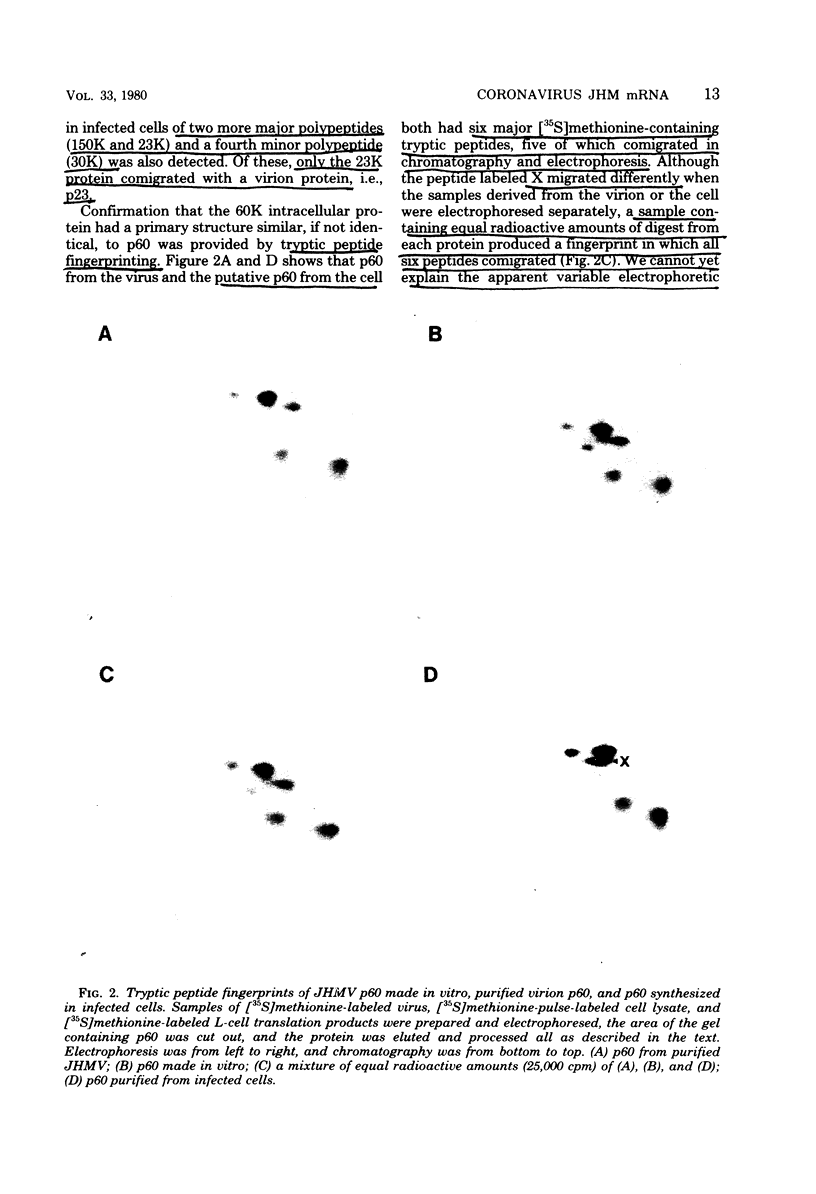
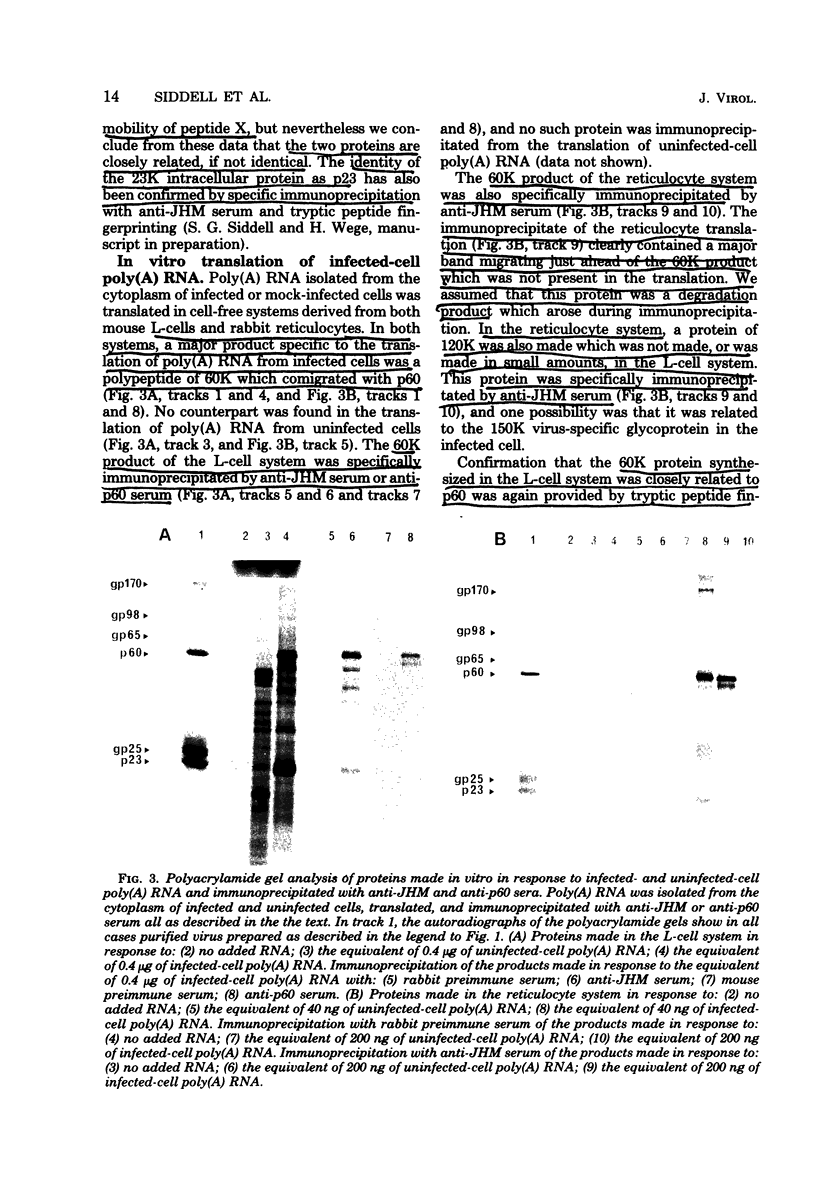
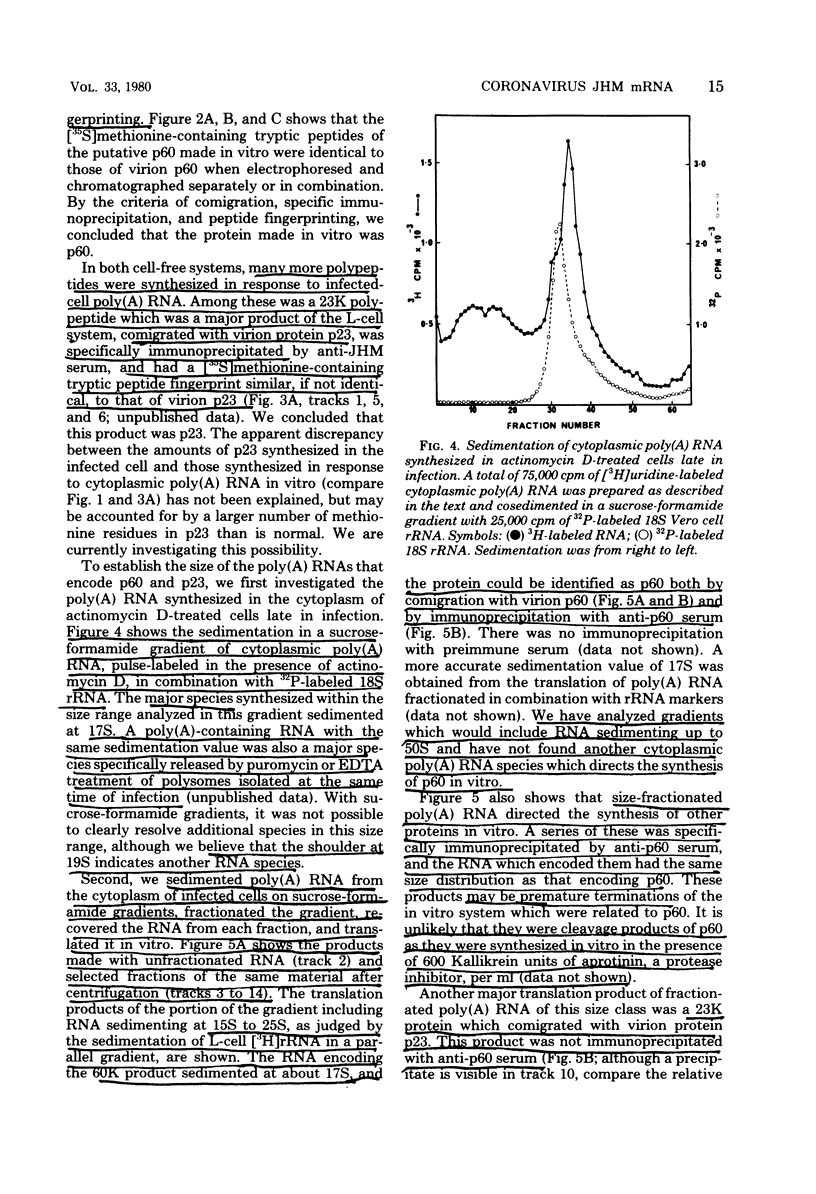
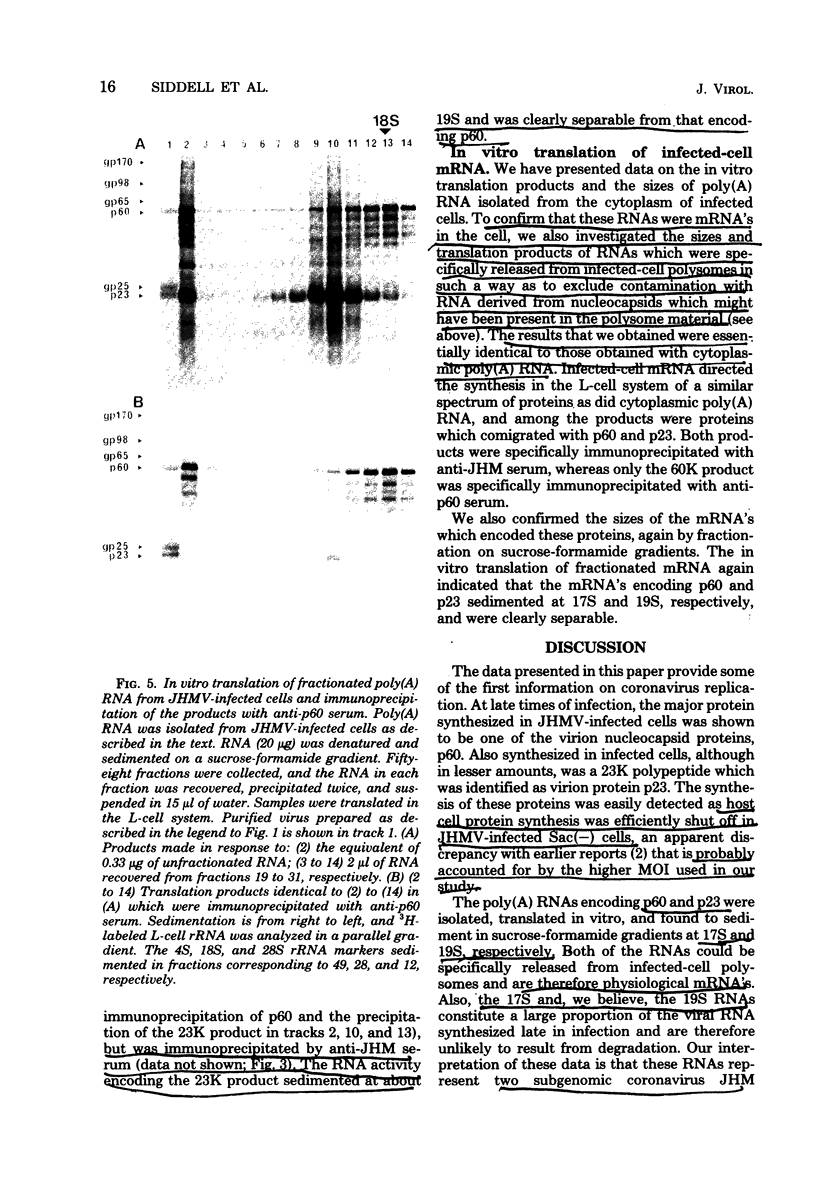
Abstract
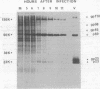
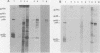
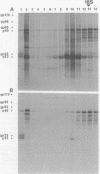
Sac(-) cells infected with murine coronavirus strain JHM shut off host cell protein synthesis and synthesized polypeptides with molecular weights of 150,000, 60,000, and 23,000. The 60,000- and 23,000-molecular-weight polypeptides comigrated with virion structural proteins p60 and p23, and the 60,000-molecular-weight protein was identified as p60 by tryptic peptide fingerprinting. Polyadenylate-containing RNA [poly(A) RNA] extracted from the cytoplasm of infected cells directed the synthesis of both 60,000- and 23,000-molecular-weight polypeptides in messenger-dependent cell-free systems derived from mouse L-cells and rabbit reticulocytes. The reticulocyte system also synthesized a 120,000-molecular-weight polypeptide that was specifically immunoprecipitated by antiserum raised against JHM virions. The identity of the 60,000- and 23,000-molecular-weight in vitro products was established by comigration with virion proteins, immunoprecipitation, and in the case of p60, tryptic peptide fingerprinting. The cytoplasmic poly(A) RNAs which encoded p60 and p23 sedimented in sucroseformamide gradients at 17S and 19S, respectively, and were clearly separable. These RNAs were among the major poly(A) RNA species synthesized in the cytoplasm of actinomycin D-treated cells late in infection, and the in vitro translation of size-fractionated RNA released from polysomes confirmed that they represent physiological mRNA's. These results suggest that the expression of the coronavirus JHM genome involves more than one subgenomic mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Dickson C. Cell-free synthesis of mouse mammary tumor virus Pr77 from virion and intracellular mRNA. J Virol. 1979 Mar;29(3):1131–1141. doi: 10.1128/jvi.29.3.1131-1141.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon R. M., Griffin D. E., McCormick U., Weiner L. P. Mouse hepatitis virus-induced recurrent demyelination. A preliminary report. Arch Neurol. 1975 Jan;32(1):32–35. doi: 10.1001/archneur.1975.00490430054008. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Stohlman S. A. RNA of mouse hepatitis virus. J Virol. 1978 May;26(2):236–242. doi: 10.1128/jvi.26.2.236-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Messenger RNA isolation with poly(U) agarose. Methods Enzymol. 1974;34:496–499. doi: 10.1016/s0076-6879(74)34061-x. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978 Oct 13;44(1):63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Demyelinating encephalomyelitis induced by a long-term corona virus infection in rats. A preliminary report. Acta Neuropathol. 1979 Mar 15;45(3):205–213. doi: 10.1007/BF00702672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Harvey R., Smith A. E. Cell-free synthesis of simian virus 40 T-antigens. J Virol. 1978 Oct;28(1):154–170. doi: 10.1128/jvi.28.1.154-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Bond C. W. Pathogenic murine coronaviruses. I. Characterization of biological behavior in vitro and virus-specific intracellular RNA of strongly neurotropic JHMV and weakly neurotropic A59V viruses. Virology. 1979 Apr 30;94(2):352–370. doi: 10.1016/0042-6822(79)90467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G. RNA hybridization to DNA coupled with cyanogen-bromide-activated sephadex. The purification of polyoma messenger RNA. Eur J Biochem. 1978 Dec;92(2):621–629. doi: 10.1111/j.1432-1033.1978.tb12785.x. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Kamen R., Mangel W. F., Shure H., Wheeler T. Location of the sequences coding for capsid proteins VP1 and VP2 on polyoma virus DNA. Cell. 1976 Nov;9(3):481–487. doi: 10.1016/0092-8674(76)90093-3. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Wheeler T., Glanville N., Käriäinen Translation of Semliki-Forest-virus 42-S RNA in a mouse cell free system to give virus-coat proteins. Eur J Biochem. 1974 Nov 1;49(1):101–110. doi: 10.1111/j.1432-1033.1974.tb03815.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. A., Alexander D. J., Almeida J. D., Cunningham C. H., Easterday B. C., Garwes D. J., Hierholzer J. C., Kapikian A., Macnaughton M. R., McIntosh K. Coronaviridae: second report. Intervirology. 1978;10(6):321–328. doi: 10.1159/000148996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Müller A., ter Meulen V. Genomic RNA of the murine coronavirus JHM. J Gen Virol. 1978 Nov;41(2):217–227. doi: 10.1099/0022-1317-41-2-217. [DOI] [PubMed] [Google Scholar]
- Wege H., Wege H., Nagashima K., ter Meulen V. Structural polypeptides of the murine coronavirus JHM. J Gen Virol. 1979 Jan;42(1):37–47. doi: 10.1099/0022-1317-42-1-37. [DOI] [PubMed] [Google Scholar]
- Weiland E., Mussgay M., Weiland F. Nonproducer malignant tumor cells with rescuable sarcoma virus genome isolated from a recurrent Moloney sarcoma. J Exp Med. 1978 Aug 1;148(2):408–423. doi: 10.1084/jem.148.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E. G. Strategy of the flavivirus genome: evidence for multiple internal initiation of translation of proteins specified by Kunjin virus in mammalian cells. Virology. 1977 Jul 15;80(2):320–335. doi: 10.1016/s0042-6822(77)80008-1. [DOI] [PubMed] [Google Scholar]
- Wheeler T., Bayley S. T., Harvey R., Crawford L. V., Smith A. E. Cell-free synthesis of polyoma virus capsid proteins VP1 and VP2. J Virol. 1977 Jan;21(1):215–224. doi: 10.1128/jvi.21.1.215-224.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]