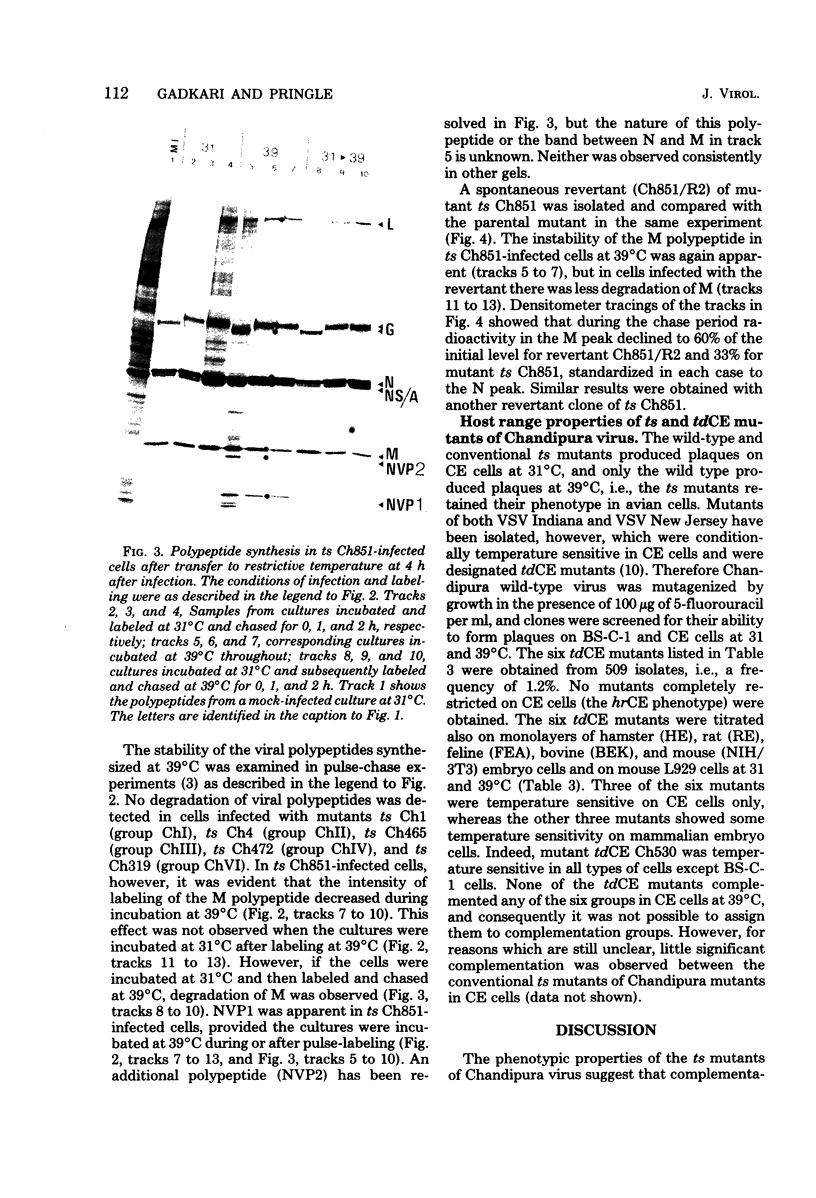
Abstract
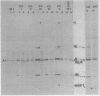
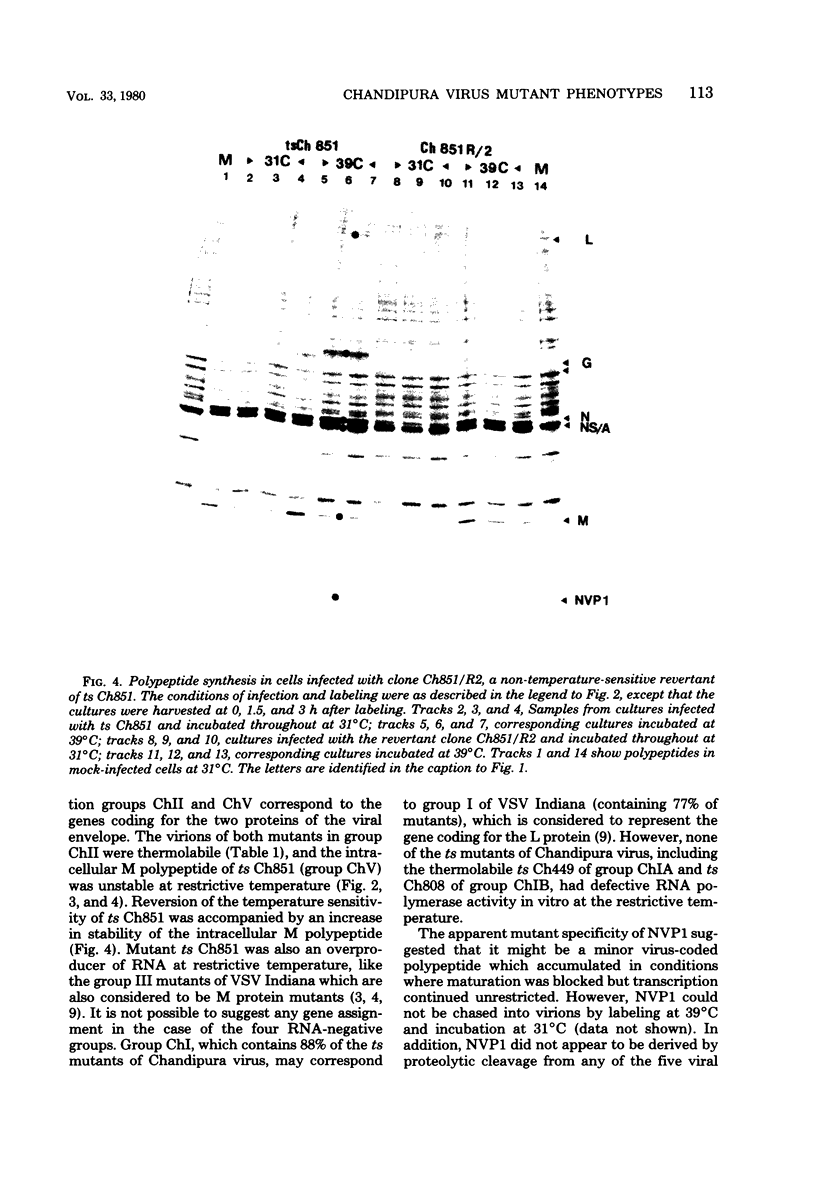
Fifty temperature-sensitive (ts) mutants of the rhabdovirus Chandipura virus have been classified into six complementation groups designated ChI to ChVI. Group ChI contains 44 mutants, group ChII contains 2 mutants, and the remaining groups have 1 mutant each. Mutants in groups ChI, ChIII, ChIV, and ChVI had RNA-negative phenotypes in experiments measuring amplification of RNA synthesis at restrictive temperature. The two mutants in group ChII had RNA-positive phenotypes, and the virions were thermolabile. Mutant ts Ch851 of group ChV was also RNA positive, and the M polypeptide of this mutant appeared to be unstable in cells incubated at restrictive temperature. It is likely, therefore, that complementation groups ChII and ChV represent the genes coding for the two viral proteins of the virion envelope. No precise assignment can be made in the case of the four RNA-negative groups, since all the mutants examined showed some polymerase activity in vitro at restrictive temperature. An attempt to obtain polymerase mutants by screening for sensitivity to rifampin was not successful. Six temperature-dependent host range mutants (the tdCE phenotype) of Chandipura virus failed to multiply in chicken embryo cells at restrictive temperature, but otherwise they differed in their host range properties from similar mutants of vesicular stomatitis virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gadkari D. A., Pringle C. R. Temperature-sensitive mutants of Chandipura virus. I. Inter- and intragroup complementation. J Virol. 1980 Jan;33(1):100–106. doi: 10.1128/jvi.33.1.100-106.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Lodish H. F., Baltimore D. Localization of two cellular forms of the vesicular stomatitis viral glycoprotein. J Virol. 1977 Mar;21(3):1121–1127. doi: 10.1128/jvi.21.3.1121-1127.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Moreau M. C. Inhibition of a vesicular stomatitis virus mutant by rifampin. J Virol. 1974 Sep;14(3):517–521. doi: 10.1128/jvi.14.3.517-521.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. The tdCE and hrCE phenotypes: host range mutants of vesicular stomatitis virus in which polymerase function is affected. Cell. 1978 Oct;15(2):597–606. doi: 10.1016/0092-8674(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutation on activity of the RNA transcriptase of vesicular stomatitis virus New Jersey. J Virol. 1979 Jun;30(3):692–700. doi: 10.1128/jvi.30.3.692-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutations on the virion-associated RNA transcriptase of vesicular stomatitis virus. J Mol Biol. 1972 Nov 14;71(2):281–291. doi: 10.1016/0022-2836(72)90351-8. [DOI] [PubMed] [Google Scholar]