Abstract
Controversy surrounds the efficacy and safety of 17β-estradiol (E2)-mimetic therapies to women for treatment of menopausal symptoms. An important question is the nature of the trophic actions of E2-mimetics in the brain for behavioral processes versus in the periphery for beneficial effects related to osteoporosis, or unwanted proliferative effects in reproductive tissues, such as mammary glands and uterus. Of recent interest are the effects of selective estrogen receptor modulators (SERMs), which can have tissue specific actions, for these processes. In the present study, the effects was determined of E2 alone, or co-administered with a SERM, raloxifene, for anxiety-like, depression-like and trophic peripheral effects in ovariectomized rats that were exposed to a chemical carcinogen (7, 12-dimethylbenz(a)anthracene; DMBA), or not. Once per week, rats were administered vehicle, E2 (0.09 mg/kg) and/or raloxifene (1 mg/kg) s.c. 44–48 hours before testing in a positive control, E2-dependent behavior (lordosis), depression (forced swim test), and anxiety (elevated plus maze) behavioral assays. In addition to behavioral endpoints, incidence and number of tumors, and tumor, pituitary gland, and uterine weight 14 weeks after carcinogen-exposure, and weekly hormone treatments, were analyzed. Rats administered DMBA had increased number and size of tumors, compared to vehicle treatment. E2+raloxifene increased the number of tumors. Administration of E2 or E2+raloxifene, but not raloxifene alone, increased pituitary and uterine weight, compared to vehicle administration. E2 or E2+raloxifene, but not raloxifene alone, also increased incidence of lordosis and reduced depression-like behavior in the forced swim test (i.e. decreased time spent immobile) compared to vehicle administration. However, administration of E2 or raloxifene reduced anxiety behavior in the elevated plus maze (i.e. increased time spent on the open arms of the maze), compared to vehicle. Together these data demonstrate that E2 and/or raloxifene can have some effects to alter behavior of ovariectomized rodents, depending upon task. As well, E2, with or without raloxifene, can also have clear trophic actions in peripheral tissues, such as carcinogen-induced tumors, uterus, and pituitary glands.
Keywords: lordosis, estrogen receptor, selective estrogen receptor modulator, DMBA, tumor
Introduction
Menopause is characterized by ovarian cessation in the secretion of steroids, such as 17β-estradiol (E2) (Lund, 2008). Given the important role of E2 throughout adult life, this abrupt reduction in ovarian E2 can produce such severe physical and psychological symptoms, in an estimated one-quarter of menopausal women, that pharmacological interventions will be sought (Dickson and Henriques, 1992; Lund, 2008). Effects on bone health, as well as mood/quality of life, are some of the major reasons for women to seek menopausal hormone treatments, which include E2-mimetics (Bhavnani and Strickler, 2005; Stovall and Pinkerton, 2008). However, these types of E2-mimetic treatments can increase risks of some women for cardiovascular complications and reproductive cancers (breast, uterine; Collaborative Group on Hormonal Factors in Breast Cancer, 1997; Colditz et al., 1995; Magnusson et al., 1999). A similar pattern is observed in preclinical rodent models. For example, we have demonstrated that E2 to female rats can have positive effects to reduce anxiety behaviors, but it can increase carcinogen-induced tumor incidence and uterine proliferation (Walf and Frye, 2009a,b). The source of positive and negative effects of estrogens in the central nervous system and peripheral tissues may be related to divergent mechanisms of action.
The greater understanding of the cellular mechanisms of estrogens in specific tissues in recent years has led to the formulation of E2-mimetics, called selective estrogen receptor modulators (SERMs). SERMs, like ER agonists or antagonists, bind estrogen receptors, and can function as either estrogen agonists or antagonists in different types of tissue (Katzenellenbogen and Katzenellenbogen, 2002). The three characteristics that interact and define SERMs are that: 1) ER expression differs across tissues, 2) ER conformation differs for ligand binding, and 3) there is different co-regulator protein expression and binding to the ER (Riggs and Hartmann, 2003). The prototypical SERM is tamoxifen, which has been used clinically to treat ER-positive breast cancer for over 50 years because of its actions as an ER antagonist in the breast (MacGregor and Jordan, 1998; Wang et al., 2009). However, tamoxifen has unwanted side effects, including increasing the risk of thromboembolisms, stroke, and endometrial cancer (Jordan and Morrow, 1999). Raloxifene is a more recently characterized SERM that was approved for use in 1997 by the U.S. Food and Drug Administration for osteoporosis prevention (MacGregor and Jordan, 1998). Raloxifene is an agonist on bone (Delmas et al., 1997; Ettinger et al., 1999) and lipid metabolism (Delmas et al., 1997), but an antagonist in the breast (Cumming et al., 1999) and uterus (Mitlak and Cohen, 1999; Delmas et al., 1997). There is some indication in clinical studies that raloxifene can be an agonist in the central nervous system. In a clinical trial of postmenopausal women with osteoporosis, raloxifene was not associated with negative changes on affect or cognition (Nickelsen et al., 1999). Among healthy, postmenopausal women, raloxifene decreased anxiety/fear self-rated scores (Strickler et al., 2000) and quality of life measures (Utian et al., 2004) at a 12-month follow-up. Similarly, depression and anxiety scores were reduced in non-depressed postmenopausal women in a randomized, double-blind study on prevention of osteoporosis by raloxifene (Jarkova et al., 2002). A question of continued interest is the role of raloxifene for central nervous system function that may be complementary to its positive effects in the bone and other ER-rich tissues.
To date, there are a handful of rodent studies that have been published that have demonstrated the beneficial effects of raloxifene for central nervous system function. In rats, raloxifene increases potassium-stimulated acetylcholine release (Gibbs et al., 2004), choline acetyltransferase activity (Wu et al., 199), and glutamate AMPA and NMDA receptor binding (Cyr et al., 2001a,b), in the hippocampus. Functionally, raloxifene produces similar taste aversion in young, ovariectomized rats as does tamoxifen (Fudge et al, 2009), and can reduce immobility of rats in a two-day forced swim test (Karahancer et al., 2008). Raloxifene may have neuroprotective effects. In support, following experimentally-induced traumatic brain injury, male rats have fewer sensorimotor and working memory deficits, despite little evidence of reduced lesion size, following post-injury administration of raloxifene (3 mg/kg at 15 minutes, and hours 24, 48, 72, and 96; Kokiko et al., 2006). Neuroprotective effects in in vitro neurotoxicity models (e.g. toxicity due to glutamate, hydrogen peroxide, β-amyloid) have been demonstrated with application of raloxifene (O’Neil et al., 2004). Although these studies suggest beneficial effects of raloxifene in the central nervous system, the effects of raloxifene for these central nervous system processes in relation to trophic effects in the body are not known. Moreover, raloxifene acts as an agonist in some tissues when no E2 is present, but can have antagonistic effects (O’Neil et al., 2004) or agonistic effects (Walf and Frye, 2006) in the presence of E2. As such, the present study tested the hypothesis that E2 and raloxifene, alone or in combination, would have different behavioral (anxiety, depression, sexual responding) and peripheral trophic effects (tumor, uterus, pituitary growth). It was predicted that E2 would have clear behavioral effects and trophic actions in peripheral tissues; whereas, raloxifene would have a greater effect on behavior than on growth in the periphery at this dosing. Furthermore, it was predicted that raloxifene in conjunction with E2 may reverse effects of E2 alone.
Methods
The methods utilized in this study conform to the accepted standards of humane animal use and were approved by the Institutional Animal Care and Use Committee at The University at Albany- SUNY.
Subjects and Housing
Subjects (N= 65) were adult (~8 weeks old) female, Long-Evans rats from our colony, with original breeders obtained from Taconic Farms (Germantown, NY). Rats were group-housed, with 3–5 rats per cage. Cages were polycarbonate (45 × 24 × 21 cm) and contained woodchips for bedding. Rats were housed in these cages in a temperature-controlled room (21 ± 1 °C), on a reversed-lighting schedule (lights off at 8:00 am), in the core Laboratory Animal Care Facility of The Life Sciences Research Building at The University at Albany-SUNY. Rats had free access to commercial rodent chow and tap water in their home cages.
Ovariectomy
All rats were ovariectomized, using typical methods, under anesthesia (xylazine 12 mg/kg; Bayer Corp., Shawnee Mission, KS and ketamine 60 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA). Surgery occurred one week before initiation of experimental protocol (described in Procedure, below).
Hormone administration
Each week, rats were administered vegetable oil vehicle or E2 (0.09 mg/kg in vegetable oil vehicle; Steraloids, Newport, RI), and/or raloxifene (1 mg/kg in propylene glycol vehicle; generously supplied by Eli Lilly & Company; Indianapolis, Indiana). These treatments were administered 44–48 hours before behavioral testing. E2 dosing was based upon previous studies in our lab that have shown that this regimen produces physiological E2 levels in plasma and brain at time of testing, decreases anxiety behavior, and increases lordosis of rats when they are tested 44–48 hours after injection (Frye et al., 1998; Walf & Frye, 2005; 2009a,b). Raloxifene dosing was based upon pilot studies done in the lab in rats and mice, and the literature (Takahata et al., 2008; Walf and Frye, 2006). Because higher dosages of raloxifene (over 1 mg/kg) disrupt estrous cyclicity, and can reduce fertility, of young rats (Hoyt et al., 1998), a lower dosage of raloxifene was intentionally utilized so that its physiologically-relevant effects and/or interactions with E2 could be observed and parsed out. Rats were administered these compounds once a week to allow for sufficient washout before re-administration and behavioral testing the following week.
DMBA-induced tumor model
Rats are most susceptible to tumor induction in mammary glands between 45 and 60 days of age because there are high rates of grandular epithelium proliferation at this time. Administration of the chemical carcinogen, 7, 12-dimethylbenz(a)anthracene (DMBA) to intact female rats between 30 and 45 days of age produces 100% tumor incidence, but the greatest tumor severity (as per the tumor-affected animal index) is reached when DMBA is administered to rats that are 46–50 days of age (Russo and Russo, 1996). As such, adult female rats, between 50 and 60 days of age, in the present study were administered a single dosing of an inert control substance (i.e. vegetable oil) or DMBA (Sigma; St. Louis, MO), as per described methods (Walf and Frye, 2009a,b).
DMBA (12.5 mg) was dissolved in vegetable oil before administration via gavage, using a curved animal feeding needle that was 3 inches long, 16-gauge, with a 3 mm diameter ball on the end. This dosing of DMBA was based upon dose-response studies of Huggins and our laboratory (Huggins, 1965; Walf and Frye, 2009a,b). With higher dosing of DMBA (20 mg/kg) all rats survive carcinogen exposure, but there are shorter latencies to tumor growth and greater incidence of tumors, at this dosing than at lower dosages. With this high dosing, there is 100% incidence of tumors between 8 and 21 weeks following administration (Russo and Russo, 1996). We have recently demonstrated that lower dosing can produce discernible differences in tumor incidence with E2-modulation of young adult, ovariectomized rats within 14 weeks (Walf and Frye, 2009a,b). Given that these past studies in our laboratory, and the present investigation, had behavioral endpoints, it was important to use sub-optimal dosing of DMBA and a shorter latency to the study endpoint. Given these parameters, tumors that were produced were small, most clearly detectable at necropsy, and did not hamper the mobility of rats in behavioral tasks, which allowed concurrent examination of behavior and peripheral trophic effects. Mean tumor wet weights are included in Table 1.
Table 1.
The number of tumors, and wet weight of tumors, pituitaries, and uteri of OVX rats administered vehicle or DMBA, and vehicle, E2 and/or raloxifene
| DMBA condition | E2 condition | n | # tumors | Tumor weight (mg) | Pituitary Weight (mg) | Uterine weight (mg) |
|---|---|---|---|---|---|---|
| vehicle | Vehicle | 8 | 0.5 ± 0.3 | 16 ± 3 | 12 ± 1 | 56 ± 6 |
| E2 | 7 | 1.0 ± 0.3 | 20 ± 6 | 14 ± 3+ | 112 ± 32* | |
| Raloxifene | 7 | 0.8 ± 0.3 | 22 ± 4 | 13 ± 1 | 73 ± 11 | |
| E2 + raloxifene | 7 | 1.7 ± 0.5* | 20 ± 4 | 14 ± 1+ | 113 ± 15* | |
| DMBA | Vehicle | 8 | 1.5 ± 0.3^ | 24 ± 4# | 11 ± 1 | 53 ± 8 |
| E2 | 8 | 1.4 ± 0.3^ | 30 ± 3# | 14 ± 1+ | 163 ± 15* | |
| Raloxifene | 10 | 1.2 ± 0.2^ | 18 ± 3# | 12 ± 1 | 67 ± 6 | |
| E2 + raloxifene | 10 | 1.8 ± 0.1^* | 25 ± 2# | 15 ± 1+ | 130 ± 14* |
Values are mean ± SEM.
indicates significant effect of DMBA vs. no carcinogen exposure (P≤0.05).
indicates a tendency for DMBA to have a significant effect versus no carcinogen exposure (P≤0.10)
indicates significant effect of E2 or E2+raloxifene versus vehicle or raloxifene alone (P≤0.05).
indicates significant effect of E2 or E2+raloxifene versus vehicle (P≤0.05).
Procedure
Rats were randomly assigned to be administered an inert vehicle or DMBA at the beginning of the study, one week after ovariectomy. Rats in these conditions were then assigned to their hormone condition, which they received on a weekly basis for 14 weeks (as per Walf and Frye, 2009a,b): vehicle, E2, raloxifene, or E2+raloxifene. Rats were behaviorally tested (as described below) and, at the end of the study, tumors (if present), pituitary glands, and uterine tissue were collected and weighed.
Behavioral Testing
Experimental rats were tested in the following tasks in the same order. Data were collected by trained observers and the Any-maze video-tracking system (Stoelting, Inc., Wood Dale, IL).
Sexual behavior
Rats were tested for sexual behavior, or the lordosis posture (dorsiflexion which allows males to intromit during mating), as a measure of an E2-dependent behavioral response. They were tested using typical methods, which involve placing the experimental female rat in a Plexiglas chamber (50 × 25 × 30 cm) with a sexually-experienced male rat (Frye et al., 1998). The lordosis quotients, or frequency of lordosis postures assumed when the female was mounted by a male, were recorded for rats for 10 mounts or 10 minutes, whichever occurred first, as is standard protocol for this task. The majority of female rats received 10 mounts by the sexually-experienced male rat (n=33 of 57), and in the unlikely event that rats received fewer than 4 mounts (n=5 of 57), their data were excluded from analyses. Technical difficulties precluded the analyses of data from 8 rats for this measure. The n’s for each group for this measure are included in the Figure 1 legend.
Figure 1.
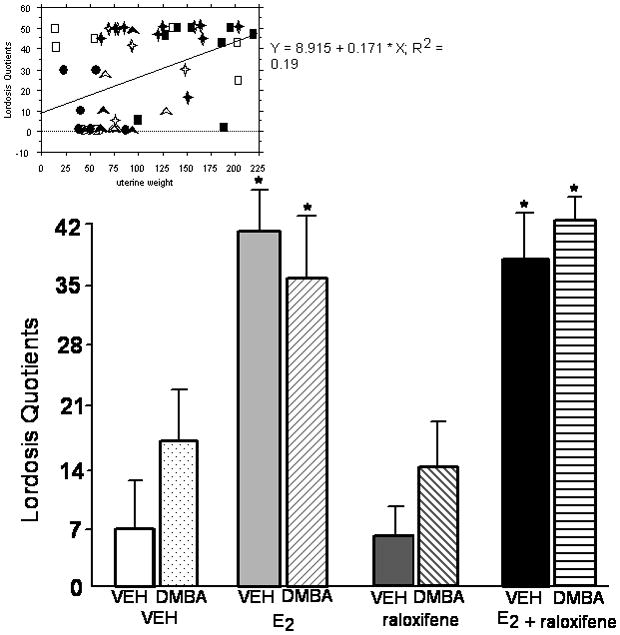
Mean (+ sem) lordosis quotients of ovariectomized rats administered vehicle or DMBA, and vehicle, E2 and/or raloxifene. * significant effect of E2 and/or raloxifene vs. vehicle (P≤ 0.05). Simple regression analyses are included at the top of the figure. Open symbols indicate the vehicle condition and closed symbols indicate the DMBA condition. Circles, squares, triangles, and diamonds indicate vehicle (n=6 DMBA, n=6 vehicle), E2 (n=8 DMBA, n=6 vehicle), raloxifene (n=5 DMBA, n=6 vehicle), and E2 + raloxifene (n=9 DMBA, n=6 vehicle), respectively.
Forced Swim Test
The forced swim test is a typically-used behavioral assay for depression-like behavior of rodents. It was done as previously described in detail (Frye and Walf, 2002). Briefly, rats were placed in a cylindrical chamber (45 cm high, 20 cm diameter; Stoelting), filled to 30 cm of room temperature (30 °C) tap water. The amount of time rats spent swimming or struggling (as measures of activity behavior) versus immobile (as a measure of depression-like behavior) following placement in the chamber was recorded for ten minutes.
Elevated Plus Maze
The elevated plus maze is a typically-used behavioral assay for anxiety-like behavior of rodents. In this task, experimental rats were placed in the center of the maze, between the two closed and two open arms (Walf and Frye, 2007a). The time spent by rats on the open arms of the maze, during the five minute test, was recorded and used a measure on reduced anxiety-like behavior. The number of total arm entries made by rats was used as an index of general motor behavior in this task.
Tissue Collection
Rats were euthanized by rapid decapitation. They were palpated and visually inspected to determine presence of tumors. If tumors were present, they were dissected out and weighed. Pituitary glands and uteri of rats was dissected out and weighed.
Statistical Analyses
Two-way analyses of variance tests (ANOVAs) were utilized to determine effects of hormone condition and DMBA condition on all endpoints. If significant main effects were found, group differences were determined by Fisher’s post hoc tests. Because uterine weight can be used as a proxy for estrogenic effects of compounds, simple regression analyses were also utilized to determine the extent to which uterine weights accounted for the variability in the behavioral effects assessed. A p-value of ≤0.05 was considered significant and a p-value of ≤ 0.10 was considered a tendency.
Results
Peripheral trophic effects
As expected, we found that exposure to the chemical carcinogen and E2 had trophic effects in peripheral tissues. DMBA exposure increased group tumor incidence of rats administered vehicle (87.5%), raloxifene (80%), E2 (87.5%), or both E2 and raloxifene (100%). There was a significant main effect of DMBA condition for the number of tumors in rats [F(1,57)=4.26, P<0.05], and a tendency for differences in mean wet weights [F(1,40)=2.95, P=0.09], in that DMBA increased both number and wet weights of tumors compared to vehicle (Table 1). There was a significant main effect of hormone condition for the number of tumors [F(3,57)=2.67, P<0.05], but not wet weight of tumors. Compared to vehicle or raloxifene alone, E2+raloxifene significantly increased the number of tumors that developed (Table 1). There were no significant interactions between hormone condition and DMBA condition for number of tumors or their wet weight.
There were no significant main effects of DMBA, or interaction between variables, for pituitary or uterine weight. Hormone condition significantly altered pituitary [F(3,54)=2.86, P<0.05] and uterine weights [F(3,57)=14.73, P<0.01]. E2 and E2+raloxifene significantly increased pituitary weights compared to vehicle (Table 1). E2 and E2+raloxifene significantly increased uterine weights compared to vehicle or raloxifene alone (Table 1).
Behavioral effects
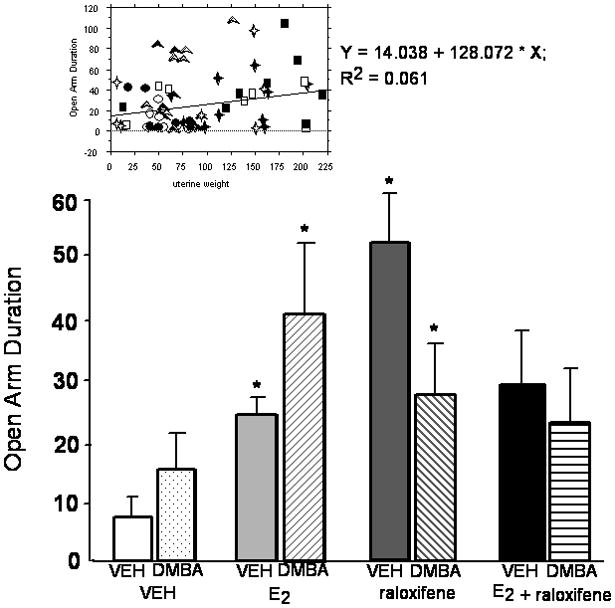
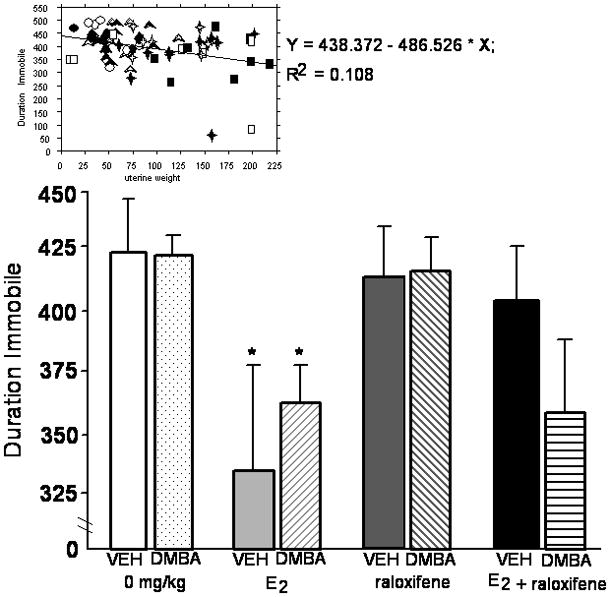
Simple regression analyses demonstrated that there were positive relationships between uterine weight and lordosis quotients (r2= 0.19, P<0.01), immobility in the forced swim test (r2= 0.11, P<0.05), and duration spent on the open arms of the elevated plus maze (r2= 0.11, P<0.05). Scatter plots of these data are included at the top of each figure for these measures (Figures 1–3).
Figure 3.
Mean (+ sem) duration spent immobile in the forced swim test of ovariectomized rats administered vehicle or DMBA, and vehicle, E2 and/or raloxifene. * significant effect of E2 and/or raloxifene vs. vehicle (P≤ 0.05). Simple regression analyses are included at the top of the figure. Open symbols indicate the vehicle condition and closed symbols indicate the DMBA condition. Circles, squares, triangles, and diamonds indicate vehicle (n=8 DMBA, n=8 vehicle), E2 (n=8 DMBA, n=7 vehicle), raloxifene (n=10 DMBA, n=7 vehicle), and E2 + raloxifene (n=10 DMBA, n=7 vehicle), respectively.
As expected, hormone condition altered behavior in the E2-dependent measure, lordosis quotients [F(3,44)=15.27, P<0.01]. E2 and E2+raloxifene significantly increased lordosis quotients compared to administration of raloxifene or vehicle (Figure 1). There was no significant main effect of DMBA, or interaction between hormone and DMBA condition, for lordosis quotients.
A similar pattern was observed in the forced swim test as was shown with lordosis. There was a significant main effect of hormone treatment, but not DMBA condition or an interaction between hormone and DMBA condition, for duration spent immobile in the forced swim test [F(3,57)=2.93, P<0.05]. Rats administered E2 spent significantly less time immobile in the forced swim test than did rats administered vehicle or raloxifene (Figure 2). There were neither significant main effects, nor interactions, of hormone and DMBA condition for duration spent struggling or swimming in this task (Table 2).
Figure 2.
Mean (+ sem) duration spent on the open arms of the elevated plus maze of ovariectomized rats administered vehicle or DMBA, and vehicle, E2 and/or raloxifene. * significant effect of E2 and/or raloxifene vs. vehicle (P≤ 0.05). Simple regression analyses are included at the top of the figure. Open symbols indicate the vehicle condition and closed symbols indicate the DMBA condition. Circles, squares, triangles, and diamonds indicate vehicle (n=8 DMBA, n=8 vehicle), E2 (n=8 DMBA, n=7 vehicle), raloxifene (n=10 DMBA, n=7 vehicle), and E2 + raloxifene (n=10 DMBA, n=7 vehicle), respectively.
Table 2.
The number of entries in the elevated plus maze, duration spent swimming and struggling in the forced swim test of OVX rats administered vehicle or DMBA, and vehicle, E2 and/or raloxifene.
| DMBA condition | Hormone condition | n | Total entries in the elevated plus maze | Duration spent swimming in the forced swim test (secs) | Duration spent struggling in the forced swim test (secs) |
|---|---|---|---|---|---|
| vehicle | Vehicle | 8 | 6.0 ± 0.9 | 51.8 ± 13.5 | 126.6 ± 17.3 |
| E2 | 7 | 9.1 ± 1.8 | 79.2 ± 42.0 | 184.6 ± 15.2 | |
| Raloxifene | 7 | 11.3 ± 1.3 | 31.8 ± 7.3 | 153.3 ± 20.8 | |
| E2 + raloxifene | 7 | 7.7 ± 1.6 | 32.2 ± 4.8 | 160.3 ± 18.0 | |
| DMBA | Vehicle | 8 | 10.1 ± 1.4 | 41.0 ± 22.3 | 136.5 ± 19.7 |
| E2 | 8 | 9.9 ± 1.1 | 66.1 ± 31.5 | 173.8 ± 27.8 | |
| Raloxifene | 10 | 8.3 ± 2.3 | 30.7 ± 9.0 | 154.6 ± 13.5 | |
| E2 + raloxifene | 10 | 12.0 ± 2.0 | 55.9 ± 26.1 | 186.6 ± 26.3 |
Values are mean ± SEM.
There was a slightly different pattern in the elevated plus maze following treatment. There was a significant effect of hormone condition [F(3,57)=2.94, P<0.05], but not DMBA condition, or the interaction between these variables, for time spent on the open arms of the elevated plus maze. Rats administered E2 or raloxifene spent more time on the open arms than did rats administered vehicle (Figure 3). There were no significant differences due to hormone or DMBA condition, or the interaction between these variables, for total entries made in this task (Table 2).
Discussion
The results of the present study partially supported our hypothesis that there would be discernible effects of E2 and raloxifene, administered alone or when co-administered, for behavior and trophic effects in peripheral, ER-rich tissues. Rats administered DMBA had increased incidence and number of tumors, compared to inert vehicle treatment. E2+raloxifene increased the number of tumors compared to vehicle or raloxifene alone. Administration of E2, or E2+raloxifene, but not raloxifene alone, increased pituitary and uterine weight, compared to vehicle administration. In our positive control E2-dependent measure of lordosis, E2 or E2+raloxifene, but not raloxifene alone, increased lordosis quotients compared to vehicle administration. Administration of E2, compared to vehicle or raloxifene alone, reduced depression-like behavior in the forced swim test (i.e. decreased time spent immobile). A different pattern emerged for anxiety-like behavior in the elevated plus maze. Administration of E2 or raloxifene alone, but not co-administration of these compounds, reduced anxiety behavior in the elevated plus maze (i.e. increased time spent on the open arms of the maze), compared to vehicle. Notably, the behavioral effects of raloxifene and E2 in the present study occurred without clear non-specific effects on motor measures in the affective tasks utilized (i.e. struggling and swimming in the forced swim test and total arm entries in the plus maze). These results demonstrate that E2 and/or raloxifene can have some effects to increase sexual responding and improve affective behavior of ovariectomized rats. E2 can also have clear trophic actions in peripheral tissues, such as carcinogen-induced tumors, uteri, and pituitary glands, which are not apparent with raloxifene administration alone or altered by raloxifene co-administration. Thus, these data of discernible trophic effects of E2 and raloxifene suggest that there may be divergent actions of these compounds at ER-rich brain and peripheral targets.
The present data confirm previous results on the trophic effects of E2 in the central nervous system and periphery. We and others have previously demonstrated that physiological levels of E2 that are akin to those observed in behavioral estrous increase lordosis and reduce anxiety- and depression-like behavior, similar to the effects observed in the present study (Pfaff, 2005; Kow and Pfaff, 2004; Mazzucco et al., 2008; Walf and Frye, 2005a,b; 2006; 2009a,b). Furthermore, there is evidence for E2 to increase spontaneous and carcinogen-induced tumors as well as uterine proliferation (Leung et al., 2003; Walf and Frye, 2009a,b), and this predicted effect was observed in the present study. However, there are some inconsistencies in the reported effects of E2 for tumor incidence and growth in rat models that need to be addressed. Low physiological E2 levels can stimulate DMBA-induced tumor growth (Walf and Frye, 2009a,b), but supraphysiological E2 dosing can inhibit this growth (Callejo et al., 2005; Ohi and Yoshida, 1992). The different regimens of E2 in these studies may account for these differences. In the present study, and in other recent studies in our laboratory, E2 (0.09 mg/kg) was administered once weekly, 44–48 hours before behavioral testing to mimic physiological rise in E2 of young intact rats across the estrous cycle (Walf and Frye, 2009a,b). This dosing increased incidence of tumors and number of tumors formed (Walf and Frye, 2009a,b). More chronic dosing, such as 5 mg/kg of estradiol valerate daily for 6 months, had an opposite effect to reduce DMBA-induced tumor growth (Callejo et al., 2005). In addition to different E2 regimen, these studies differ in the strain of rats and DMBA dosing utilized, which may also account for some of the inconsistencies observed for modulation of tumor progression by E2.
Another question that needs to be addressed is the mechanism for these effects on peripheral tissues and behavior. For instance, studies to date suggest that these effects of E2 on lordosis and affective behavior may partly be due to actions of E2 via ERs, specifically at the ERα isoform in the hypothalamus and ERβ in the hippocampus, respectively (Etgen, 1987; Mazzucco et al., 2008; Ogawa et al., 1998; Walf, 2010; Walf and Frye, 2007b; 2008). The influence of other ER-rich brain targets for these effects on anxiety and depression behavior, such as the amygdala, raphe nucleus, and frontal cortex, are also of interest (Donner and Handa, 2009; Hughes et al., 2008; Krezel et al., 2001). In the present study, the mechanisms for observed behavioral and peripheral effects of E2 and raloxifene were not elucidated, but recent evidence suggest that effects of E2 or SERMs in the brain and periphery may act at ERβ and ERα, respectively, for their trophic actions (Jensen et al., 2010; Walf, 2010). Ongoing studies in our laboratory are focused on elucidating whether actions at these ER forms, and the downstream pathways that they activate, may be related to the discernible effects of E2 and SERMs for brain function and proliferation in peripheral tissues.
The present results support the few studies to date that have investigated the functional effects of raloxifene on central nervous system processes. Similar to studies in women (Jarkova et al., 2002; Strickler et al., 2000), raloxifene reduced anxiety-like behavior in the elevated plus maze in a manner congruous to E2; however, there was no effect of this dosing of raloxifene for depression-like behavior in the present study, using the one-trial forced swimming test. Twelve days of daily treatment with raloxifene (1 mg/kg), to Sprague-Dawely rats that were ovariectomized for 21 days before treatment was initiated, showed anti-depressant-like effects in the two-day version of the forced swim test (Karahancer et al., 2008). As described as follows, other studies have demonstrated that raloxifene has modest, or no, effects, compared to E2 (Berendsen et al., 2001; Gibbs et al., 2004; Karahancer et al., 2008; Pinilla et al., 2002). Despite evidence for anti-depressant-like effects of raloxifene in the aforementioned study, there were no effects of this dosing of raloxifene for Morris water maze learning (Karahancer et al., 2008). In a delayed-matching-to-sample task, E2, but not raloxifene, improved performance with chronic dosing (Gibbs et al., 2004). In another study, unlike E2, raloxifene did not reduce tail temperature in an ovariectomy-induced “hot flash” rat model (Berendsen et al., 2001). Similar to effects on lordosis in the present study using once weekly, low dosing of raloxifene, no effects of low dosing of subchronic (3 days) raloxifene on lordosis behavior of adult ovariectomized rats were observed (Pinilla et al., 2002). Some of these differences in the patterns of effects may be due to dosing utilized. A low dosing of raloxifene was utilized in the present study. At high dosages (over 1 mg/kg) raloxifene disrupted estrous cyclicity, and reduced fertility; these effects could be reversed with discontinuation of treatment (Hoyt et al., 1998). As such, a lower dosage of raloxifene was intentionally utilized so that physiologically-relevant effects and/or interactions with E2 could be observed and parsed out. Furthermore, the present results in rats extend the previous studies on the effects of E2 and raloxifene co-administration for affective behavior in mice to begin to address whether there may be some species-specificity. We investigated the effects of E2 and raloxifene for affective behavior among aged (24–28 months old) congenic female mice administered vehicle, E2 (~0.1 mg/kg), and/or raloxifene (3 mg/kg). Unlike the present results in young, ovariectomized rats, we found that co-administration of E2+raloxifene had greater efficacy than E2 or raloxifene alone to decrease anxiety- and depression-like behavior of these aged mice across several affective tasks (Walf and Frye, 2006). These data suggest that there may be species-specificity effects for the effects of E2 and raloxifene co-administration that need to be investigated further. Together, these data suggest that E2 can enhance lordosis, and have effects to reduce anxiety and depression behavior, of rats, irrespective of co-administration of raloxifene; yet, raloxifene has more robust anxiety-reducing effects than effects to alter sexual or depression behavior in ovariectomized rats at the dosing utilized.
The results of this study confirm and extend previous studies investigating the trophic actions of raloxifene in peripheral tissues. Unlike E2, and the typically-prescribed SERM, tamoxifen, raloxifene is well-known for not increasing uterine proliferation, and the pattern of effects that we found in the present study supports these previous findings (Stygar et al., 2003; Yamamoto et al., 2005). A question for further consideration is the role of raloxifene for tumorigenesis in our model. As in the present results, other studies have demonstrated that DMBA typically produces adenocarcinomas and hormone-dependent tumors (Cheung et al., 2003; Russo et al., 1990; Russo and Russo, 1996; 1998). Also, E2 in high dosages alone, or when administered to DMBA-exposed rats, can increase tumorigenesis of female rats (Leung et al., 2003; Walf and Frye, 2009a,b). Effects of E2, alone or with raloxifene, in the present study, were more robust than those of raloxifene alone to increase tumor number and weight. Similarly, raloxifene does not reduce tumor burden from DMBA-induction among Sencar mice (Wurz et al., 2005). However, raloxifene (3 mg/kg daily) to ovariectomized rats reduced tumorigenesis, compared to that observed in intact Sprague-Dawley rats (a more tumor prone rat model than Long-Evans rats) at the study endpoint of 6 months in another study using DMBA-induction (Callejo et al., 2005). In the present study, raloxifene was administered in more moderate dosing, which could account for why raloxifene did not block the effects of E2, and/or have its own effects, on tumorigenesis. Also, synergistic effects of E2 and raloxifene were not found. It may be of some use for future studies to examine dose-response of raloxifene for these effects; however, raloxifene alone altered open arm time in the plus maze, which suggests that dosing utilized had some efficacy. Together, these data demonstrate that E2, but not raloxifene, can have clear trophic effects in uterine tissues and carcinogen-induced tumors.
Conclusions
This study is novel because an animal model was utilized to investigate the behavioral responses as well as peripheral trophic effects of E2 and raloxifene, which is a typical menopause hormone therapy for osteoporosis prevention. This study has clinical relevance as important health concerns for many postmenopausal women are breast cancer and osteoporosis with fractures, which increases morbidity and mortality substantially. Despite efficacy in treating osteoporosis, menopausal hormone therapy use is not advisable for many women who may be at increased risk for breast cancer as these therapies can further increase this risk. There is some recent evidence that raloxifene may even reduce primary breast cancer incidence in some high risk women, but these results are not unequivocal to date (Nelson et al., 2009; Thomsen and Kolesar, 2008). Some of the effects of raloxifene for tumors in reproductive tissues, bone density, and cholesterol levels may become refractory once treatment has ended, but long-term effects on central nervous system processes are not known. This study demonstrates that the magnitude of the effects of raloxifene in an animal model may differ for central nervous system function and in peripheral tissues. The present results partially supported the a priori hypothesis that there would be discernible effects of E2 and raloxifene, administered alone or when co-administered, for beneficial behavioral effects (anxiety, depression, sexual responding) versus trophic effects in peripheral, ER-rich tissues. The prediction that E2 would have clear behavioral effects and trophic actions in peripheral tissues, and raloxifene would have a greater effect on behavioral effects than on growth in the periphery, was supported by these results. These results also show that this dosing of raloxifene did not alter the effects of E2 to increase tumor, pituitary, or uterine weight, but had an apparent, non-statistically-significant effect to reduce the efficacy of E2 for decreasing anxiety- and depression-like behavior. Further investigation of the mechanisms, and functional trophic effects in the brain and periphery, of E2-mimetic therapies, such as raloxifene, is necessary.
Acknowledgments
This research was supported, in part, by grants from the Department of Defense CDMRP Breast Cancer Research Program and National Institute of Mental Health. Assistance, provided by Amy Kohtz, Carolyn Koonce, Danielle Llaneza, and Danielle Osborne, is greatly appreciated.
References
- Berendsen HH, Weekers AH, Kloosterboer HJ. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol. 2001;419:47–54. doi: 10.1016/s0014-2999(01)00966-9. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR, Strickler RC. Menopausal hormone therapy. J Obstet Gynaecol Can. 2005;27:137–62. doi: 10.1016/s1701-2163(16)30186-4. [DOI] [PubMed] [Google Scholar]
- Callejo J, Cano A, Medina M, Villaronga M, Gonzalez-Bosquet E, Sabria J, Lailla JM. Hormonal environment in the induction of breast cancer in castrated rats using dimethylbenzanthracene: influence of the presence or absence of ovarian activity and of treatment with estradiol, tibolone, and raloxifene. Menopause. 2005;12:601–8. doi: 10.1097/01.gme.0000172269.32573.34. [DOI] [PubMed] [Google Scholar]
- Cheung SY, Yuen MT, Choi HL, Cheng HK, Huang Y, Chen S, Chan FL. An expression study of hormone receptors in spontaneously developed, carcinogen-induced and hormone-induced mammary tumors in female Noble rats. Int J Oncol. 2003;22:1383–95. [PubMed] [Google Scholar]
- Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Stampfer MJ, Rosner B, Hennekens CH, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–9. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–59. [PubMed] [Google Scholar]
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- Cyr M, Morissette M, Landry M, Di Paolo T. Estrogenic activity of tamoxifen and raloxifene on rat brain AMPA receptors. Neuroreport. 2001a;12:535–9. doi: 10.1097/00001756-200103050-00021. [DOI] [PubMed] [Google Scholar]
- Cyr M, Thibault C, Morissette M, Landry M, Di Paolo T. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology. 2001b;25:242–57. doi: 10.1016/S0893-133X(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–7. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- Dickson A, Henriques N. Menopause: The Woman’s View. London: Quartet Books; 1992. [Google Scholar]
- Donner N, Handa RJ. Estrogen receptor β regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–18. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–15. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnami NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Fudge MA, Kavaliers M, Baird JP, Ossenkopp KP. Tamoxifen and raloxifene produce conditioned taste avoidance in female rats: a microstructural analysis of licking patterns. Life Sci. 2009;84:282–9. doi: 10.1016/j.lfs.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–8. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Hoyt JA, Fisher LF, Swisher DK, Byrd RA, Francis PC. The selective estrogen receptor modulator, raloxifene: reproductive assessments in adult male rats. Reprod Toxicol. 1998;12:223–32. doi: 10.1016/s0890-6238(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Huggins C. Two principles in endocrine therapy of cancers: hormone deprival and hormone interference. Can Res. 1965;25:1163–1167. [PubMed] [Google Scholar]
- Hughes ZA, Liu F, Platt BJ, Dwyer JM, Pulicicchio CM, Zhang G, Schechter LE, Rosenzweig-Lipson S, Day M. WAY-200070, a selective agonist of estrogen receptor beta as a potential novel anxiolytic/antidepressant agent. Neuropharmacology. 2008;54:1136–42. doi: 10.1016/j.neuropharm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Jarkova NB, Martenyi F, Masanauskaite D, Walls EL, Smetnik VP, Pavo I. Mood effect of raloxifene in postmenopausal women. Maturitas. 2002;42:71–5. doi: 10.1016/s0378-5122(01)00303-6. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Jacobson HI, Walf AA, Frye CA. Estrogen action: A historic perspective on the implications of considering alternative approaches. Physiol Behav. 2010;99:151–162. doi: 10.1016/j.physbeh.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC, Morrow M. Tamoxifen, raloxifene, and the prevention of breast cancer. Endocr Rev. 1999;20:253–78. doi: 10.1210/edrv.20.3.0368. [DOI] [PubMed] [Google Scholar]
- Karahancer M, Cirpan T, Kanit L, Terek MC, Dikmen Y, Ozsener S. The effects of raloxifen on depression and cognition in ovariectomized rats. Fertil Steril. 2008;89:240–2. doi: 10.1016/j.fertnstert.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Katzenellenbogen JA. Biomedicine. Defining the “S” in SERMs. Science. 2002;295:2380–1. doi: 10.1126/science.1070442. [DOI] [PubMed] [Google Scholar]
- Kokiko ON, Murashov AK, Hoane MR. Administration of raloxifene reduces sensorimotor and working memory deficits following traumatic brain injury. Behav Brain Res. 2006;170:233–40. doi: 10.1016/j.bbr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–7. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–82. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G, Tsao SW, Wong YC. Sex hormone-induced mammary carcinogenesis in female Noble rats: detection of differentially expressed genes. Breast Cancer Res Treat. 2003;77:49–63. doi: 10.1023/a:1021123914339. [DOI] [PubMed] [Google Scholar]
- Lund KJ. Menopause and the menopausal transition. Med Clin North Am. 2008;92:1253–71. doi: 10.1016/j.mcna.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Magnusson C, Baron JA, Correia N, Bergström R, Adami HO, Persson I. Breast-cancer risk following long-term oestrogen- and oestrogen-progestin-replacement therapy. Int J Cancer. 1999;81:339–44. doi: 10.1002/(sici)1097-0215(19990505)81:3<339::aid-ijc5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mazzucco CA, Walker HA, Pawluski JL, Lieblich SE, Galea LA. ERα, but not ERβ, mediates the expression of sexual behavior in the female rat. Behav Brain Res. 2008;191:111–7. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Mitlak BH, Cohen FJ. Selective estrogen receptor modulators: a look ahead. Drugs. 1999;57:653–63. doi: 10.2165/00003495-199957050-00001. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703–15. doi: 10.7326/0003-4819-151-10-200911170-00147. [DOI] [PubMed] [Google Scholar]
- Nickelsen T, Lufkin EG, Riggs BL, Cox DA, Crook TH. Raloxifene hydrochloride, a selective estrogen receptor modulator: safety assessment of effects on cognitive function and mood in postmenopausal women. Psychoneuroendocrinology. 1999;24:115–28. doi: 10.1016/s0306-4530(98)00041-9. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–81. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yoshida H. Influence of estrogen and progesterone on the induction of mammary carcinomas by 7,12-dimethylbenz(a)anthracene in ovariectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:365–70. doi: 10.1007/BF02899705. [DOI] [PubMed] [Google Scholar]
- O’Neill K, Chen S, Brinton RD. Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer’s disease. Exp Neurol. 2004;185:63–80. doi: 10.1016/j.expneurol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Barreiro ML, Tena-Sempere M, Aguilar E. Raloxifene effects upon the neuronal system controlling sexual receptivity in female rats. Neurosci Lett. 2002;329:285–8. doi: 10.1016/s0304-3940(02)00685-7. [DOI] [PubMed] [Google Scholar]
- Pfaff D. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. J Endocrinol. 2005;184:447–53. doi: 10.1677/joe.1.05897. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Hartmann LC. Selective estrogen-receptor modulators -- mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- Russo IH, Koszalka M, Gimotty PA, Russo J. Protective effect of chorionic gonadotropin on DMBA-induced mammary carcinogenesis. Br J Cancer. 1990;62:243–7. doi: 10.1038/bjc.1990.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- Stovall DW, Pinkerton JV. Estrogen agonists/antagonists in combination with estrogen for prevention and treatment of menopause-associated signs and symptoms. Womens Health. 2008;4:257–68. doi: 10.2217/17455057.4.3.257. [DOI] [PubMed] [Google Scholar]
- Strickler R, Stovall DW, Merritt D, Shen W, Wong M, Silfen SL. Raloxifene and estrogen effects on quality of life in healthy postmenopausal women: a placebo-controlled randomized trial. Obstet Gynecol. 2000;96:359–65. doi: 10.1016/s0029-7844(00)00937-6. [DOI] [PubMed] [Google Scholar]
- Stygar D, Muravitskaya N, Eriksson B, Eriksson H, Sahlin L. Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus. Reprod Biol Endocrinol. 2003;1:40. doi: 10.1186/1477-7827-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A, Kolesar JM. Chemoprevention of breast cancer. Am J Health Syst Pharm. 2008;65:2221–8. doi: 10.2146/ajhp070663. [DOI] [PubMed] [Google Scholar]
- Utian WH, Janata JW, Barbier S, Rosen AS, Mayer MH, Taylor MB. Effect of raloxifene on quality of life: a prospective study using the Utian Quality of Life (UQOL) Scale. Menopause. 2004;11:275–80. doi: 10.1097/01.gme.0000109295.37664.0e. [DOI] [PubMed] [Google Scholar]
- Walf AA. Oestrogen receptor β is involved in the actions of oestrogens in the brain for affective behaviour, but not trophic effects in peripheral tissues. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2009.01945.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol’s effects to reduce anxiety and depressive behavior may be mediated by estradiol dose and restraint stress. Neuropsychopharmacology. 2005a;30:1288–301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005b;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of: Mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006a;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007a;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor β-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007b;86:407–14. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol enhances sociosexual behavior and augments carcinogen-induced tumorigenesis in ovariectomized rats. AGE. 2009a;31:221–9. doi: 10.1007/s11357-008-9079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gender Medicine. 2009b;6:300–11. doi: 10.1016/j.genm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, You Q, Huang FS, Xiang H. Recent advances in selective estrogen receptor modulators for breast cancer. Mini Rev Med Chem. 2009;9:1191–201. doi: 10.2174/138955709789055207. [DOI] [PubMed] [Google Scholar]
- Wu X, Glinn MA, Ostrowski NL, Su Y, Ni B, Cole HW, Bryant HU, Paul SM. Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 1999;847:98–104. doi: 10.1016/s0006-8993(99)02062-4. [DOI] [PubMed] [Google Scholar]
- Wurz GT, Read KC, Marchisano-Karpman C, Gregg JP, Beckett LA, Yu Q, Degregorio MW. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol. 2005;97:230–40. doi: 10.1016/j.jsbmb.2005.06.027. [DOI] [PubMed] [Google Scholar]


