Abstract
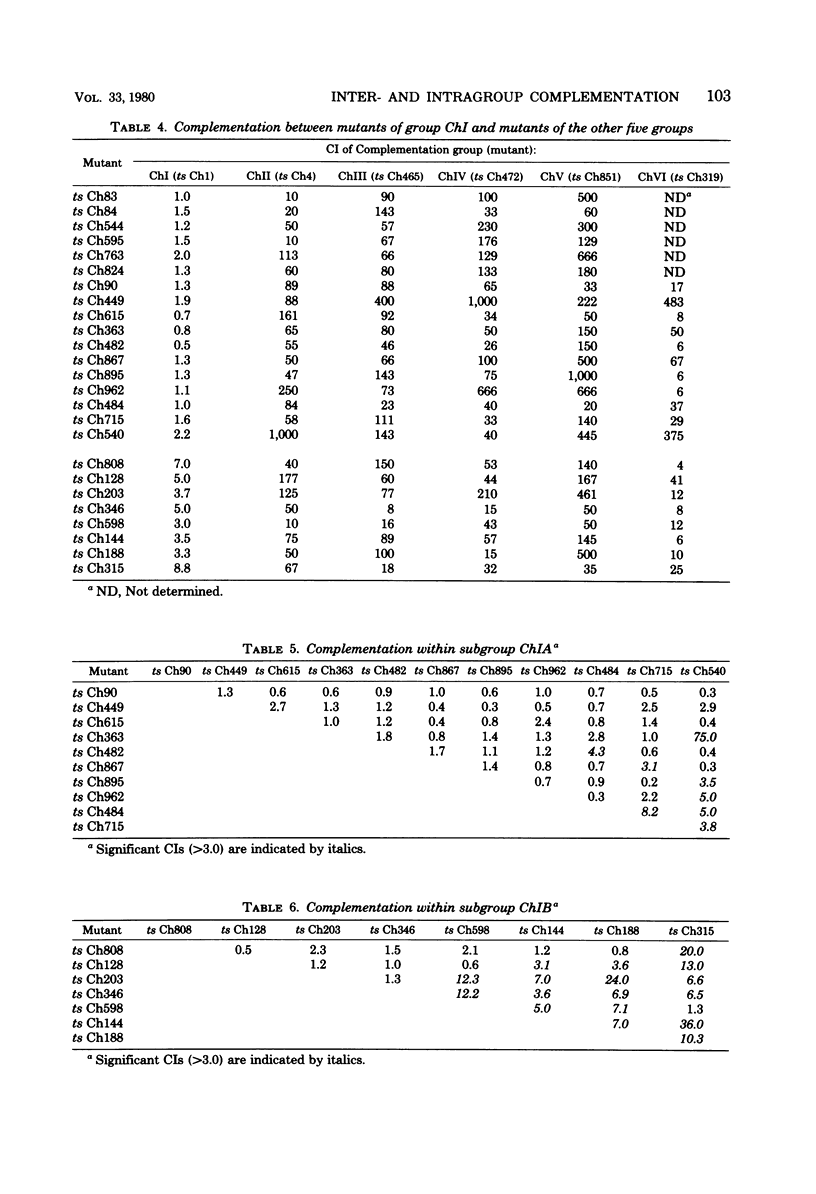
Fifty temperature-sensitive (ts) mutants of Chandipura virus, a human rhabdovirus, have been classified into six complementation groups, designated ChI, ChII, ChII, ChIV, ChV, and ChVI and containing 44, 2, 1, 1, 1, and 1 mutants, respectively. Weak complementation was observed within group ChI, allowing the division of the group into subgroups ChIA and ChIB. Intragroup complementation was most extensive within subgroup ChIB, and one mutant in this subgroup complemented all but one (ts Ch598) of the mutants in group ChI. If ts Ch598 had been omitted from the analysis the number of complementation groups would have been increased to seven. Consequently, in circumstances where intragenic and intergenic complementation cannot be clearly distinguished, the number of complementation groups identified in rhabdoviruses could be overestimated. The identification of six complementation groups in three different rhabdoviruses need not imply the existence of an as yet unidentified sixth virus-specified polypeptide. The extensive intragroup complementation observed in Chandipura virus suggests that the functional form of one at least of the virion proteins of Chandipura virus is a multimer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatt P. N., Rodrigues F. M. Chandipura: a new Arbovirus isolated in India from patients with febrile illness. Indian J Med Res. 1967 Dec;55(12):1295–1305. [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972 Sep;16(3):391–398. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. Virus-induced demyelination. Production by a viral temperature-sensitive mutant. J Neurol Sci. 1979 Jun;42(1):155–168. doi: 10.1016/0022-510x(79)90159-x. [DOI] [PubMed] [Google Scholar]
- Dragúnová J., Závada J. Cross-neutralization between vesicular stomatitis virus type Indiana and Chandipura virus. Acta Virol. 1979 Jul;23(4):319–328. [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkari D. A., Pringle C. R. Temperature-sensitive mutants of Chandipura virus. II. Phenotypic characteristics of the six complementation groups. J Virol. 1980 Jan;33(1):107–114. doi: 10.1128/jvi.33.1.107-114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez H. B., Pringle C. R. Seven complementation groups of respiratory syncytial virus temperature-sensitive mutants. J Virol. 1978 Sep;27(3):459–464. doi: 10.1128/jvi.27.3.459-464.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Interallelic complementation of mutants of herpes simplex virus deficient in deoxypyrimidine kinase activity. Virology. 1978 Mar;85(1):109–117. doi: 10.1016/0042-6822(78)90415-4. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Langerman N. R., Darnall D. W. Quaternary structure of proteins. Annu Rev Biochem. 1970;39:25–62. doi: 10.1146/annurev.bi.39.070170.000325. [DOI] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Weiss R. A. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979 Apr;30(1):177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet C., Combard A., Printz-Ané C., Printz P. Envelope proteins and replication of vesicular stomatitis virus: in vivo effects of RNA+ temperature-sensitive mutations on viral RNA synthesis. J Virol. 1979 Jan;29(1):123–133. doi: 10.1128/jvi.29.1.123-133.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. The tdCE and hrCE phenotypes: host range mutants of vesicular stomatitis virus in which polymerase function is affected. Cell. 1978 Oct;15(2):597–606. doi: 10.1016/0092-8674(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Wunner W. H. Genetic and physiological properties of temperature-sensitive mutants of Cocal virus. J Virol. 1973 Oct;12(4):677–683. doi: 10.1128/jvi.12.4.677-683.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Dumont R., Baltimore D. Screening procedure for complementation-dependent mutants of vesicular stomatitis virus. J Virol. 1975 Jan;15(1):41–49. doi: 10.1128/jvi.15.1.41-49.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutation on activity of the RNA transcriptase of vesicular stomatitis virus New Jersey. J Virol. 1979 Jun;30(3):692–700. doi: 10.1128/jvi.30.3.692-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Virion trascriptase activity differences in host range mutants of vesicular stomatitis virus. J Virol. 1975 Oct;16(4):927–936. doi: 10.1128/jvi.16.4.927-936.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Holloway A. F., Cormack D. V. Characterization of three complementation groups of vesicular stomatitis virus. Virology. 1972 Dec;50(3):829–840. doi: 10.1016/0042-6822(72)90437-0. [DOI] [PubMed] [Google Scholar]
- Zabin I., Villarejo M. R. Protein complementation. Annu Rev Biochem. 1975;44:295–313. doi: 10.1146/annurev.bi.44.070175.001455. [DOI] [PubMed] [Google Scholar]


