Abstract
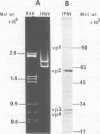
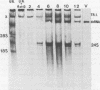
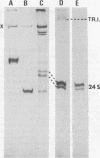
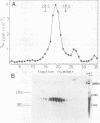
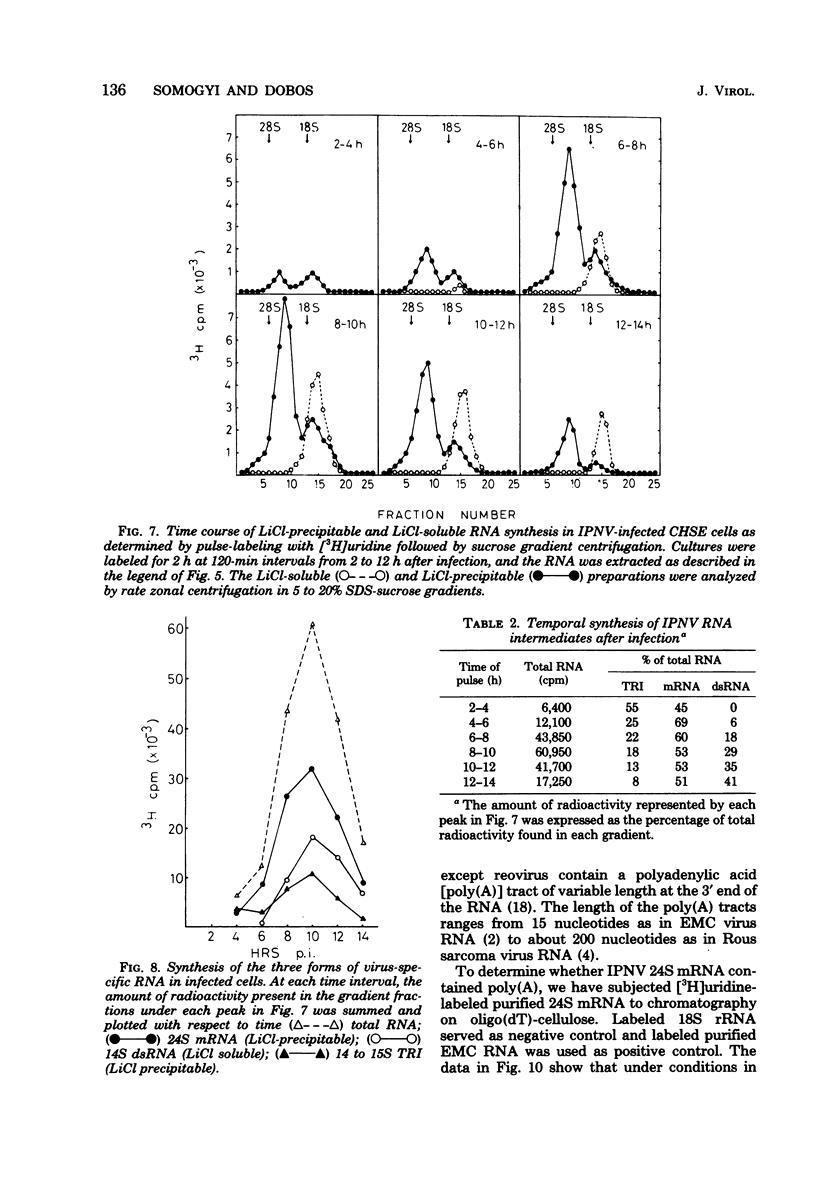
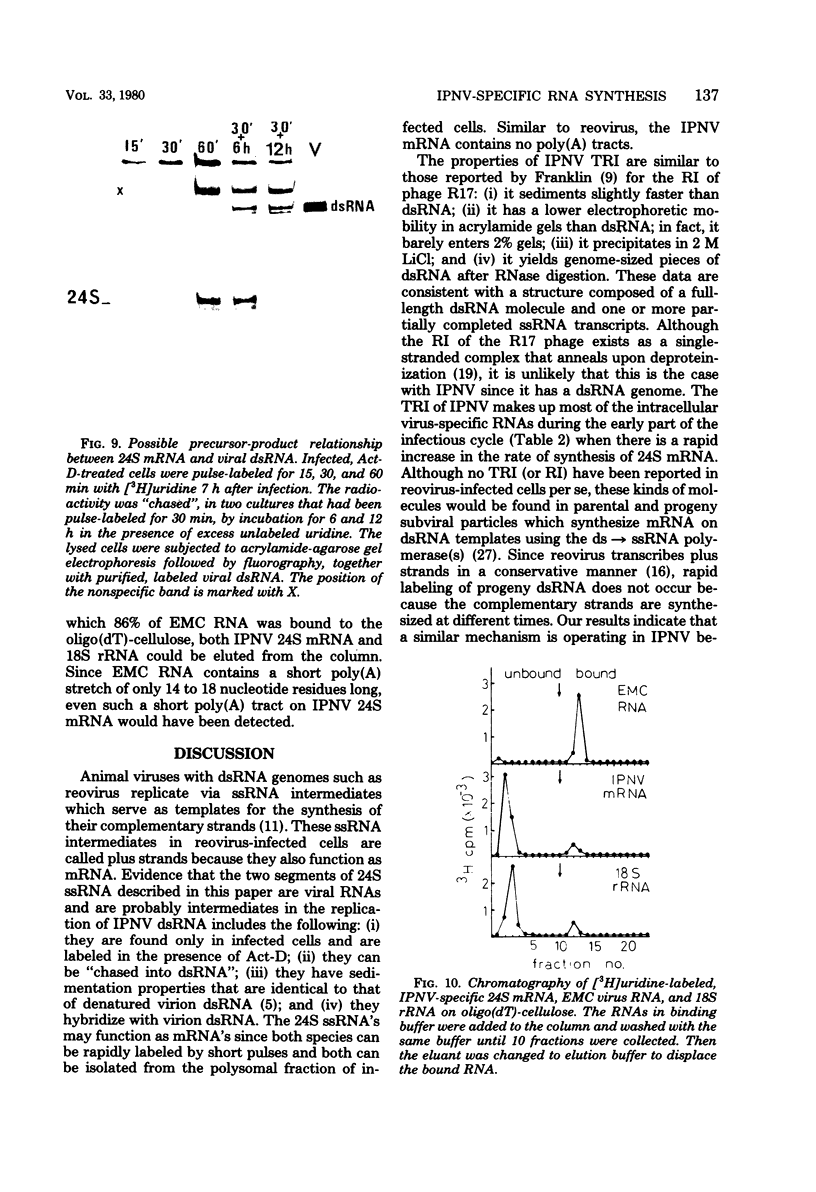
Pulse-labeling experiments with [3H]uridine revealed that the rate of infections pancreatic necrosis virus-specific RNA synthesis was maximal at 8 to 10 h after infection and was completely diminished by 12 to 14 h. Three forms of RNA intermediates were detected: (i) a putative transcription intermediate (TRI) which comigrated in acrylamide gels with virion double-stranded RNA (dsRNA) after RNase treatment; (ii) a 24S genome length mRNA which could be resolved into two bands by polyacrylamide gel electrophoresis; and (iii) a 14S dsRNA component indistinguishable from virion RNA by gradient centrifugation and gel electrophoresis. The TRI (i) was LiCl precipitable; (ii) sedimented slightly faster and broader (14 to 16S) than the 14S virion dsRNA; (iii) had a lower electrophoretic mobility in acrylamide gels than dsRNA, barely entering acrylamide gels as a heterogenous component; (iv) yielded genome-sized pieces of dsRNA after RNase digestion; and (v) was the most abundant RNA form early in the infectious cycle. The 24S single-stranded RNA was thought to be the viral mRNA since it: (i) became labeled during short pulses; (ii) was found in the polysomal fraction of infected cells; and (iii) hybridized to denatured viral RNA, forming two segments of RNase-resistant RNA that comigrated with virion dsRNA in gels. The 24S mRNA component was formed before the synthesis of dsRNA, and radioactivity could be chased from 24S single-stranded RNA to dsRNA, indicating that 24S RNA may serve as template for the synthesis of complementary strands to form dsRNA. Similar to reovirus, infectious pancreatic necrosis viral 24S mRNA contained no polyadenylic acid tracts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I. U., Duffy E. M., Bhalla R. B., Goldstein N. O. The size and location of the poly(A) tract in EMC virus RNA. J Gen Virol. 1977 Feb;34(2):331–344. doi: 10.1099/0022-1317-34-2-331. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Villa-Komaroff L., Lodish H. F., Schlesinger M. Initiation sites for translation of sindbis virus 42S and 26S messenger RNAs. Cell. 1975 Oct;6(2):215–222. doi: 10.1016/0092-8674(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Dobos P., Hallett R., Kells D. T., Sorensen O., Rowe D. Biophysical studies of infectious pancreatic necrosis virus. J Virol. 1977 Apr;22(1):150–159. doi: 10.1128/jvi.22.1.150-159.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Rowe D. Peptide map comparison of infectious pancreatic necrosis virus-specific polypeptides. J Virol. 1977 Dec;24(3):805–820. doi: 10.1128/jvi.24.3.805-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Size and structure of the genome of infectious pancreatic necrosis virus. Nucleic Acids Res. 1976 Aug;3(8):1903–1924. doi: 10.1093/nar/3.8.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Virus-specific protein synthesis in cells infected by infectious pancreatic necrosis virus. J Virol. 1977 Jan;21(1):242–258. doi: 10.1128/jvi.21.1.242-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jense H., Knauert F., Ehrenfeld E. Two initiation sites for translation of poliovirus RNA in vitro: comparison of LSc and Mahoney strains. J Virol. 1978 Oct;28(1):387–394. doi: 10.1128/jvi.28.1.387-394.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Yamamoto T. The structure of infectious pancreatic necrosis virus RNA. J Gen Virol. 1977 Feb;34(2):235–247. doi: 10.1099/0022-1317-34-2-235. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg M., Silverstein S. C., Levin D. H., Acs G. Asynchronous synthesis of the complementary strands of the reovirus genome. Proc Natl Acad Sci U S A. 1971 Feb;68(2):505–508. doi: 10.1073/pnas.68.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. F., Mindich L. RNA synthesis during infection with bacteriophage phi6. Virology. 1976 Nov;75(1):209–217. doi: 10.1016/0042-6822(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Shatkin A. J., Banerjee A. K. Absence of polyadenylic acid from reovirus messenger ribonucleic acid. J Biol Chem. 1973 Dec 10;248(23):7993–7998. [PubMed] [Google Scholar]
- Thach S. S., Thach R. E. Mechanism of viral replication. I. Structure of replication complexes of R17 bacteriophage. J Mol Biol. 1973 Dec 15;81(3):367–380. doi: 10.1016/0022-2836(73)90147-2. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. Strategy of the flavivirus genome: evidence for multiple internal initiation of translation of proteins specified by Kunjin virus in mammalian cells. Virology. 1977 Jul 15;80(2):320–335. doi: 10.1016/s0042-6822(77)80008-1. [DOI] [PubMed] [Google Scholar]
- Wiegers U., Hilz H. Rapid isolation of undegraded polysomal RNA without phenol. FEBS Lett. 1972 Jun 1;23(1):77–82. doi: 10.1016/0014-5793(72)80289-8. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Ito Y., Matsuhisa T. Synthesis of reovirus double-stranded RNA within virionlike particles. Virology. 1972 Nov;50(2):349–358. doi: 10.1016/0042-6822(72)90386-8. [DOI] [PubMed] [Google Scholar]