Abstract
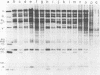
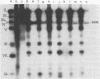
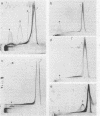
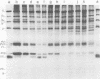
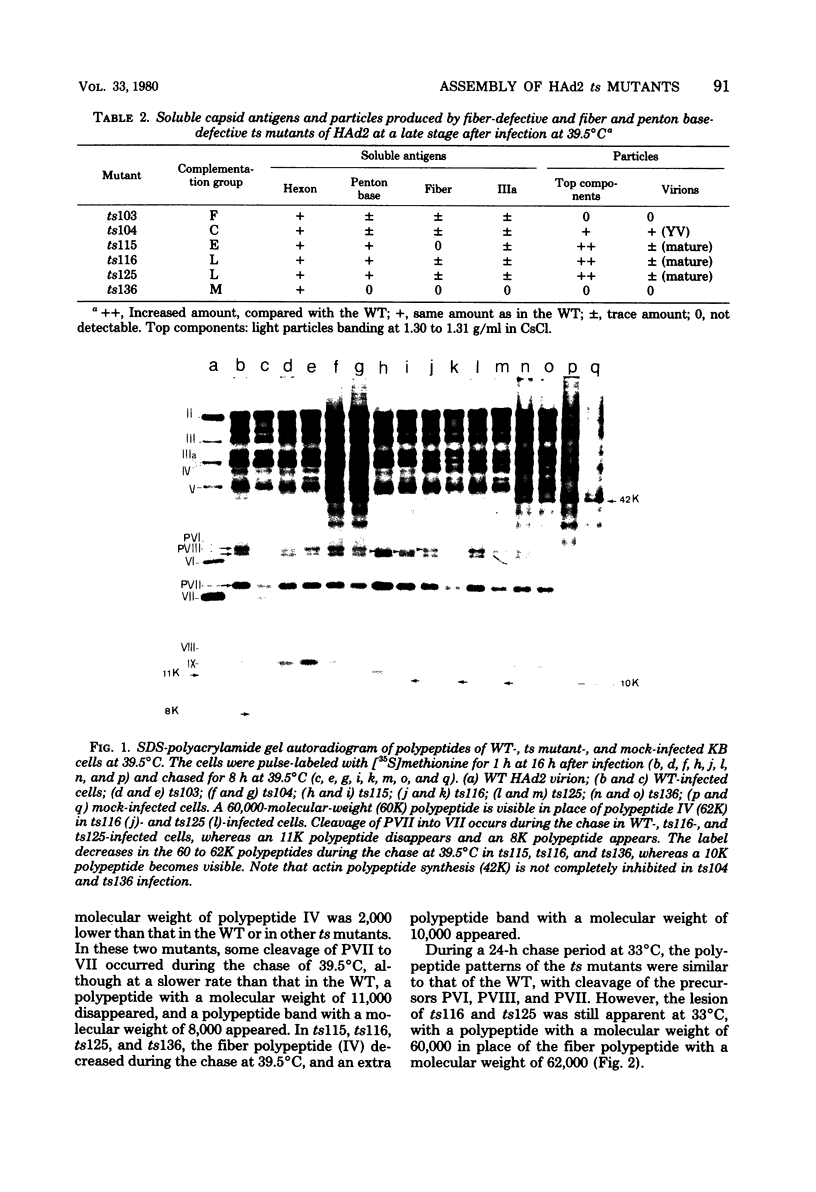
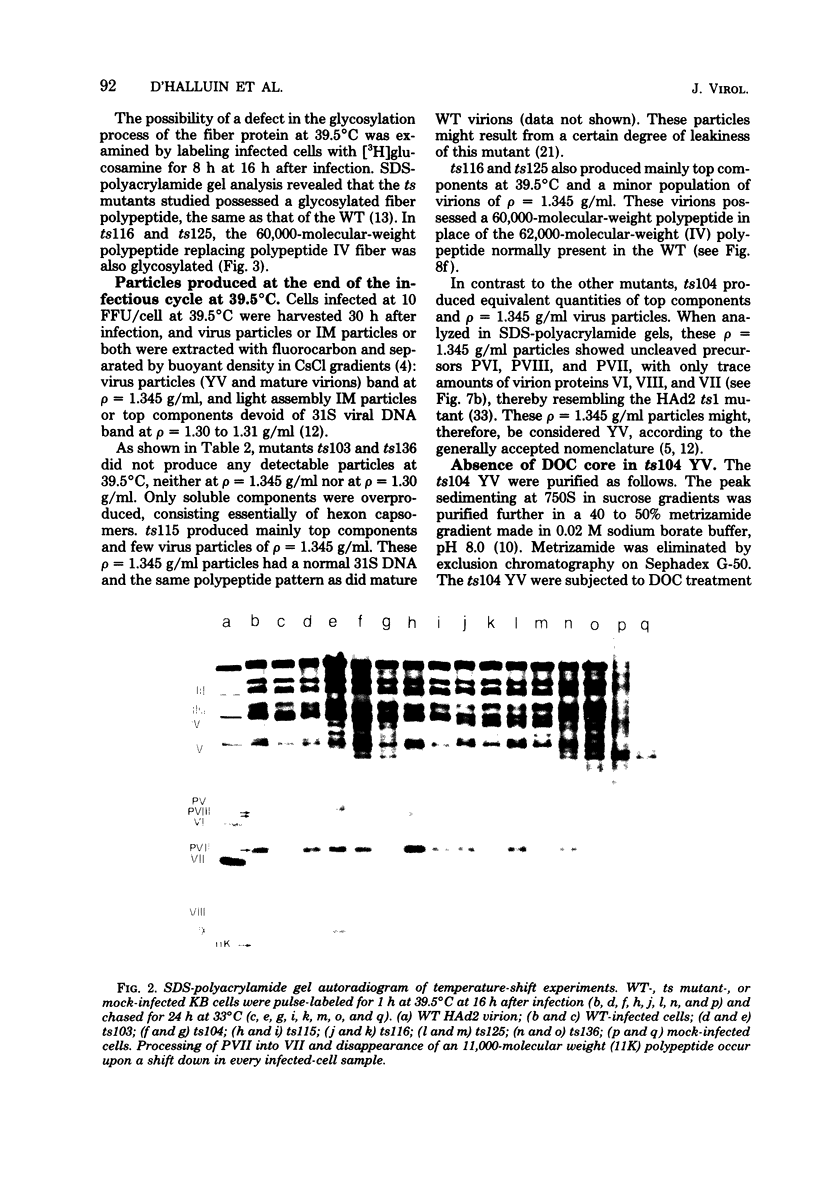
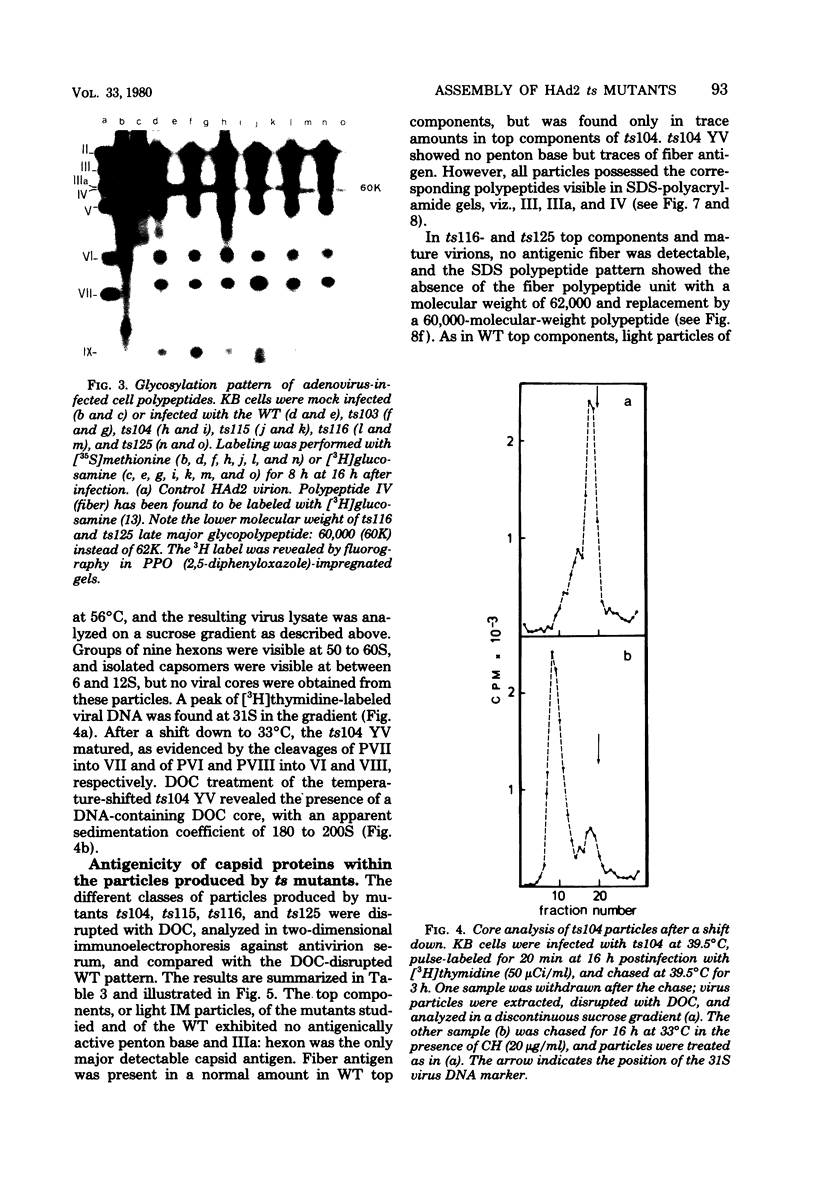
The nature, polypeptide composition, and antigenic composition of the particles formed by six human adenovirus type 2 temperature-sensitive (ts) mutants were studied. ts115, ts116, and ts125 were phenotypically fiber-defective mutants, and ts103, ts104, and ts136 failed to synthesize detectable amounts of fiber plus penton base at 39.5 degrees C. The mutants belonged to five complementation groups, one group including ts116 and ts125. Except for ts103 and ts136, the other mutants were capable of producing particles at 39.5 degrees C. ts116 and ts125 accumulated light assembly intermediate particles (or top components) at nonpermissive temperatures, with few virus particles. The sodium dodecyl sulfate polypeptide pattern of ts116- or ts125-infected cells, intermediate particles, and virus particles showed that polypeptide IV (fiber) was smaller by a molecular weight of 2,000 than that in the wild-type virion and was glycosylated. In fiber plus penton base-defective ts104-infected cells, equivalent quantities of top components and viruses with a buoyant density (rho) of 1.345 g/ml (rho = 1.345 particles) were produced at 39.5 degrees C. These rho = 1.345 particles corresponded to young virions, as evidenced by the presence of uncleaved precursors to proteins VI, VIII, and VII. These young virions matured upon a shift down. Virus capsid vertex antigenic components underwent a phase of eclipse during their incorporation into mature virus particles. No antigenic penton base or IIa was detected in intermediate particles of all the ts mutants tested. Only hexon and traces of fiber antigens were found in ts104 young virions. Penton base and IIIa appeared as fully antigenically expressed capsid subunits in mature wild-type virions or ts104 virions after a shift down. The ts104 lesion is postulated to affect a regulatory function related in some way to penton base and fiber overproduction and the maturation processing of precursors PVI, PVII, and PVII.
Full text
PDF



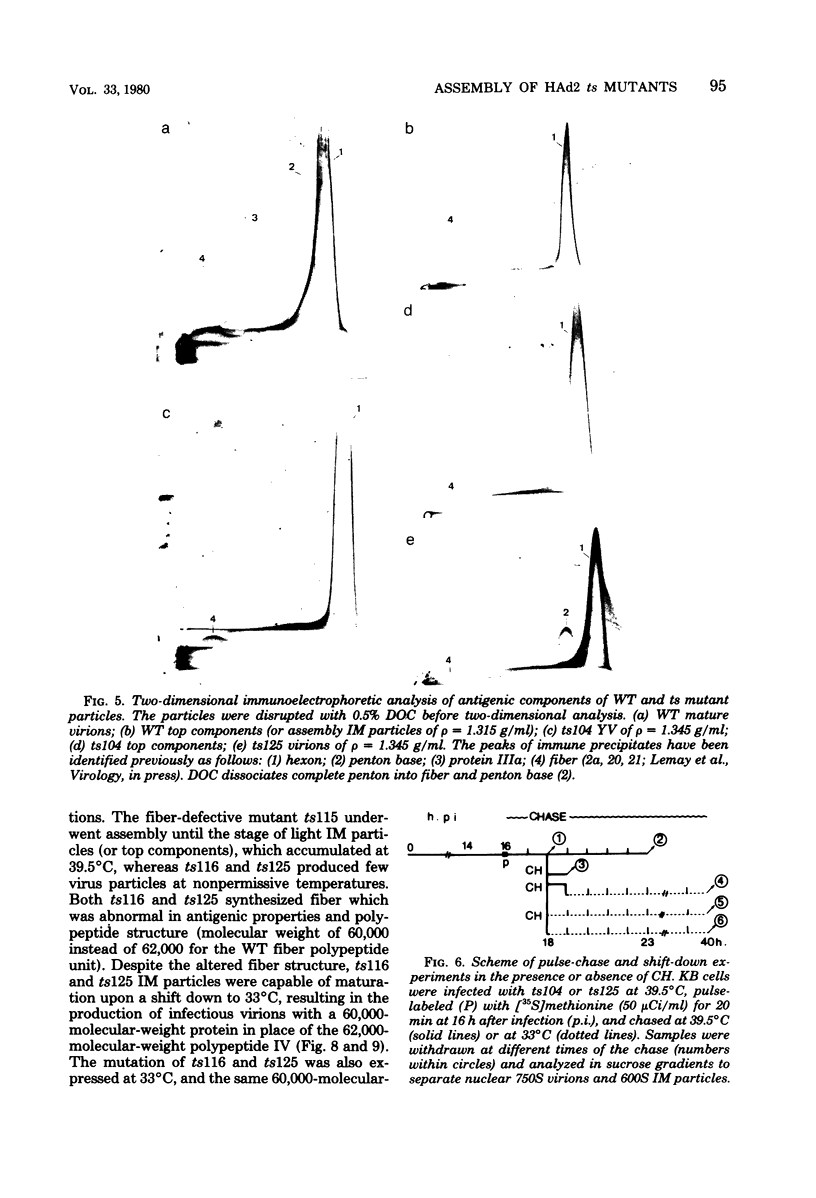
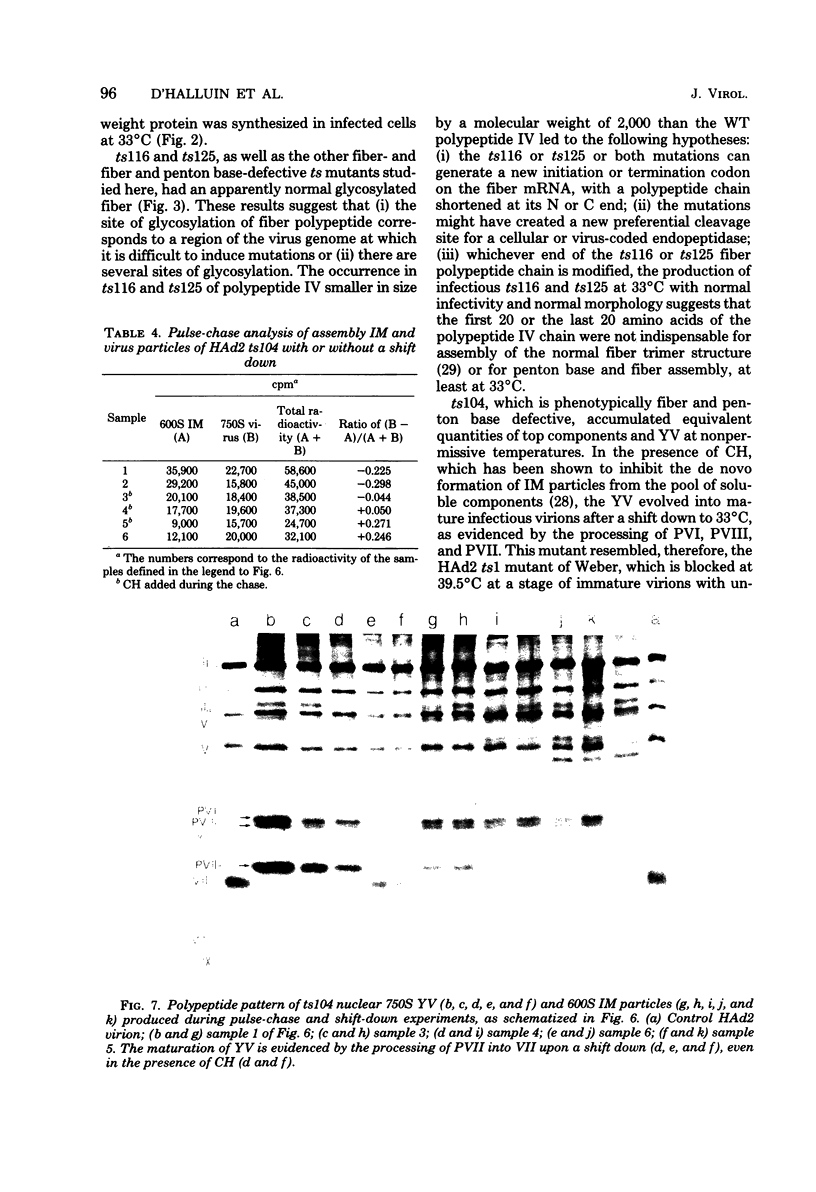

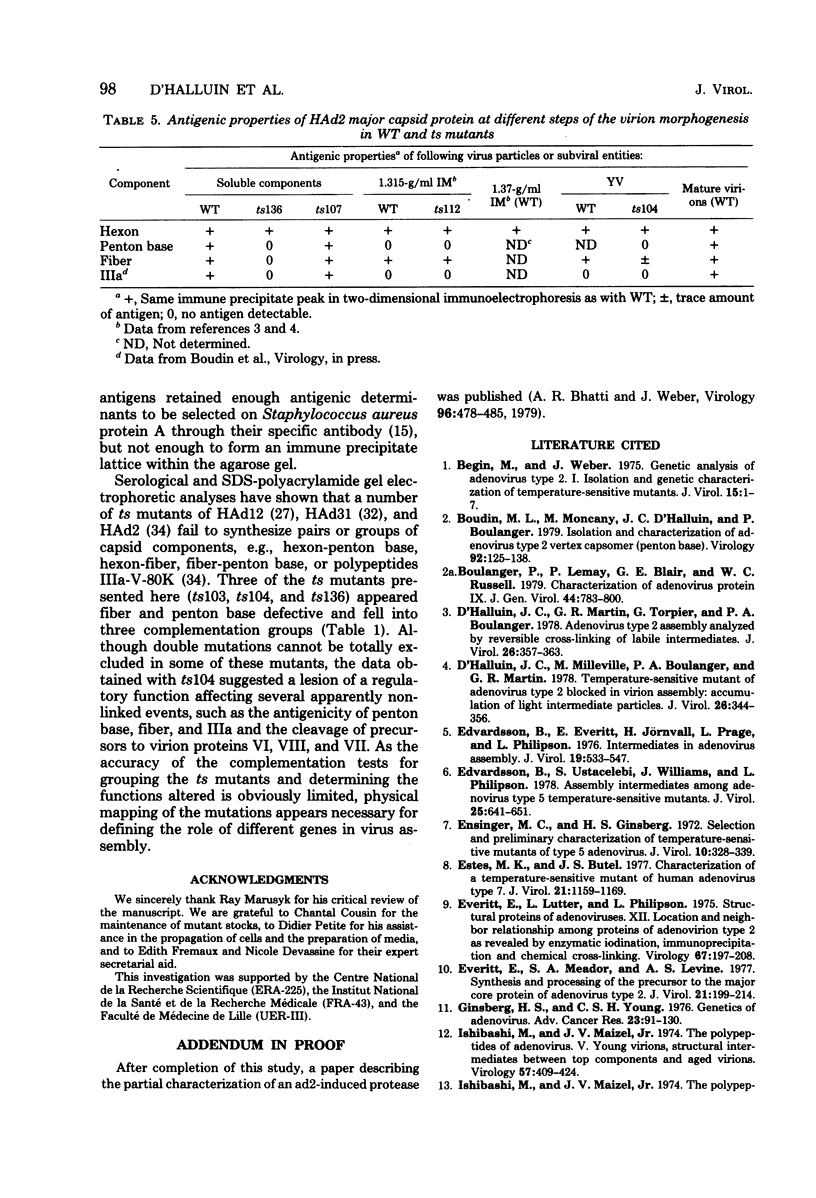

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin M., Mirza A., Weber J. Genetic analysis of adenovirus type 2. VII. Cleavage-modified affinity for DNA of internal virion proteins. Virology. 1977 Jul 1;80(1):83–97. doi: 10.1016/0042-6822(77)90382-8. [DOI] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2: partial characterization. Virology. 1979 Jul 30;96(2):478–485. doi: 10.1016/0042-6822(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., Moncany M., D'Halluin J. C., Boulanger P. A. Isolation and characterization of adenovirus type 2 vertex capsomer (penton base). Virology. 1979 Jan 15;92(1):125–138. doi: 10.1016/0042-6822(79)90219-8. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Lemay P., Blair G. E., Russell W. C. Characterization of adenovirus protein IX. J Gen Virol. 1979 Sep;44(3):783–800. doi: 10.1099/0022-1317-44-3-783. [DOI] [PubMed] [Google Scholar]
- Bégin M., Weber J. Genetic analysis of adenovirus type 2. I. Isolation and genetic characterization of temperature-sensitive mutants. J Virol. 1975 Jan;15(1):1–7. doi: 10.1128/jvi.15.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Martin G. R., Torpier G., Boulanger P. A. Adenovirus type 2 assembly analyzed by reversible cross-linking of labile intermediates. J Virol. 1978 May;26(2):357–363. doi: 10.1128/jvi.26.2.357-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. A., Martin G. R. Temperature-sensitive mutant of adenovirus type 2 blocked in virion assembly: accumulation of light intermediate particles. J Virol. 1978 May;26(2):344–356. doi: 10.1128/jvi.26.2.344-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson B., Ustacelebi S., Williams J., Philipson L. Assembly intermediates among adenovirus type 5 temperature-sensitive mutants. J Virol. 1978 Feb;25(2):641–651. doi: 10.1128/jvi.25.2.641-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Butel J. S. Characterization of a temperature-sensitive mutant of human adenovirus type 7. J Virol. 1977 Mar;21(3):1159–1169. doi: 10.1128/jvi.21.3.1159-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Young C. S. Genetics of adenoviruses. Adv Cancer Res. 1976;23:91–130. doi: 10.1016/s0065-230x(08)60544-8. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. V. Young virions, structural intermediate between top components and aged virions. Virology. 1974 Feb;57(2):409–424. doi: 10.1016/0042-6822(74)90181-0. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. VI. Early and late glycopolypeptides. Virology. 1974 Apr;58(2):345–361. doi: 10.1016/0042-6822(74)90070-1. [DOI] [PubMed] [Google Scholar]
- Kathmann P., Schick J., Winnacker E. L., Doerfler W. Isolation and characterization of temperature-sensitive mutants of adenovirus type2. J Virol. 1976 Jul;19(1):43–53. doi: 10.1128/jvi.19.1.43-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Wrigley N. G., Pereira H. G. Removal of pentons from particles of adenovirus type 2. Virology. 1969 Nov;39(3):599–604. doi: 10.1016/0042-6822(69)90111-1. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Temperature-sensitive mutants of adenovirus type 12 defective in viral DNA synthesis. J Virol. 1974 Sep;14(3):457–468. doi: 10.1128/jvi.14.3.457-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Boulanger P. A. Quantitation of adenovirus soluble antigens by crossed immunoelectrophoresis: application to serological characterization of mutants. Intervirology. 1975;5(3-4):162–172. doi: 10.1159/000149893. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Cousin C., D'Halluin J. C., Boulanger P. A. Isolation and phenotypic characterization of human adenovirus type 2 temperature-sensitive mutants. J Gen Virol. 1978 Nov;41(2):303–314. doi: 10.1099/0022-1317-41-2-303. [DOI] [PubMed] [Google Scholar]
- McGuire J. C., Pène J. J., Barrow-Carraway J. Gene expression during the development of bacteriophage phi 29. 3. Analysis of viral-specific protein synthesis with suppressible mutants. J Virol. 1974 Mar;13(3):690–698. doi: 10.1128/jvi.13.3.690-698.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. G., Wrigley N. G. In vitro reconstitution, hexon bonding and handedness of incomplete adenovirus capsid. J Mol Biol. 1974 Jan 5;85(4):617–630. doi: 10.1016/0022-2836(74)90319-2. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel W. C., Newman C., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5--serology. J Gen Virol. 1972 Dec;17(3):265–279. doi: 10.1099/0022-1317-17-3-265. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Irisawa J., Shimojo H. Isolation and a preliminary characterization of temperature-sensitive mutants of adenovirus 12. Virology. 1972 Jul;49(1):1–11. doi: 10.1016/s0042-6822(72)80002-3. [DOI] [PubMed] [Google Scholar]
- Sundquist B., Everitt E., Philipson L., Hoglund S. Assembly of adenoviruses. J Virol. 1973 Mar;11(3):449–459. doi: 10.1128/jvi.11.3.449-459.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M. T., Lischwe M. A., Richards J. C., Hosokawa K. Adenovirus chromatin I. Isolation and characterization of the major core protein VII and precursor Pro-VII. J Biol Chem. 1977 Jul 25;252(14):4981–4987. [PubMed] [Google Scholar]
- Sunquist B., Pettersson U., Thelander L., Philipson L. Structural proteins of adenoviruses. IX. Molecular weight and subunit composition of adenovirus type 2 fiber. Virology. 1973 Jan;51(1):252–256. doi: 10.1016/0042-6822(73)90389-9. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H., Moritsugu Y. Isolation and a preliminary characterization of temperature-sensitive mutants of adenovirus 31. Virology. 1972 Aug;49(2):426–438. doi: 10.1016/0042-6822(72)90495-3. [DOI] [PubMed] [Google Scholar]
- Weber J., Begin M., Carstens E. B. Genetic analysis of adneovirus type 2. IV. Coordinate regulation of polypeptides 80K, IIIa, and V. Virology. 1977 Feb;76(2):709–724. doi: 10.1016/0042-6822(77)90253-7. [DOI] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F., Gharpure M., Ustacelebi S., McDonald S. Isolation of temperature-sensitive mutants of adenovirus type 5. J Gen Virol. 1971 May;11(2):95–101. doi: 10.1099/0022-1317-11-2-95. [DOI] [PubMed] [Google Scholar]
- Wills E. J., Russell W. C., Williams J. F. Adenovirus-induced crystals: studies with temperature-sensitive mutants. J Gen Virol. 1973 Sep;20(3):407–412. doi: 10.1099/0022-1317-20-3-407. [DOI] [PubMed] [Google Scholar]