Abstract
Systemic administration of cystamine is known to protect from both chemical and genetic models of neurotoxicity. Despite positive effects in laboratory models, cystamine has not been successfully translated to clinical application for neurodegenerative disease. Furthermore, the long held assumption that cystamine protects through tissue-transglutaminase inhibition has recently been challenged. The studies described here examine other potential mechanisms of cystamine-mediated protection in an attempt to reveal molecular targets for neurodegenerative therapy. Based on previously described effects of cystamine, we examined the potential for activation of NF-E2 related factor 2 (Nrf2) mediated signaling through the antioxidant response element (ARE). We found that cystamine activates Nrf2/ARE both in cell culture and in brain tissue and then probed the mechanism of activation in cell culture. In live animals, we show that neuroprotection from 3-nitropropionic acid (3NP) toxicity is Nrf2-dependent. Therefore, these findings provide strong evidence that Nrf2 signaling may be an effective target for prevention of neurodegeneration.
Keywords: Nrf2, cystamine, oxidative stress, 3-nitropropionic acid
Introduction
Cystamine and its reduced form, cysteamine, are known to have multiple effects in biological systems. Several enzymes are inhibited in culture and in vivo by cystamine including tissue transglutaminase (Lorand and Conrad, 1984), gamma-glutamyl cysteine synthetase (Lebo and Kredich, 1978) and Caspase 3 (Lesort, et al., 2003). Additionally cystamine is known to increase glutathione levels in cultured cells (Lesort, et al., 2003). Levels of cystamine, cytsteamine or the eventual metabolite taurine are not measurably increased in mouse brain after systemic cystamine administration (Pinto, et al., 2005). Despite the lack of accumulation in brain, systemic administration of cystamine is known to diminish neural toxicity associated with 3-nitropropionic acid (3-NP) (Fox, et al., 2004), methylphenyl-1,2,4,6-tetrahydropyridine (MPTP) (Stack, et al., 2008, Tremblay, et al., 2006), 6-hydroxydopamine (6-OHDA) (Stack, et al., 2008), and intracerebral hemorrhage (Okauchi, et al., 2008). Furthermore, cystamine protects against neurodegeneration, and extends lifespan in genetic models of Huntington’s disease (HD), including R6/2 (Dedeoglu, et al., 2002, Fox, et al., 2004, Karpuj, et al., 2002, Wang, et al., 2005) and the full-length YAC128 (Van Raamsdonk, et al., 2005) models. The putative hypothesis that protection in the R6/2 model is due to tissue transglutaminase inhibition has recently been tested directly and called into question (Bailey and Johnson, 2006). This result has stimulated investigation into other functions of cystamine in hopes that discovering the definitive mechanism of action might lead to rational drug design for HD and other neurodegenerative conditions. Most recently, cystamine has been shown to increase levels of brain derived neurotrophic factor (BDNF) in the striatum of HD knock-in mice and in primate blood. Furthermore, cystamine does not effectively extend lifespan in R6/1 mice with a BDNF deficient background (Borrell-Pages, et al., 2006). While this hypothesis is certainly promising in terms of therapeutic potential for HD, cystamine is known to be a multifunctional chemical and most likely exhibits multiple modes of action. Therefore, it is of interest to elucidate additional effects of cystamine, including the potential for induction of antioxidant defenses.
Expression of antioxidant genes is often induced via the transcription factor Nrf2 [recently reviewed in (Osburn and Kensler, 2008)]. In fact, Nrf2 is considered one of the major regulators of cytoprotective genes and confers antioxidant defense in vitro and in vivo. Activating Nrf2 has been shown to protect against neurotoxicity in a variety of models (Calkins, et al., 2008, Johnson, et al., 2008). In primary neural cultures, Nrf2 activation is neuroprotective against hydrogen peroxide, tert-butyl hydroperoxide, 6-OHDA, 3-NP, methylpyridinium ion (MPP+), and rotenone (Calabrese, et al., 2005, Calkins, et al., 2005, Jakel, et al., 2005, Kraft, et al., 2004, Lee, et al., 2003, Shih, et al., 2003). In these reports, Nrf2 activation reduced toxicity and Nrf2 deficiency potentiated toxicity. Nrf2 functions by binding the antioxidant response element (ARE), a cis-acting enhancer found in the promoter of many cytoprotective genes. Not only is Nrf2 implicated as a major factor limiting toxicity in cell culture, but in vivo, Nrf2 is known to play a role in protection from neurotoxicity from malonate, 3-NP, kainic acid, 6-OHDA, MPTP, ischemia-reperfusion injury, traumatic brain injury, and genetic models of Amyotrophic Lateral Sclerosis (Burton, et al., 2006, Chen, et al., 2009, Jakel, et al., 2007, Kraft, et al., 2006, Satoh, et al., 2008, Satoh, et al., 2006, Shih, et al., 2005, Shih, et al., 2005, Vargas, et al., 2008, Zhao, et al., 2007, Zhao, et al., 2007).
One effect of cystamine in cell culture is to increase glutathione (GSH) levels (Lesort, et al., 2003). Moreover, in Vanin deficient mouse models, glutathione s-transferase (GST) protein levels are increased by cystamine administration (Di Leandro, et al., 2008). Because Nrf2 activation is known to regulate both GSH synthesis and GST expression, we investigated the potential for cystamine treatment to initiate Nrf2 driven transcription in neural cell cultures and in brain. Additionally, we tested the Nrf2-dependence of cystamine protection against striatal toxicity from the mitochondrial complex II inhibitor 3-NP.
Materials and Methods
Animals
ARE-hPAP transgenic and Nrf2−/− mice were bred separately on a BL6/SJL background. ARE-hPAP mice were created by insertion of a 51 basepair segment of the promoter from rat NAD(P)H Quinone Oxidoreductase-1 (NQO1) gene, which contains the core ARE sequence, upstream of a minimal promoter and the gene for the heat stable human Placental Alkaline Phosphatase (hPAP) (Johnson, et al., 2002). Nrf2−/− mice were created by targeted disruption of the Nrf2 gene (Chan, et al., 1996) and maintained on a BL6/SJL background. In vivo experiments were performed using male animals. All experiments were approved by and performed according to the ethical guidelines provided by the Animal Care and Use Committee at the University of Wisconsin Medical School.
Neuron enriched primary cultures
Mixed cortical neural cultures were prepared as previously described (Kraft, et al., 2004). Briefly, cortices were isolated from E15 embryos and pooled in Hank’s Balanced Salt Solution without Ca++ and Mg++ (HBSS). Tissue was minced and then incubated in HBSS with 0.05% trypsin shaking at 37°C for 10 minutes. After trypsinization, the tissue was washed three times with HBSS and then triturated into a single cell suspension in CEMEM (Eagle’s MEM, 10% Horse Serum, 10% Fetal Bovine Serum, 1% Penicillin/Streptomycin). The suspension was passed through a 70µm mesh, after which, cells were plated at 320,000 cells/cm2 in 6-well or 96-well plates coated with poly-d-lysine or 8-well CC2 coated chamber slides (LabTech). Forty-five minutes after plating CEMEM was replaced. After 48 hours, media was changed to Neurobasal with B27 and 1mM glutamine in order to inhibit glial cell growth. Cells were maintained in Neurobasal medium and experiments were initiated after five days in culture.
Glia enriched cultures
Cortical glial cultures were prepared as previously described (Lee, et al., 2003). Briefly, cortices were dissected from P1 pups and menenges removed. Tissue was minced and then incubated at 37°C for 10 minutes in HBSS and 0.05% trypsin. After trypsinization, tissue was washed three times with HBSS and triturated to a single cell suspension in CEMEM. Cells were filtered through a 70µm mesh and suspended in 5ml CEMEM per cortical hemisphere. Cells were seeded onto collagen coated plates at 3ml per well of a 6 well dish or 100 µl per well of a 96-well plate. After approximately five days, cultures were routinely confluent with GFAP positive astrocytes comprising at least 90% of the population (data not shown). Treatments began seven days after plating.
Cell Viability
Cell viability was measured by the Cell Titer 96 Aqueous Assay according to manufacturer’s instructions. This assay measures [3- (4,5- Dimethylthiazol- 2- yl)- 5- (3-carboxymethyoxyphenyl)- 2- (4- sulfophenyl)- 2H- tetrazoliem salt (MTS; Promega) reduction by cellular dehydrogenases.
Glutathione
Glutathione content in live cultures was determined using monochlorobimane using a protocol previously described and validated (Sun, et al., 2005). Select results were verified using the well known GSSG recycling method previously described (Tietze, 1969) (data not shown). Cystamine or cysteamine were added to samples or standards to determine whether these thiols interfered with the recycling assay. We determined that these chemicals do not interfere with GSH measurement (data not shown). For tissue samples, glutathione was measured using the GSSG recycling assay previously described (Tietze, 1969).
hPAP Activity
Activity of the hPAP reporter was measured as previously described (Johnson, et al., 2002). Briefly, cells were homogenized in TMN (50mM Tris, 5mM MgCl2, 100mM NaCl) with 1% CHAPS by a single freeze thaw cycle. Tissues were homogenized in TMN with 4% CHAPS with a T8 tissue homogenizer (IKA Works, Inc.) at approximately 1–10 mg wet weight per ml. An aliquot of the lysate was added to 0.2M diethanolamine (DEA) and heated to 65°C for 20–30 minutes to inactivate endogenous alkaline phosphatase activity. After heat inactivation CSPD® and Emerald™ (Applied Biosystems) were used as luminescent substrate and enhancer respectively. Luminescence was measured in 96 well plates with a Berthold luminometer and corrected for soluble protein in the case of tissue homogenate. Cell cultures were routinely monitored for consistency and viability using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega) according to manufacturer’s instructions.
hPAP histochemistry
Histochemistry for the hPAP reporter was performed as previously described (Calkins, et al., 2005, Jakel, et al., 2005, Johnson, et al., 2002). Animals were killed with CO2 and then transcardially perfused first with phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Perfused animals were set aside for five minutes and then brains were removed into PBS at 4°C overnight. Tissue was cryoprotected with 30% sucrose in PBS and then embedded in OCT. Slides were prepared with PFA fixed tissue from hPAP transgenic or wildtype control tissue and rehydrated in TMN for 10 minutes. Slides were incubated at 65°C in preheated TMN for 20 minutes, cooled and then removed to TMN with BCIP/NBT at 37°C or else Vector Red substrate in the provided buffer (Vector Labs). Color development was monitored and samples were counterstained and cover-slipped as appropriate.
Immunofluorescence
Immunohistochemistry was performed by standard methods. Cells were fixed with 4% PFA. Blocking was performed with 2% serum from the animal secondary antibody was produced as well as 1% BSA and 0.1% Triton X-100 for 2 hours at room temperature. Primary antibody was applied overnight at 4°C in blocking solution. Secondary antibody was applied for 2 hours at room temperature.
NQO1 Activity
Activity of NQO1 was measured in tissue homogenate as previously described (Prochaska and Santamaria, 1988). Mice were killed with CO2 after which tissue was dissected and immediately flash frozen in liquid nitrogen. Frozen tissue was homogenized in ice cold 1% NP-40 in PBS for 20 seconds using a T8 homogenizer and refrozen on dry-ice. After one freeze thaw cycle samples were centrifuged at 7500×g for five minutes, and soluble protein was used for the NQO1 activity assay. Assay conditions were as follows: 25mM Tris pH7.4, 0.01% Tween 20, 5µM FAD, 30µM NADP, 1mM Glucose-6-phosphate, 2U/ml yeast glucose-6-phosphate dehydrogenase, 1mg/ml BSA, 0.3mg/ml MTT, 0.5mM menadione. Stop solution was composed of 0.6mM dicoumarol, 0.5% DMSO and 5mM Potassium phosphate pH 7.4. Homogenates were diluted 10µl into 200µl reaction buffer in 96-well format and incubated for approximately 10 minutes. After incubation 50µl stop solution was added to each sample. Background was determined for each sample by preincubating tissue homogenate with stop solution. Absorbance was read at 610nm and background was subtracted. Soluble protein was measured from the homogenate using the BCA method by manufacturer’s instructions. Rate of absorbance increase was corrected by protein concentration.
3NP administration
Nrf2 deficient and wildtype animals were given either PBS or cystamine (112 mg/kg/day) i.p. at 3PM for seven days. On the eighth day of the study, animals received 3NP at 60 mg/kg every twelve hours. 3NP was administered at 9AM and 9PM, while cystamine administration was continued at 3PM. After four doses, 3NP dosing was discontinued entirely in the Nrf2 deficient animals and the 9AM dose was discontinued in the wildtype animals. The wildtype animals were given two additional 3NP doses. The cumulative dose in the Nrf2 deficient animals was 240mg/kg while the wildtype animals received 360mg/kg.
Thirty-six hours after the final 3NP dose, animals were assessed using a subjective clinical grading scale set forth previously (Gabrielson et al., 2001). Briefly, this scale consists of three stages that mice pass through after 3NP administration. Stage I presents with normal posture and gait, but reduced motor activity including reduced grooming and interaction with other mice. During stage II, mice become increasingly active in response to handling with significant alterations in gait and posture. At this stage mice exhibit deficits in balance (wobbly gait) a more hunched posture and sometimes significant weight loss (greater than 10%). Stage III mice are generally considered moribund. Once mice reached stage III, they were euthanized according to animal care and use standards.
The 3NP dosing schedule was based on previous literature (Fox, et al., 2004), with 60 mg/kg doses administered every twelve hours. Dosing was discontinued due to development of morbidity in the cystamine and 3NP combination experimental groups. After 3NP treatment was ended, mice were sacrificed 36 hours after the last dose. Animals were euthanized with CO2 and transcardially perfused with PBS followed by 4% PFA. After approximately five minutes, brains were removed to PBS and stored at 4°C overnight. Fixed tissue was transferred to 30% sucrose for cryoprotection, after which it was frozen and sectioned at 40µm on a cryostat (Leica). Sections taken every 0.2mm were stained with cresyl violet to visualize lesions. Lesion area for each section was calculated using Zeiss Axiovision software and lesion volume was estimated by multiplying the individual lesion areas by the distance between sampled sections.
Statistics
Data from cell cultures are shown from representative experiments repeated at least three times. All cultures were grown from tissue derived from multiple pups or embryos. In vivo experiments were performed on mice with littermate controls populating all groups. Statistical comparisons were made between parametric data using student’s t-test with p < 0.05 considered statistically significant. Data are presented as average +/− SEM. Non-parametric data (clinical scoring of 3NP treated animals) was compared using the Mann-Whitney U-test, with p < 0.05 considered statistically significant.
Results
Cystamine activates Nrf2 dependent transcription in astrocytes
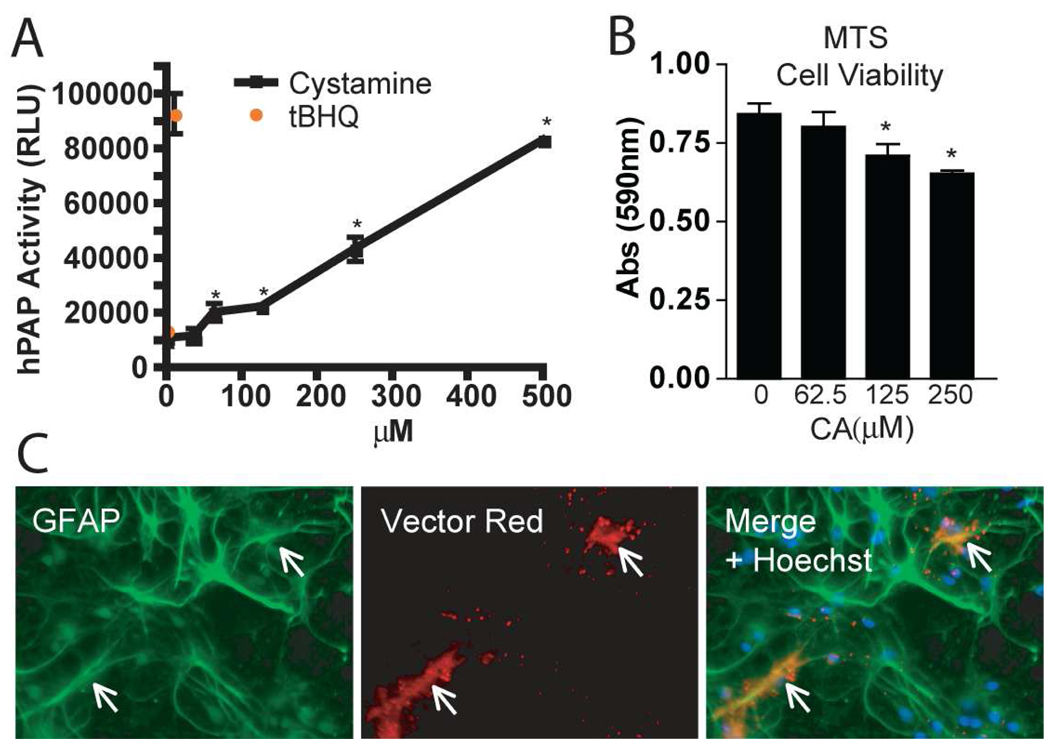
Primary cultures of mixed neurons and astrocytes were prepared using combined cortical tissue from embryos that were derived from wildtype female mice crossed with male hemizygous transgenic ARE-hPAP reporter mice. Cultures were allowed to differentiate for five days, after which they were treated with cystamine or the known Nrf2/ARE inducing chemical tert-butyl hydroquinone (tBHQ). Cells were harvested and hPAP activity was assayed after 48 hours of treatment. Significant induction of Nrf2/ARE-mediated transcription was detected at cell culture doses often used in literature to inhibit tissue transglutaminase (Figure 1A). At these doses however, we found a minor reduction in cell viability as measured by MTS (Figure 1B). In neuronal cultures treated with the maximum dose of cystamine (250µM) cell viability was reduced by 22.5%.
Figure 1. Cystamine activates the Nrf2-ARE pathway in astrocytes of mixed cultures.
(A) Primary neural cultures composed of neurons and glia were treated with 10µM tBHQ or cystamine at several doses. hPAP reporter activity was measured using the luminescent assay and reported as RLU. (B) Cultures treated with varying doses of cystamine were evaluated for cell viability by MTS. (C) Cultures treated with 250µM cystamine were stained immunohistochemically for GFAP and hPAP histochemistry was conducted using vector red. Panels A and B are from experiments with n = 4 in each group. * p < 0.05 compared to vehicle treated.
Cell type specificity of Nrf2/ARE activation was investigated by using a substrate for the alkaline phosphatase reporter that produces a fluorescent precipitate, and co-localization with fluorescent immunohistochemical staining for GFAP. hPAP activity occurred only in cells that labeled for GFAP (Figure 1C). When co-localization of hPAP activity was performed using the neuronal marker β-III tubulin, no co-labeled cells were observed (data not shown). It should be noted that not all of the GFAP-positive astrocytes were labeled by the reporter substrate. This may be due to two reasons. First, it is possible that not all of the cells label for reporter activity because not all of the astrocytes in the culture exhibit activated Nrf2. Additionally the breeding strategy we used to produce embryos for culture (ARE-hPAP hemizygous male crossed with wildtype female) results in only approximately half of the pups positive for the hPAP reporter gene. Therefore it was only possible to detect ARE-hPAP expression in a portion of the cultured cells. Importantly, the Nrf2 response that we were able to observe was localized to astrocytes.
Cystamine activation of Nrf2/ARE in culture is tied closely to GSH levels
In order to better understand the mechanism by which cystamine activates Nrf2 in neural cultures, the role of GSH homeostasis on induction of Nrf2-ARE-mediated transcription was investigated. Primary astrocyte cultures were prepared from ARE-hPAP reporter mice. Cultures were allowed to grow into a confluent monolayer (usually between three and five days) and were treated after one week in culture.
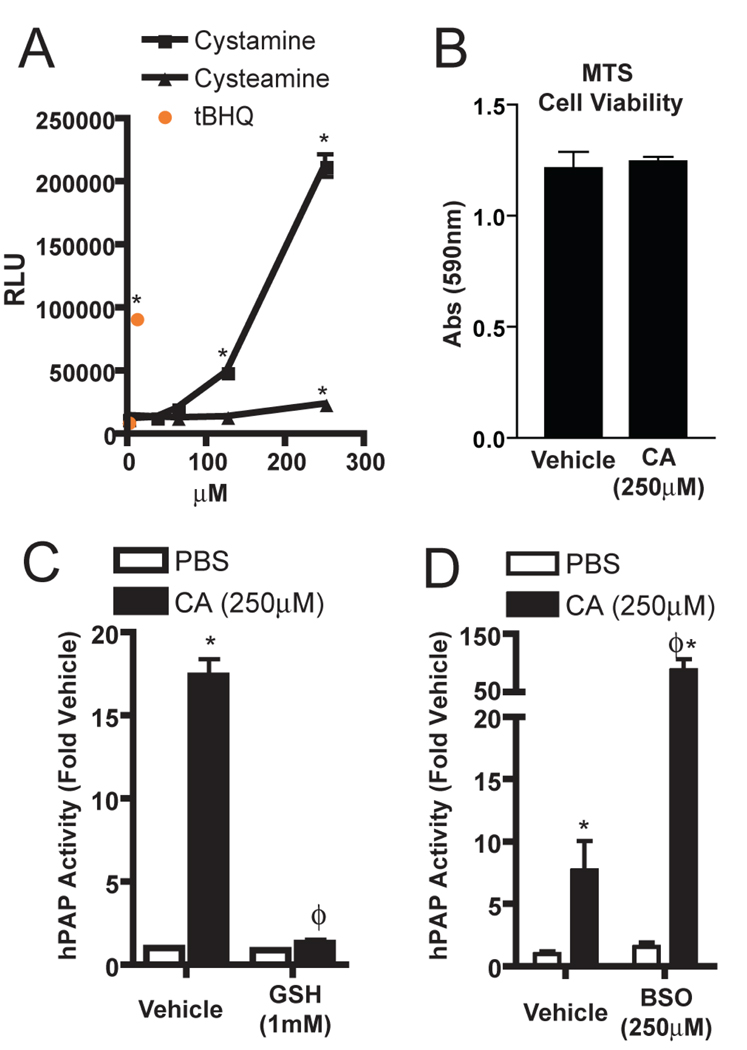
Cystamine induced the ARE-hPAP reporter in astrocytes at similar doses to those observed in mixed primary neural cultures. The reduced form of the molecule, cysteamine, however, induced ARE-hPAP reporter only slightly in comparison (Figure 2A). Since we intended to pursue studies with the 250µM dose, we verified that it did not cause toxicity in the glial cultures (Figure 2B). The presence of 1mM GSH in the culture medium abolished the ability of 250µM cystamine to activate Nrf2-ARE signaling (Figure 2C). When GSH levels were reduced by 24 hour pretreatment with the glutathione synthetase inhibitor buthionine sulfoximine (BSO), ARE-hPAP induction by cystamine was potentiated significantly (Figure 2D). Cystamine is metabolized first to the reduced form cysteamine, and then ultimately to taurine in cells. When cystamine metabolites (cysteamine and taurine) were applied to the cultures, only cystamine showed significant activation of the ARE-hPAP reporter (data not shown). Together these results indicate that ARE-hPAP induction in culture is likely due to some property of the dithiol. Interestingly, other dithols, such as GSSG and cystine, at similar concentrations do not activate ARE-hPAP (data not shown).
Figure 2. Cystamine activation of the Nrf2-ARE pathway in astrocytes is tied to glutathione content of the culture.
(A) Primary glial cultures were treated with tBHQ, cystamine, or cysteamine for 24 hours. hPAP reporter activity was measured using a luminescent assay. (B) Cultures treated with 250µM cystamine were evaluated for cell viability by MTS. (C) Cultures were treated with PBS or cystamine in combination with PBS or 1mM GSH for 24 hours. (D) Cultures were pretreated with BSO (250µM) for 24 hours and then treated with 250µM cystamine for 24 hours. hPAP reporter activity was measured using a luminescent assay. All data are from experiments with n = 4 in each group. * p < 0.05 compared to PBS control. ϕ p < 0.05 compared to vehicle control
Cystamine administration increases glutathione content in cells independently of Nrf2
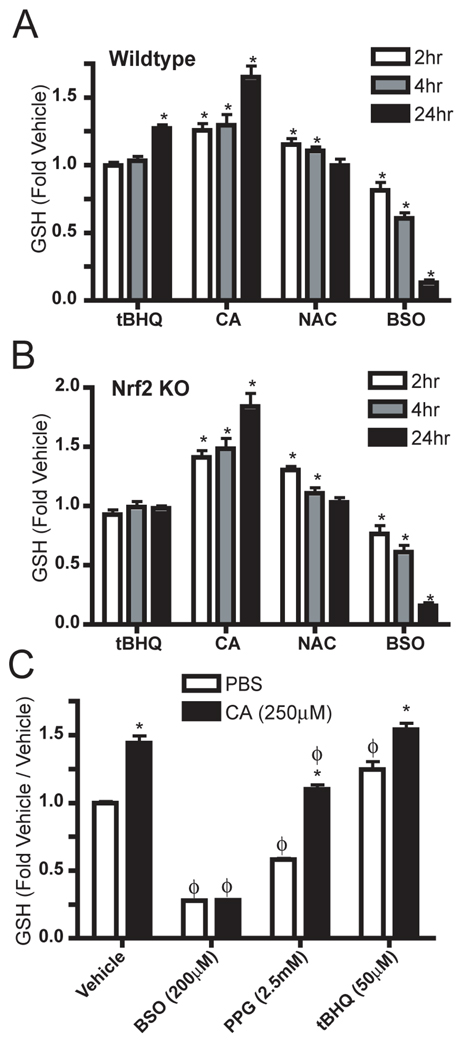
Primary astrocytes derived from wildtype or Nrf2 deficient mice were treated with either tBHQ, cystamine, NAC, BSO, or the appropriate vehicle. GSH content in the live culture was measured two, four or 24 hours post treatment using monochlorobimane (MCB). After 24 hours of tBHQ treatment, a significant increase in GSH level was observed in the wildtype cultures (Figure 3A). Conversely, in Nrf2 deficient cells, tBHQ had no effect on GSH levels at any measured time point (Figure 3B). Both Nrf2 knockout and wildtype cultures treated with cystamine exhibited increased GSH at all time points examined, thus showing that cystamine increases GSH in cell culture independently of Nrf2. Supplemental studies in wildtype cultures indicate that cystamine mediated increases in GSH occur over the course of approximately 30 minutes to one hour with no GSH depletion evident at any measured time point (data not shown).
Figure 3. Glutathione increases by cystamine are independent of Nrf2 activation, but are dependent on de novo synthesis.
(A,B) Primary glial cultures from wildtype pups (A) or Nrf2 deficient pups (B) were treated with tBHQ, cystamine, N-Acetyl cysteine, or BSO for 2, 4, or 24 hours. Glutathione was measured by monochlorobimane conjugation, and normalized to cultures treated with vehicle for the same time. (C) Primary glial cultures prepared from wildtype pups were pretreated with vehicle, BSO, PPG, or tBHQ for 24 hours and then treated with vehicle or cystamine for 24 hours. Glutathione was measured by monochlorobimane conjugation and normalized to cultures that were pretreated and treated with vehicle. All data are from experiments with n = 4 in each group. * p < 0.05 compared to PBS or vehicle treated. ϕ p < 0.05 compared to vehicle pretreatment.
In order to verify the method of GSH measurement, N-acetyl cysteine (NAC) and BSO were administered and predicted results were observed. NAC produced a transient increase in GSH concentration, due to increase in free cysteine, and BSO inhibited glutathione synthesis resulting in decreased GSH concentrations over time (Figures 3A, 3B). These effects were observable in both widltype and Nrf2 knockout cultures.
To determine whether the increase in GSH was dependent on cysteine availability or GSH synthesis, cultures were pretreated with BSO or propargyl glycine (PPG) for 24 hours followed by vehicle or cystamine treatment for 24 hours (Figure 3C). PPG is an irreversible inhibitor of cystathionase and prevents methionine conversion to cysteine by the transsulfuration pathway. We observed that after BSO pretreatment, cystamine administration had no effect on GSH levels in the culture. This observation further validates the method of measurement by demonstrating that addition of cystamine alone to the culture cannot affect the assay and give a false positive fluorescence. These data also indicate that cystamine mediated increases in GSH are dependent on de novo GSH synthesis. Furthermore, since PPG pretreatment does not attenuate GSH increases (Figure 3C), cystamine does not appear to increase GSH by increasing cysteine synthesis through the transsulfuration pathway. The model Nrf2 activator, tBHQ, was used as a positive control to demonstrate increases in GSH.
Systemic administration of cystamine induces ARE mediated transcription in brain
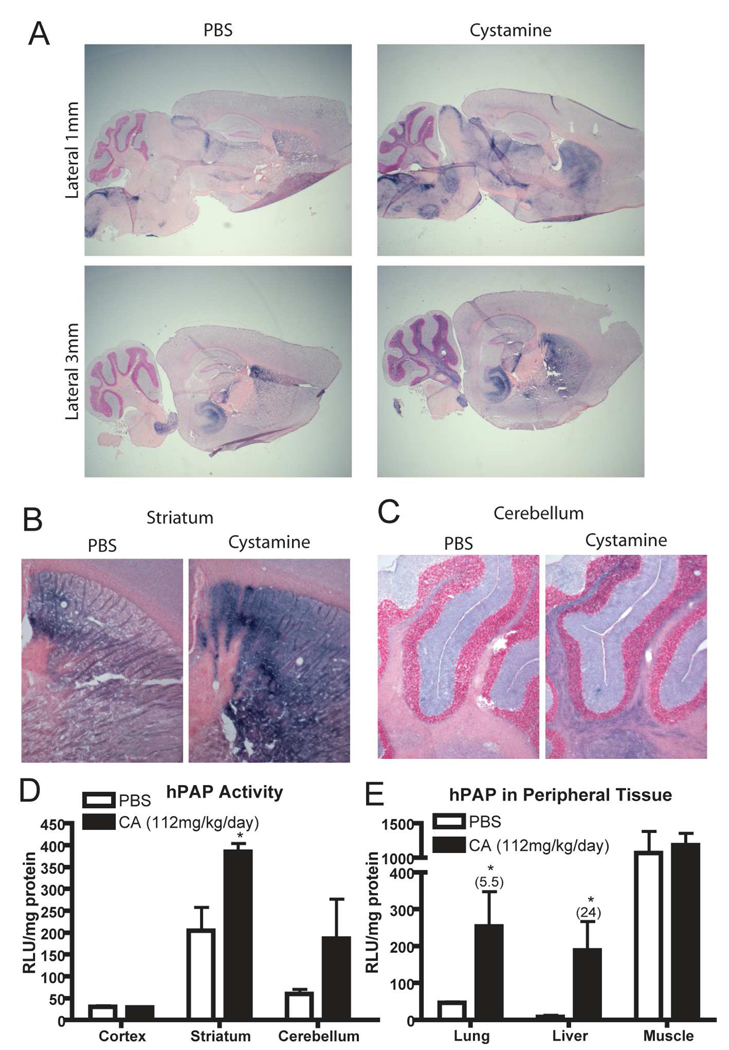
Wildtype mice or mice carrying the ARE-hPAP reporter gene were injected with 112mg/kg/day cystamine for seven days after which brain, lung, liver and muscle were harvested approximately four hours after the last dose. Cryosectioned sagittal brain slices were histochemically analyzed for hPAP activity by BCIP/NBT and counterstained with Nuclear Fast Red. Two regions appeared to exhibit increased activation of the hPAP reporter gene. The first was striatum where staining was diffuse but localized entirely within the striatum, bounded by the corpus callosum and ventricles (Figure 4A). The striatum exhibits a higher basal level of ARE-hPAP activity when compared to cortex. Striatal ARE-hPAP staining was consistently increased in the cystamine treated animals. The second region where cystamine treated animals stained more robustly for the hPAP reporter was cerebellum (Figure 4B). Increased staining was observed in the arbor vitae, which is the cerebellar white matter and consists mainly of axonal projections and glial cells. We focused on these two regions because of their relevance to neurodegenerative conditions caused by CAG repeat elongations.
Figure 4. Systemic administration of cystamine activates Nrf2-ARE signaling in brain and peripheral tissues.
Mice were treated with 112mg/kg/day cystamine for 7 days. (A,B) Brain was harvested and sectioned sagittally. Histochemical staining for the ARE-hPAP reporter was performed using BCIP/NBT and sections were counterstained with nuclear fast red. Brain regions with differential staining after cystamine treatment included (A) striatum and (B) cerebellum. (C) Specific brain regions were dissected and hPAP activity was assessed using a luminescent substrate. Relative light units were normalized to protein content and then background as determined from ARE-hPAP non-transgenic animals was subtracted (n = 3). (D) Peripheral tissues were dissected and analyzed for ARE-hPAP reporter activity by the same method as was used for brain tissue (n = 6). * p < 0.05 compared to PBS treated animals.
Quantification of hPAP activity in three regions of the brain was performed, and it was confirmed that while cortex had no difference in activity, striatal activity was increased significantly. Cerebellum showed a trend toward increased activity (Figure 4C). Finally, peripheral tissues were analyzed for hPAP activity. Lung and liver exhibited increased hPAP reporter activity after cystamine treatment (5.5 fold and 24 fold respectively; Figure 4D). On the other hand, no evidence was seen of hPAP increase in skeletal muscle. These results show that the Nrf2 response to systemic cystamine administration is not only localized to brain.
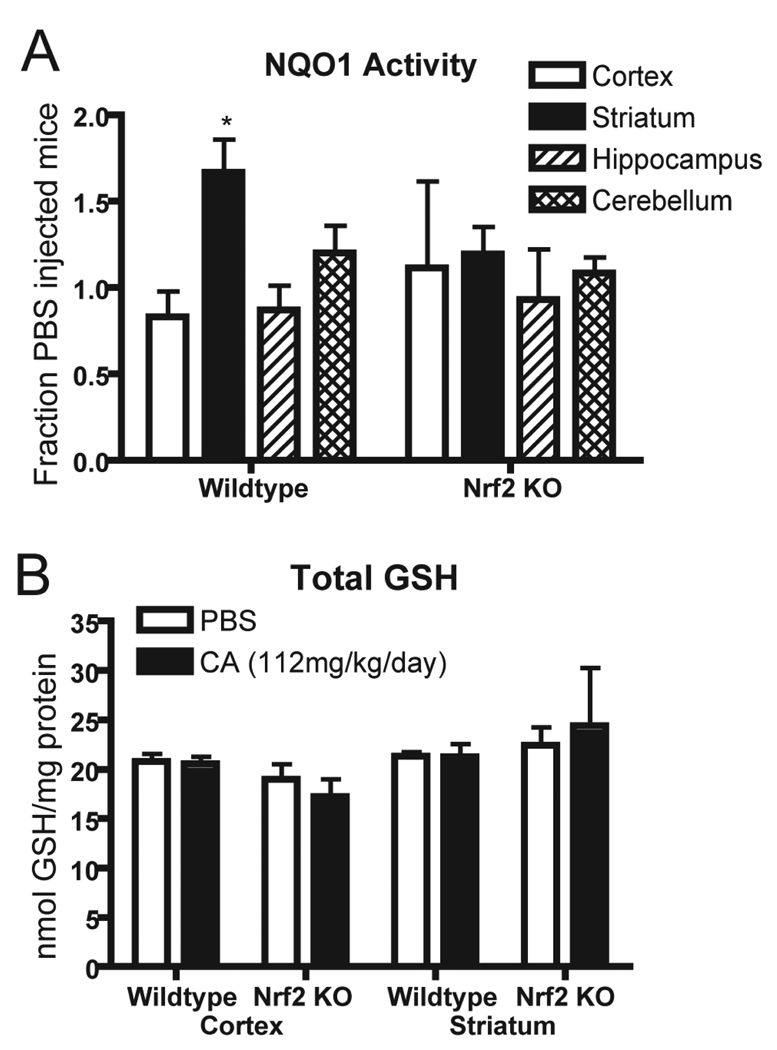
In order to verify that the increased reporter activity was reflective of physiologically relevant Nrf2-dependent changes and to demonstrate Nrf2 dependence of the changes, NQO1 activity was measured in wildtype and Nrf2 knockout mice treated with either vehicle or cystamine. Of the four brain regions analyzed, only the striatum showed significant activation of NQO1 activity in wildtype mice. There was a trend toward increase in the cerebellum, matching the hPAP activity data (Figure 5B). Nrf2 deficient mice did not exhibit cystamine induced increase in NQO1 activity in any region examined. This result supports the hypothesis that NQO1 is increased in striatum by an Nrf2 dependent mechanism.
Figure 5. Nrf2-ARE activation by cystamine results in increased NQO1 activity in striatum but not increased GSH.
(A) NQO1 activity was measured by the enzymatic assay using dissected brain regions from wildtype or Nrf2 deficient mice. Data are normalized to NQO1 activity in tissue from PBS treated animals. n = 5, 5 wildtype; n = 5, 5 Nrf2 knockout. (B) Glutathione content of cortex and striatum was measured in wildtype and Nrf2 deficient mice and normalized to protein. n = 5, 5 wildtype; n = 3, 3 Nrf2 knockout. * p < 0.05 compared to PBS treated animals
Because Nrf2 is known to regulate glutathione synthesis genes and L-cysteine is known to be increased in brain after cystamine administration, we measured levels of total GSH in striatum and cortex. L-cysteine availability and glutamate-cysteine ligase activity are widely regarded as the rate limiting factors in GSH production. Previous reports have shown that GSH does not change in brain after cystamine administration; however, these reports have largely used whole brain or forebrain homogenates. We hypothesized that because striatal Nrf2 activity was specifically upregulated, any effects on striatal GSH may be diluted by including other brain regions in the analysis. Therefore, striatum and cortex were dissected from Nrf2 deficient and wildtype mice receiving either PBS or cystamine for 7 days, and total GSH was evaluated. In accordance with previously published results, we found no increase in brain tissue GSH after cystamine administration (Figure 5C).
Cystamine protection from 3-nitropropionic acid (3NP) is Nrf2 dependent
In order to test whether the induction of Nrf2 activity via cystamine treatment in striatum conferred neuroprotection, mice were treated with cystamine (112 mg/kg/day) for seven days after which we began a regimen of 3NP. The cystamine and 3NP dosing schedule was selected to conform to a previous demonstration of cystamine protection from 3NP lesions (Fox, et al., 2004). Wildtype mice received a total dose of 360mg 3NP/kg while Nrf2 deficient mice received 240mg 3NP/kg. This regimen was used in an attempt to normalize lesion size between wildtype and Nrf2 deficient PBS-3NP mice. Mice were kept for thirty-six hours after the last 3NP dose and then sacrificed. Until sacrifice, all mice continued to receive cystamine or PBS daily.
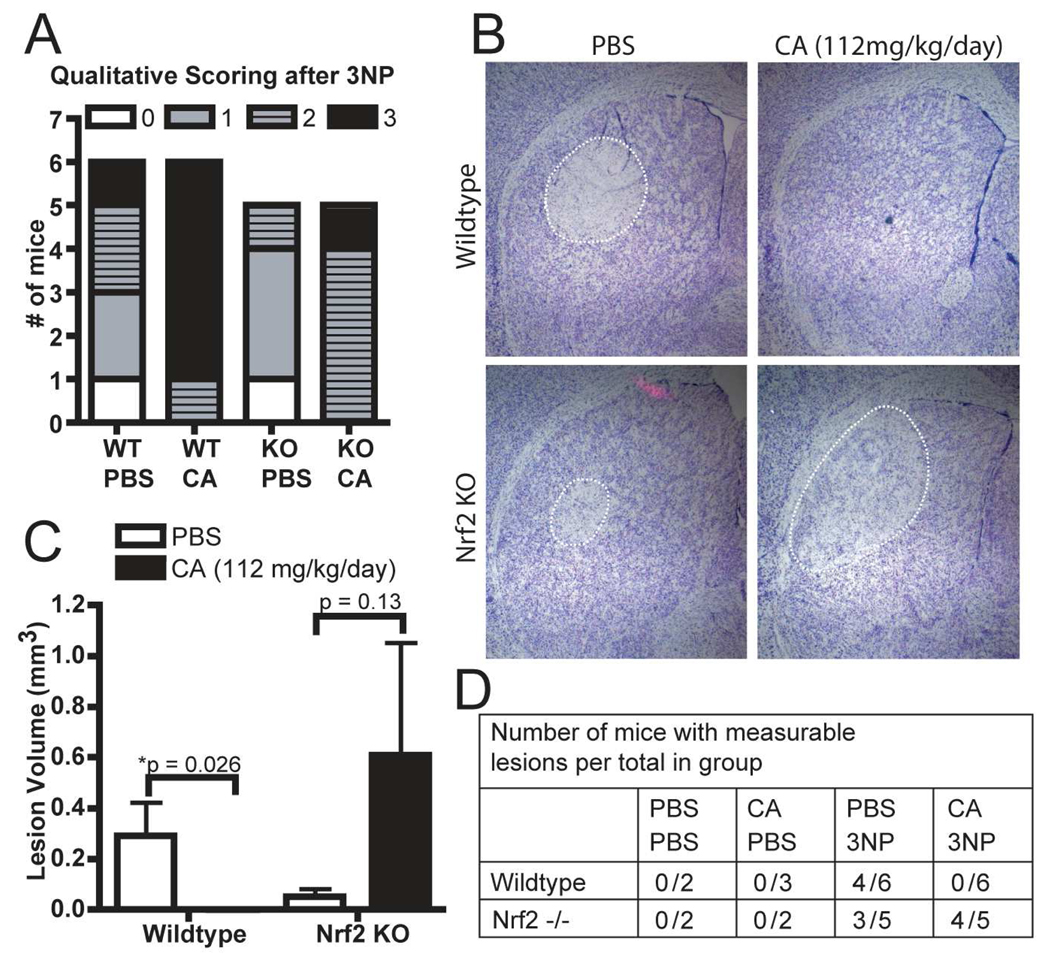
At the time of sacrifice, each mouse was assessed according to the clinical scoring system previously developed (Gabrielson, et al., 2001) (Figure 6A). Under this system, a score of zero indicates no impairment and a score of three indicates a severely impaired animal. After four total doses of 3NP the Nrf2 deficient mice receiving PBS had an average clinical score of 1.0 +/− 0.32 while those receiving the combination of cystamine and 3NP had an average clinical score of 2.20 +/− 0.20. A similar distribution was found in the corresponding wildtype population after six total doses (1.50 +/− 0.46 in the 3NP only group with the combination group at 2.83 +/− 0.17). Interestingly, in both wildtype and Nrf2 deficient mice, those groups receiving cystamine and 3NP treatment presented higher clinical scores than those that received PBS and 3NP. These data indicate that cystamine treatment can increase the clinical score resulting from 3NP administration regardless of Nrf2 deficiency.
Figure 6. Cystamine mediated protection from 3NP lesioning is dependent on Nrf2.
Mice were treated with 112mg/kg/day cystamine for 7 days after which 3NP treatment began. (A) Dosing was halted and wildtype and Nrf2 knockout mice were sacrificed after the 3-NP and cystamine treated groups showed overt toxicity based on a clinical scoring system. Wildtype mice received a total dose of mg 360mg 3-NP/kg over the course of 5 days. Nrf2 deficient mice received a total dose of 240mg 3-NP/kg over the course of 3 days. Clinical scores were significantly increased (p < 0.05 by the Mann-Whitney U-test) in both widltype and Nrf2 knockout 3NP treated mice with combined cystamine treatment. (B) After sacrifice, brains were harvested and sectioned coronally for cresyl violet staining and visualization of lesions. Representative images of lesions are shown (C) Lesion volume was quantified by measuring lesion area in sections 0.2mm apart, and then multiplying the area by 0.2mm. (D) Number of mice with measurable lesions per total number in the group is reported. * p < 0.05 compared to PBS treated of the same genotype.
The increased clinical scoring in 3NP-cystamine combination mice required that we halt dosing earlier than normal. Mice were harvested and brains were analyzed for striatal lesions by cresyl violet staining (Figure 6B). By comparing lesion volumes, we found that cystamine pretreatment protects from 3NP in wildtype mice. Although not quite statistically significant due to lesion volume variation, the data suggest that cystamine may enhance toxicity in Nrf2 deficient mice (Figure 6C). Four of six wildtype mice treated with PBS had measurable 3NP-induced lesions (average volume: 0.29 +/− 0.13 mm3) while none of the wildtype mice receiving cystamine had 3NP-induced lesions. In groups of Nrf2 deficient mice receiving PBS or cystamine, three and four of five mice had measurable 3NP-induced lesions, respectively. Knockout mice receiving PBS had relatively small lesions (average volume: 0.05 +/− 0.03mm3) while Nrf2 KO mice that were cystamine treated had highly variable lesion volumes (average volume: 0.61 +/− 0.45mm3). While the increase in lesion size was not statistically significant in the Nrf2 KO mice, the results clearly show that cystamine administration cannot protect from 3NP lesions in the absence of Nrf2. Moreover, these results demonstrate that cystamine mediated protection from 3NP is dependent on Nrf2.
Discussion
Overall, these experiments indicate that Nrf2 is involved in the protective capacity of cystamine against neurodegeneration. This observation is interesting because Nrf2 has previously not been implicated in the biological activity of cystamine. While other protective mechanisms have been identified and considered, such as transglutaminase inhibition and BDNF induction (Bailey and Johnson, 2006, Borrell-Pages, et al., 2006), cystamine is known to be a multifunctional chemical. Therefore it is of interest to elucidate and evaluate the relative importance of the multiple potential therapeutic mechanisms of this dynamic small molecule.
We found that cystamine in neural cell cultures leads to a robust activation of the Nrf2 pathway in astrocytes. In cultures with both neurons and astrocytes, Nrf2 activation was observed in the astrocytes alone. This finding is in agreement with observations from other Nrf2 activating molecules such as sulforaphane (Kraft, et al., 2004) and tBHQ (Johnson, et al., 2002). While it is possible to activate Nrf2 signaling in the neurons within primary cultures (Johnson, et al., 2002, Satoh, et al., 2008, Satoh, et al., 2006), we find that in primary cortical cultures, Nrf2 activation occurs preferentially in astrocytes. The data presented herein further this observation. In order to probe the mechanism by which cystamine leads to Nrf2 activation, we modified the cellular environment by either increasing or decreasing GSH. Cystamine-mediated activation of Nrf2 was found to be inversely correlated with the GSH content of the culture. BSO pretreatment led to significantly increased ARE-hPAP induction, while excess GSH in the media significantly reduced the amount of ARE-hPAP induction. Taken together, these observations clearly place GSH as a central factor that may prevent cystamine induction of Nrf2 signaling in cell culture. One interpretation may be that GSH is necessary for the detoxification of cystamine, and in the absence of sufficient GSH, cystamine activates Nrf2 in an effort to promote its own detoxification. It is possible to speculate on molecular mechanisms by which this type of phenomenon could occur. For example, cystamine may cause initial GSH depletion and subsequent Nrf2 activation which leads to GSH overshoot and recovery. However in the initial studies we conducted, cystamine treatment did not decrease GSH levels, even as soon as 30 minutes after administration (data not shown). Alternatively, because reactive thiols in the Keap1 protein are known to be involved in Nrf2 induction (Itoh, et al., 2004), it is possible that cystamine may modify these thiols directly. GSH is known to bind cystamine and therefore, sufficient GSH in the cell or the media may prevent interaction of cystamine with cellular proteins. Regardless of the mechanism, it is clear that cystamine activates Nrf2 by a pathway that is sensitive to the GSH status of the culture system. This mechanism is in contrast to tBHQ activation, which is generally insensitive to GSH in the culture (Lee, et al., 2001).
Because Nrf2 activation is known to lead to GSH increases via the induction of GSH synthesis genes, we evaluated the Nrf2 dependence of cystamine-mediated GSH increases. We found that even in Nrf2 deficient cultures, cystamine administration leads to increased GSH. One potential explanation for this phenomenon would be an increase in cysteine levels, similar to that which has been observed previously in cell cultures and in brain tissue (Fox, et al., 2004, Pinto, et al., 2005). We probed this potential mechanism by inactivating the trassulfuration pathway. The transsulfuraton pathway is a major route of cysteine synthesis. However, even after inactivation of the transsulfuration pathway, we were able to find increases in GSH levels after cystamine treatment. These results indicate that the GSH increase is not dependent on cysteine synthesis by the transsulfuration pathway, but they do not rule out the involvement of cysteine increase by other mechanisms. One hypothetical mechanism for cysteine increase after cystamine administration is via the breaking of cystine disulfide bonds to form a mixed disulfide and cysteine (Thoene, et al., 1976). We also tested whether GSH was increased by de novo synthesis or by a stabilization event. In order to do so, we pretreated cultures with BSO and found cystamine was unable to increase GSH in this condition. Together, these data lead us to conclude that cystamine increases GSH synthesis by providing an alternative source of substrate, separate from normal cysteine synthesis mechanisms. Cysteine is the most likely substrate because its availability is known to be rate limiting in GSH synthesis in cultured cells (Deneke and Fanburg, 1989). Furthermore, cysteine concentrations are known to increase after cystamine administration in cells and in animals (Fox, et al., 2004).
Although it is unlikely that cystamine, or even cysteamine, accumulates to appreciable levels in brain (Pinto, et al., 2005), we evaluated the potential for cystamine to induce ARE mediated transcription after systemic administration. We found that cystamine activates ARE mediated transcription in multiple tissues, including brain. The in vitro data suggest that the disulfide moiety in cystamine is responsible for Nrf2 activation. Presumably, metabolic products of cystamine (downstream of cysteamine) in astrocytes do not produce Nrf2 activation. Based on the in vitro mechanism of Nrf2 activation, it is therefore surprising that systemic administration of cystamine leads to Nrf2 activation in brain. The observed activity may be due to biotransformation of cystamine in peripheral tissues, or another similar cause. At present we are unable to suggest a likely chemical mediator of cystamine-induced Nrf2 activation in the central nervous system. Our observation that cystamine activates Nrf2 signaling in brain illustrates the importance of performing studies in vivo. Based on our in vitro data, we would predict that cystamine would not be able to activate Nrf2 in brain due to the requirement of the intact disulfide moiety. Performing in vivo experiments, in spite of the predictions from the in vitro results, allowed us to indentify a role for Nrf2 in cystamine mediated neuroprotection.
In our study, we were unable to confirm Nrf2 activation using immunostaining methods. In human postmortem brain, Nrf2 activation was detected with immunostaining in Alzheimer’s disease, Lewy body variant of Alzheimer’s disease, and Parkinson’s disease (Ramsey, et al., 2007). Recently, the immunostaining protocol for Nrf2 in human brain has been standardized, however the investigators note the difficulty with which this low abundance protein is detected (Lindl and Jordan-Sciutto, 2008). Furthermore, the same group has been thus far unsuccessful using their protocol to specifically detect Nrf2 in murine tissue (personal communication). The technical gap is especially unfortunate because we are unable to provide a direct comparison between murine models and human tissue, leaving open the possibility that Nrf2 activation by cystamine may be specific to mouse models.
Systemic cystamine administration leads to Nrf2-dependent ARE activation, especially in striatum. Because striatum is a target tissue for mutant huntingtin toxicity as well as mitochondrial complex II inhibitor toxicity, we hypothesized that cystamine protection from striatal degeneration due to these toxic insults would be Nrf2-dependent. In order to test this hypothesis, we administered cystamine to wildtype and Nrf2 deficient mice for one week and then began treatment with 3NP. We found that wildtype mice were protected from 3NP lesioning by cystamine treatment. None of the wildtype mice that received cystamine exhibited lesions from 3NP treatment. If the mechanism of this protection were independent of Nrf2, we would predict that the smaller lesions produced by lower 3NP doses in Nrf2 deficient mice would be completely protected against. However, our data show the opposite. In the Nrf2 deficient animals, lesions are still present and exhibit high inter-animal variability with combined cystamine treatment. Therefore we conclude that Nrf2 is essential for cystamine protection from striatal lesioning by 3NP. Because both chemicals are administered systemically and Nrf2 deficiency is also systemic, we are unable to conclude that striatal Nrf2 activation specifically leads to protection. However, circumstantial evidence suggests that Nrf2 induction in brain is central to this protection. Systemic cystamine induces Nrf2 activation more effectively in striatum than other brain tissues. Striatum is the target tissue for 3NP, and based on previously published results, local striatal Nrf2 activation protects from striatal toxicity (Calkins, et al., 2005, Jakel, et al., 2007).
We used a dosing regimen that pre-exposed animals to cystamine for seven days prior to 3NP treatment. This treatment regimen was employed in order to replicate the findings reported by Fox et al. (2004). It is possible that shorter treatments with cystamine would also lead to protection from 3NP, however we did not examine shorter pretreatments in this work. We performed pilot experiments treating the ARE-hPAP animals with cystamine for three days instead of seven. Similar to seven days, we saw signs of Nrf2 activation in these mice (data not shown). If ARE activation is sufficient to protect from 3NP lesioning, we would expect that three day pretreatments would also be protective.
Recently, it has been shown that 3NP treatment in rats depletes glutathione throughout the brain (Kumar and Kumar, 2009, Kumar and Kumar, 2009, Yang, et al., 2009) and further that supplementing glutathione can prevent 3NP lesions (Fontaine, et al., 2000). It is possible that cystamine may prevent glutathione depletion by 3NP and thereby exert protection. This hypothesis would be supported by our result that cystamine does not protect in Nrf2 deficient mice. Nrf2 is a key factor in the regulation of glutathione synthesis genes and deficiency should lead to an inability of the tissue to effectively upregulate glutathione synthesis. Cystamine-induced upregulation of glutathione synthesis genes via Nrf2 activation coupled with demonstrated increases L-cysteine levels could be expected to attenuate glutathione depletion by 3NP.
Surprisingly, we found that combining cystamine and 3NP treatments led to a significant elevation in clinical signs of illness in the mice. This observation was independent of Nrf2 and the extent of difference appears to correlate with length of administration. However, cystamine does protect from striatal 3NP lesioning in wildtype mice. These paired observations clearly separate clinical appearance of symptoms from striatal lesion volume. There are several potential explanations as to how clinical signs might be increased while lesion volume is decreased. For instance, it is possible that less overt damage is occurring in the nervous system and went undetected by the cresyl violet staining method. This type of damage may include but is not limited to neuritic damage or changes in neural function. Alternatively, clinical signs observed in mice after 3NP lesioning may result from toxicity outside of the central nervous system. Other groups have clearly documented that peripheral toxicity, especially in heart, occurs in mice after systemic 3NP administration (Gabrielson, et al., 2001, Lopez, et al., 1998, Zwingmann and Bilodeau, 2006). If peripheral toxicity manifests as clinical signs, then these signs would be largely uninformative from a neurotoxicological standpoint. It is unclear why the clinical signs would be increased in concert with apparently decreased striatal neurotoxicity in 3NP-cystamine combination treated animals. Therefore, this study highlights the potential value of establishing a rubric of extrapyramidal symptoms in mouse models that clearly indicates dysfunction in specific brain regions.
This work shows that Nrf2 is an important mediator of the neuroprotective effect of cystamine. Because the chemical exhibits multiple effects in cell culture and in mouse models, it would be beneficial to distinguish the effects and therapeutically target the discovered mechanisms. Cystamine activation of Nrf2 in striatum, and the necessity of Nrf2 for striatal protection from 3NP have been demonstrated. These results support the conclusion that Nrf2 activation is an excellent therapeutic target for neurodegenerative conditions.
Acknowledgements
The authors would like to thank Dr. Neal C. Burton for providing critical comments and editing this manuscript. The authors would also like to thank Jon M. Resch, Hoa Ahn Phan, and Sara Amirahmadi for maintaining mouse colonies. These studies were funded by grants from the National Institute of Environmental Health Sciences (ES08089 and ES10042) and the Hereditary Disease Foundation to JAJ. MJC was also supported by a Molecular and Environmental Toxicology pre-doctoral training grant (T32 ES007015).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging. 2006;27:871–879. doi: 10.1016/j.neurobiolaging.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Borrell-Pages M, Canals JM, Cordelieres FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Neri C, Alberch J, Brouillet E, Saudou F, Humbert S. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79:509–521. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- 5.Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PC, Vargas MR, Pani A, Johnson DA, Smeyne R, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: a critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813361106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 11.Di Leandro L, Maras B, Schinina ME, Dupre S, Koutris I, Martin FM, Naquet P, Galland F, Pitari G. Cystamine restores GSTA3 levels in Vanin-1 null mice. Free Radic Biol Med. 2008;44:1088–1096. doi: 10.1016/j.freeradbiomed.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine MA, Geddes JW, Banks A, Butterfield DA. Effect of exogenous and endogenous antioxidants on 3-nitropionic acid-induced in vivo oxidative stress and striatal lesions: insights into Huntington's disease. J Neurochem. 2000;75:1709–1715. doi: 10.1046/j.1471-4159.2000.0751709.x. [DOI] [PubMed] [Google Scholar]
- 13.Fox JH, Barber DS, Singh B, Zucker B, Swindell MK, Norflus F, Buzescu R, Chopra R, Ferrante RJ, Kazantsev A, Hersch SM. Cystamine increases L-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J Neurochem. 2004;91:413–422. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabrielson KL, Hogue BA, Bohr VA, Cardounel AJ, Nakajima W, Kofler J, Zweier JL, Rodriguez ER, Martin LJ, de Souza-Pinto NC, Bressler J. Mitochondrial toxin 3-nitropropionic acid induces cardiac and neurotoxicity differentially in mice. Am J Pathol. 2001;159:1507–1520. doi: 10.1016/S0002-9440(10)62536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 16.Jakel RJ, Kern JT, Johnson DA, Johnson JA. Induction of the protective antioxidant response element pathway by 6-hydroxydopamine in vivo and in vitro. Toxicol Sci. 2005;87:176–186. doi: 10.1093/toxsci/kfi241. [DOI] [PubMed] [Google Scholar]
- 17.Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- 21.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft AD, Lee JM, Johnson DA, Kan YW, Johnson JA. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J Neurochem. 2006;98:1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Kumar A. Effect of lycopene and epigallocatechin-3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: A novel nitric oxide mechanism. Food Chem Toxicol. 2009 doi: 10.1016/j.fct.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Kumar P, Kumar A. Neuroprotective effect of cyclosporine and FK506 against 3-nitropropionic acid induced cognitive dysfunction and glutathione redox in rat: possible role of nitric oxide. Neurosci Res. 2009;63:302–314. doi: 10.1016/j.neures.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Lebo RV, Kredich NM. Inactivation of human gamma-glutamylcysteine synthetase by cystamine. Demonstration and quantification of enzyme-ligand complexes. J Biol Chem. 1978;253:2615–2623. [PubMed] [Google Scholar]
- 26.Lee JM, Moehlenkamp JD, Hanson JM, Johnson JA. Nrf2-dependent activation of the antioxidant responsive element by tert-butylhydroquinone is independent of oxidative stress in IMR-32 human neuroblastoma cells. Biochem Biophys Res Commun. 2001;280:286–292. doi: 10.1006/bbrc.2000.4106. [DOI] [PubMed] [Google Scholar]
- 27.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 28.Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- 29.Lindl KA, Jordan-Sciutto KL. Examining the endogenous antioxidant response through immunofluorescent analysis of Nrf2 in tissue. Methods Mol Biol. 2008;477:229–243. doi: 10.1007/978-1-60327-517-0_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez PS, Castillo CH, Pastelin GH, Hernandez MR, Suarez MJ, Sanchez ML, Escalante BA. Characterization of 3-nitropropionic acid-induced bradycardia in isolated atria. Toxicol Appl Pharmacol. 1998;148:1–6. doi: 10.1006/taap.1997.8284. [DOI] [PubMed] [Google Scholar]
- 31.Lorand L, Conrad SM. Transglutaminases. Mol Cell Biochem. 1984;58:9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- 32.Okauchi M, Xi G, Keep RF, Hua Y. Tissue-type transglutaminase and the effects of cystamine on intracerebral hemorrhage-induced brain edema and neurological deficits. Brain Res. 2008 doi: 10.1016/j.brainres.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto JT, Van Raamsdonk JM, Leavitt BR, Hayden MR, Jeitner TM, Thaler HT, Krasnikov BF, Cooper AJ. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. J Neurochem. 2005;94:1087–1101. doi: 10.1111/j.1471-4159.2005.03255.x. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL. Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci U S A. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 39.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stack EC, Ferro JL, Kim J, Del Signore SJ, Goodrich S, Matson S, Hunt BB, Cormier K, Smith K, Matson WR, Ryu H, Ferrante RJ. Therapeutic attenuation of mitochondrial dysfunction and oxidative stress in neurotoxin models of Parkinson's disease. Biochim Biophys Acta. 2008;1782:151–162. doi: 10.1016/j.bbadis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Erb H, Murphy TH. Coordinate regulation of glutathione metabolism in astrocytes by Nrf2. Biochem Biophys Res Commun. 2005;326:371–377. doi: 10.1016/j.bbrc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 43.Thoene JG, Oshima RG, Crawhall JC, Olson DL, Schneider JA. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976;58:180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 45.Tremblay ME, Saint-Pierre M, Bourhis E, Levesque D, Rouillard C, Cicchetti F. Neuroprotective effects of cystamine in aged parkinsonian mice. Neurobiol Aging. 2006;27:862–870. doi: 10.1016/j.neurobiolaging.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Van Raamsdonk JM, Pearson J, Bailey CD, Rogers DA, Johnson GV, Hayden MR, Leavitt BR. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J Neurochem. 2005;95:210–220. doi: 10.1111/j.1471-4159.2005.03357.x. [DOI] [PubMed] [Google Scholar]
- 47.Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Sarkar A, Cicchetti F, Yu M, Zhu A, Jokivarsi K, Saint-Pierre M, Brownell AL. Cerebral PET imaging and histological evidence of transglutaminase inhibitor cystamine induced neuroprotection in transgenic R6/2 mouse model of Huntington's disease. J Neurol Sci. 2005;231:57–66. doi: 10.1016/j.jns.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem. 2009;109:1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 52.Zwingmann C, Bilodeau M. Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology. 2006;43:454–463. doi: 10.1002/hep.21075. [DOI] [PubMed] [Google Scholar]