Reprod. 1988 Nov;39(4):989–997. doi: 10.1095/biolreprod39.4.989. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., McDaniel M. L. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992 Aug;41(8):897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Wang J. L., Hughes J. H., Wolf B. A., Sweetland M. A., Lancaster J. R., Jr, McDaniel M. L. Nitric oxide and cyclic GMP formation induced by interleukin 1 beta in islets of Langerhans. Evidence for an effector role of nitric oxide in islet dysfunction. Biochem J. 1992 Oct 1;287(Pt 1):229–235. doi: 10.1042/bj2870229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Nacy C. A., Meltzer M. S. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991 Jul;50(1):93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- Hughes J. H., Colca J. R., Easom R. A., Turk J., McDaniel M. L. Interleukin 1 inhibits insulin secretion from isolated rat pancreatic islets by a process that requires gene transcription and mRNA translation. J Clin Invest. 1990 Sep;86(3):856–863. doi: 10.1172/JCI114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz A., Hernandez E. R., Payne D. W., Dharmarajan A. M., Adashi E. Y. Interleukin-1 is both morphogenic and cytotoxic to cultured rat ovarian cells: obligatory role for heterologous, contact-independent cell-cell interaction. Endocrinology. 1992 Oct;131(4):1643–1649. doi: 10.1210/endo.131.4.1396309. [DOI] [PubMed] [Google Scholar]
- Hurwitz A., Loukides J., Ricciarelli E., Botero L., Katz E., McAllister J. M., Garcia J. E., Rohan R., Adashi E. Y., Hernandez E. R. Human intraovarian interleukin-1 (IL-1) system: highly compartmentalized and hormonally dependent regulation of the genes encoding IL-1, its receptor, and its receptor antagonist. J Clin Invest. 1992 Jun;89(6):1746–1754. doi: 10.1172/JCI115777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Schmidt K., Hallin P., Di Pauli R., De Geyter C., Nieschlag E. Human testis cytosol and ovarian follicular fluid contain high amounts of interleukin-1-like factor(s). Mol Cell Endocrinol. 1988 Aug;58(2-3):221–230. doi: 10.1016/0303-7207(88)90158-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magoffin D. A., Erickson G. F. Mechanism by which 17 beta-estradiol inhibits ovarian androgen production in the rat. Endocrinology. 1981 Mar;108(3):962–969. doi: 10.1210/endo-108-3-962. [DOI] [PubMed] [Google Scholar]
- Magoffin D. A., Erickson G. F. Primary culture of differentiating ovarian androgen-producing cells in defined medium. J Biol Chem. 1982 Apr 25;257(8):4507–4513. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Rajfer J., Aronson W. J., Bush P. A., Dorey F. J., Ignarro L. J. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992 Jan 9;326(2):90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- Takakura K., Taii S., Fukuoka M., Yasuda K., Tagaya Y., Yodoi J., Mori T. Interleukin-2 receptor/p55(Tac)-inducing activity in porcine follicular fluids. Endocrinology. 1989 Aug;125(2):618–623. doi: 10.1210/endo-125-2-618. [DOI] [PubMed] [Google Scholar]