Abstract
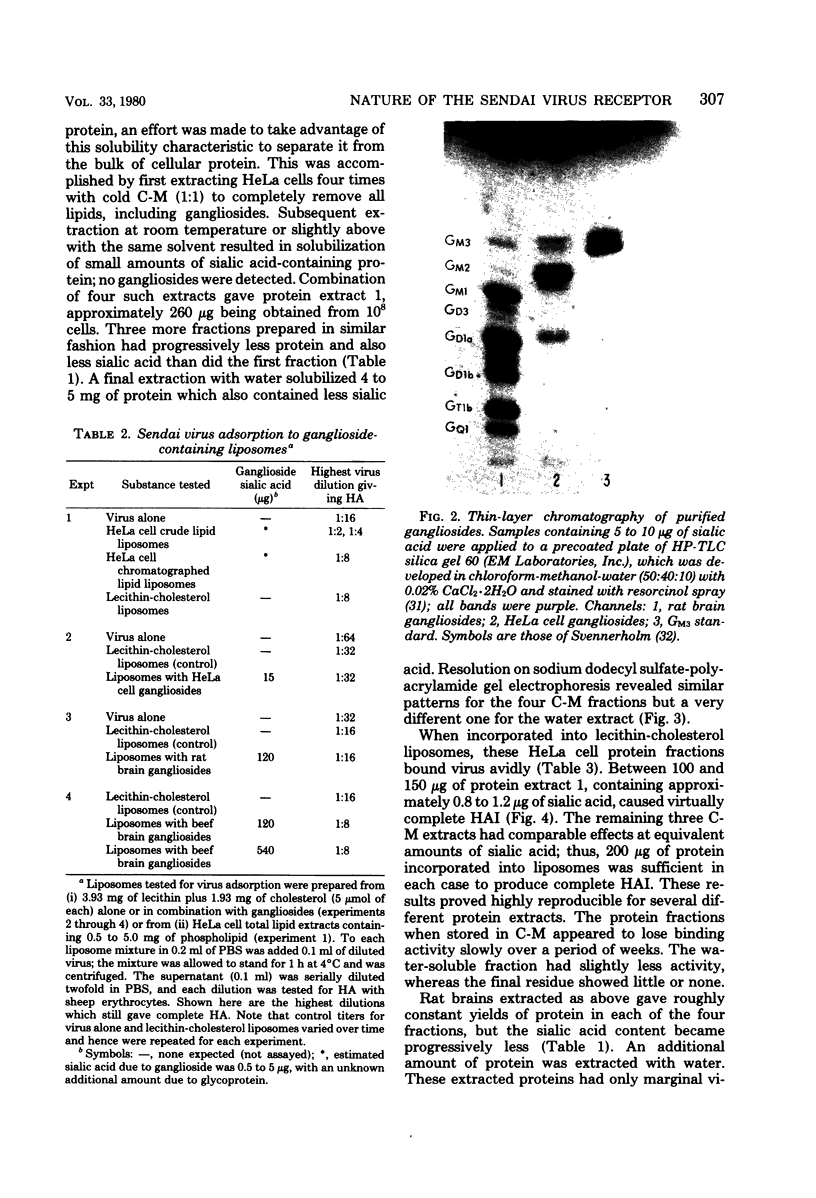
Gangliosides were compared with glycoproteins as potential receptors for Sendai virus by incorporating measured amounts of the glycoconjugates into lecithin-cholesterol liposomes and measuring binding by a hemagglutination assay with sheep erythrocytes. HeLa cell gangliosides showed no binding activity toward the virus up to 15 micrograms of sialic acid per 5 mumol of lecithin-cholesterol, whereas HeLa cell glycoproteins incorporated into similar liposomes caused avid virus binding below 1 microgram of sialic acid. These sialoglycoproteins could be separated from the bulk of cell proteins by multiple chloroform-methanol extractions. Purified rat brain gangliosides at a level of 120 micrograms of sialic acid in liposomes did not bind virus, whereas chloroform-methanol-extracted rat brain proteins caused only marginal binding. Bovine brain gangliosides differed slightly from the rat brain mixture in showing weak binding properties. Our results thus indicate that glycoproteins, rather than gangliosides, are the natural receptors for Sendai virus and that tissues differ as to the quantity of such protein receptors.
Full text
PDF


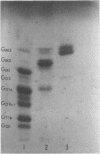
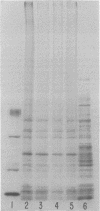
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besancon F., Ankel H., Basu S. Specificity and reversibility of interferon ganglioside interaction. Nature. 1976 Feb 19;259(5544):576–578. doi: 10.1038/259576a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Drzeniek R. Wechselwirkungen zwischen Myxoviren und Zelloberflächen. II. Reversible und irreversible Bindung von Myxoviren an Erythrocyten. Z Med Mikrobiol Immunol. 1970;155(4):335–351. [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Ghidoni R., Sonnino S., Tettamanti G., Wiegandt H., Zambotti V. On the structure of two new gangliosides from beef brain. J Neurochem. 1976 Aug;27(2):511–515. doi: 10.1111/j.1471-4159.1976.tb12275.x. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Schaumburg H. H., Norton W. T. Isolation and characterization of glial filaments from human brain. J Cell Biol. 1978 Aug;78(2):426–440. doi: 10.1083/jcb.78.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. W., McConnell H. M. Glycophorin in lipid bilayers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4653–4657. doi: 10.1073/pnas.71.12.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. I. Differential reactivities of fetal and adult human erythrocytes to antisera directed against glycolipids of human erythrocytes. Vox Sang. 1969 Jun;16(6):478–485. doi: 10.1111/j.1423-0410.1969.tb04775.x. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Characteristics of Sendai virus receptors in a model membrane. J Mol Biol. 1974 Mar 15;83(4):427–436. doi: 10.1016/0022-2836(74)90504-x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledeen R. W. Ganglioside structures and distribution: are they localized at the nerve ending? J Supramol Struct. 1978;8(1):1–17. doi: 10.1002/jss.400080102. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K., Eng L. F. Gangliosides of human myelin: sialosylgalactosylceramide (G7) as a major component. J Neurochem. 1973 Oct;21(4):829–839. doi: 10.1111/j.1471-4159.1973.tb07527.x. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- MARINETTI G. V., ERBLAND J., STOTZ E. The quantitative analysis of plasmalogens by paper chromatography. Biochim Biophys Acta. 1959 Jan;31(1):251–252. doi: 10.1016/0006-3002(59)90464-0. [DOI] [PubMed] [Google Scholar]
- Mooney J. J., Dalrymple J. M., Alving C. R., Russell P. K. Interaction of Sindbis virus with liposomal model membranes. J Virol. 1975 Feb;15(2):225–231. doi: 10.1128/jvi.15.2.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Sharom F. J., Barratt D. G., Thede A. E., Grant C. W. Glycolipids in model membranes. Spin label and freeze-etch studies. Biochim Biophys Acta. 1976 Dec 2;455(2):485–492. doi: 10.1016/0005-2736(76)90319-9. [DOI] [PubMed] [Google Scholar]
- Spiegelstein P. F., Haimsohn M., Gitelman J., Kohn A. Early changes in the membrane of HeLa cells adsorbing Sendai virus under conditions of fusion. J Cell Physiol. 1978 May;95(2):223–233. doi: 10.1002/jcp.1040950212. [DOI] [PubMed] [Google Scholar]
- Stein K. E., Schwarting G. A., Marcus D. M. Glycolipid markers of murine lymphocyte subpopulations. J Immunol. 1978 Feb;120(2):676–679. [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E. Tentative identification of the tetanus toxin recptor in nervous tissue. J Gen Microbiol. 1959 Apr;20(2):310–320. doi: 10.1099/00221287-20-2-310. [DOI] [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gas--liquid chromatographic assay of lipid-bound sialic acids: measurement of gangliosides in brain of several species. J Lipid Res. 1970 Nov;11(6):506–516. [PubMed] [Google Scholar]