Abstract
In ventricular myocytes, activation of protein kinase A (PKA) by 3′-5′cyclic adenosine-monophosphate (cAMP) increases the force of contraction by increasing L-type Ca2+ channel currents (ICa) and sarcoplasmic reticulum (SR) Ca2+ release during excitation-contraction coupling. Cyclic-nucleotide phosphodiesterases (PDEs) comprise a large family of enzymes whose role in the cell is to regulate the spatial and temporal profile of cAMP signals by controlling the degradation of this second messenger. At present, however, the molecular identity and functional roles of the PDEs expressed in ventricular myocytes are incompletely understood. Here, we tested the hypothesis that PDE8A plays a critical role in the modulation of at least one compartment of cAMP and hence PKA activity during β-adrenergic receptor (βAR) activation in ventricular myocytes.
Consistent with this hypothesis, we found that PDE8A transcript and protein are expressed in ventricular myocytes. Our data indicate that evoked [Ca2+]i transients and ICa increased to a much larger extent in PDE8A null (PDE8A−/−) that in wild type (WT) myocytes during β-adrenergic signaling activation. In addition, Ca2+ spark activity was higher in PDE8A−/− than in WT myocytes.
Our data indicate that PDE8A is a novel cardiac PDE that controls one or more pools of cAMP implicated in regulation of Ca2+ movement through cardiomyocyte.
Keywords: Phosphodiesterase, cAMP, EC coupling, L-type Ca2+ channels, Ca2+ transient, Ca2+ sparks
INTRODUCTION
3′-5′cyclic adenosine-monophosphate (cAMP) is one of the most important second messengers in the heart because it regulates many physiological and pathological processes such as cardiac contractility, relaxation, and the onset and progression of cardiac hypertrophy. Upon stimulation of β-adrenergic receptors (βAR), increased cAMP via its main effector, protein kinase A (PKA), influences the activity of several proteins involved in excitation-contraction (EC) coupling, including the L-type Ca2+ channel, phospholamban (PLB) and phospholemman (PLM), the ryanodine receptor (RyR) and Troponin I [1]. Effects of an increase in cAMP include: increases in Ca2+ current (ICa) and sarcoplasmic reticulum (SR) Ca2+ uptake and release, as well as desensitization of myofilaments to Ca2+.
The magnitude, duration, and spatial spread of cAMP signals are regulated by cyclic nucleotide phosphodiesterases (PDEs). PDEs form a superfamily of 11 homologous gene-families that have highly conserved C-terminal catalytic domains [2]. At present, members of at least four families of cAMP-hydrolizing PDEs (PDE1, PDE2, PDE3 and PDE4) are known to be expressed in ventricular myocytes[3]. All can catalyze the hydrolysis of cAMP, thus lowering its concentration in the vicinity of its effector enzymes. However the mechanism of activation, regulation, and subcellular localization differ between the isoforms [4, 5]. Lack of understanding of the localization and functional roles for each specific PDE expressed in the heart, however, limits the general impact of this model. In particular, essentially nothing is reported regarding the roles of PDE8 in cardiac functions. Here, we describe the expression of the cAMP-specific PDE, PDE8A, in cardiac myocytes. Our data indicate that PDE8A transcript and protein are expressed and catalytically active in ventricular myocytes, and that PDE8A deletion potentiates cAMP/PKA elicited increases in ICa and SR Ca2+ release during β-adrenergic stimulation.
METHODS
Mice
For a description on the generation of the PDE8A knock-out (PDE8A−/−) mouse line refer to Vasta et al. 2006 (see Supplemental Material). For the experiments reported, age-matched wild-type or littermate control mice and PDE8A−/− mice between 2 and 4 months of age were used.
Real Time PCR
cDNA was prepared from total RNA from wild-type and PDE8A-null mouse ventricles or isolated myocytes by using SuperScript III and Oligo dT (Invitrogen Corp., Carlsbad, CA). Primers (IDT, Coralville, IA) for the different PDE isoforms, directed to the catalytic domain, are listed in supplemental Table 1.
Ventricular Myocyte Dissociation
Isolated myocytes from adult mice were obtained using a standard retrograde perfusion as previously described [6]. After dissociation, ventricular myocytes were maintained in solution with 2mM Ca2+ at room temperature (25°C) until used. All experiments were performed at room temperature.
β-Galactosidase Staining
Freshly frozen mouse hearts were embedded in Tissue-Tek OCT compound and then sectioned on a cryostat at 20 μm per slice. Isolated myocytes were plated on laminin-coated coverslip and allowed to attach for 1 hour. Cells or tissue sections were then fixed in 0.2% glutaraldehyde solution, washed three times in PBS and then incubated at 37°C for 12-16 hours in X-Gal Staining Solution (5mmol/L K4Fe(CN)6, 5mmol/L. K3Fe(CN)6, 2mmol/L MgCl2, 0.02% NP40, 0.01% Deoxycholic Acid, 5mmol/L EGTA, 1mg/ml X-Gal in PBS 1X pH 7.4). Sections were then counterstained with Eosin and mounted in Permount mounting medium (Fisher Scientific).
PDE8A Immunoprecipitation
Immunoprecipitation of PDE8A from mouse ventricles or isolated myocytes was performed as previously reported[7]. Immunoprecipitate were then run on SDS-PAGE gels for immuno-blot detection with a PDE8A-specific antibody (121-AP Fabgennix, Frisco, TX).
Electrophysiology
Ionic currents and membrane potentials were recorded using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Union City, CA). Signals were digitized and stored on a computer running the pCLAMP 8 software suite (Axon Instruments, Union City, CA). Analysis of electrophysiological records was performed using the CLAMPFIT module of pCLAMP 8. For experiments measuring Ca2+ currents (ICa), cells were superfused with physiological saline solution. After whole-cell voltage clamp was achieved, the superfusion solution was changed to one containing (in mmol/L): 140 NaCl, 5 CsCl, 2 CaCl2, 1 MgCl2, 10 Glucose, 10 HEPES, 0.010 TTX. The pipette solution used in these experiments contained (in mmol/L) 130 CsCl, 10 TEA-Cl, 5 Mg-ATP and 10 HEPES. Identical solutions (without TTX) were used for simultaneous recording of ICa and [Ca2+]i.
Field-stimulation
Field stimulation was performed via 2 platinum wires (0.5 cm separation) placed at the bottom of the perfusion chamber. An IonOptix Myopacer (IonOptix Corp, Milton, MA, USA) stimulator was used to deliver square voltage pulses (4 ms duration) with amplitude of 1.5X threshold at a frequency of 1 Hz.
Ca2+ Measurements
We measured changes in [Ca2+]i in myocytes loaded with the membrane permeable acetoxymethyl-ester form of Fluo-4 (Fluo-4 AM) or Fura-2 as previously described [8].
Confocal imaging of whole-cell [Ca2+]i and Ca2+ sparks was performed using a BioRad Radiance 2000 confocal system (Cambridge, MA, USA) coupled to a Nikon TE300 inverted microscope equipped with a Nikon 60X oil immersion lens (NA = 1.4). Images were analyzed with custom software written in IDL language (Research Systems, Boulder, CO, USA). Ca2+ sparks were identified using a computer algorithm similar to the one described by Cheng et al. [9]. Ca2+ spark mass was calculated as described elsewhere [10]. The amplitude of the [Ca2+]i transient evoked by the application of a Ca2+- and Na+-free (substituted with N-methyl-D-glucamine) solution containing 20 mmol/L caffeine (via a picospritzer) was used as an indicator of SR Ca2+ content [11].
Statistics
The GraphPad Prism software, version 4.0a, was used for statistical analysis. All values are presented as mean ± SEM. To assess statistical significance comparison between groups, genotypes and/or stimulation conditions was performed by using an unpaired Student’s t-test. Non-significant (n.s.) differences are indicated by P-values greater than 0.05.
RESULTS and DISCUSSION
Although PDE8A is known to be expressed most highly in testis, its presence in several other tissues, including heart, had been indicated by Northern Blot analysis in human and mouse specimens [7, 12, 13]. Thus, we utilized several methods to determine if PDE8A transcript is expressed in ventricular myocytes. First we performed a quantitative real-time PCR profile for all known cAMP hydrolyzing PDEs in whole mouse ventricle and also in isolated myocytes. We found that, beside the most abundant PDE mRNAs for PDE2A and PDE1C, the PDE8A mRNA level is similar to that of other important cardiac PDEs including PDE3A and PDE4B (supplementary Fig.1).
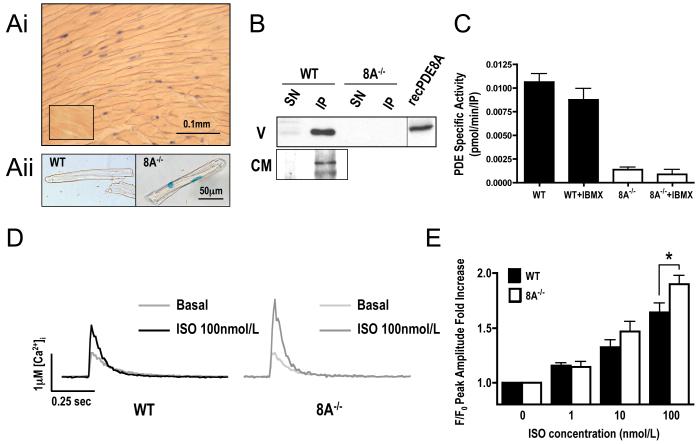
Next, we used a mouse genetically engineered to lack PDE8A expression (see Methods section and Vasta et al.[7] for details on the generation of PDE8A−/− mice). In these animals, the PDE8A gene-targeting construct contains a LacZ-Neo cassette that replaces one of the catalytic domain exons thus assuring loss of PDE8A catalytic activity. This cassette also contains a nuclear localization signal that allows determination of PDE8A mRNA expression by immunocytochemical visualization of nuclear β-Galactosidase staining. As shown in Figure 1, the nuclei of ventricular myocytes stained positive, indicating active transcription of the PDE8A gene in these cells (Fig. 1Ai and 1Aii and Supplementary Fig.2).
Figure 1.
PDE8A expression in cardiac tissue and effect its absence on Ca2+ transient. (A) PDE8A immunohistochemistry detection in mouse ventricles. (Ai) PDE8A−/− ventricle section stained for β-Gal activity. Lower left inset shows a section from a WT animal used as negative control. (Aii) staining on an isolated WT and PDE8A−/− myocyte (see also supplementary Fig.2). Due to the presence of a nuclear localization sequence (NLS) fused at the 3′ of the LacZ gene, β-Gal activity is confined to the nucleus of PDE8A expressing cells. (B) Western Blot gels of PDE8A immunoprecipitates (IP) and their supernatants (SN) from ventricle (V) or cardiomyocytes (CM) from WT and PDE8A−/− animals. Recombinant PDE8A is used as positive control (n=3). (C) PDE activity assay on IP in presence of 10nmol/L cAMP as substrate and absence or presence of 100μmol/L IBMX (n=3). (D) Typical fluo-4 traces of Ca2+ transients at 2 mmol/L Ca2+ and 1.0-Hz field stimulation, in basal state and after 2 min of 100 nmol/L Isoproterenol (ISO) stimulation. (E) Statistics for Ca2+ transient peak amplitude fold increase over basal at increasing concentration of ISO (n=31 to 51 cells from 5 to 6 hearts for each group; * P<0.05 WT vs. 8A−/−).
Consistent with the transcript data above, using Western analysis we detected a protein with the expected molecular weight of PDE8A (95 kDa) in immunopecipitates from protein extracts obtained from either total ventricles or isolated ventricular myocytes. This 95 kDa protein co-migrated with recombinant PDE8A and was missing in extracts from PDE8A−/− tissue (Figure 1B). We next measured PDE activity in ventricular PDE8A immunoprecipitates. As expected for PDE8A, we found that the phosphodiesterase activity in this immunoprecipitate was insensitive to a high dose (100μmol/L) of the PDE non-selective inhibitor 3-isobutyl-1-metyl-xanthine (IBMX)(PDE8 is the only family of cAMP selective PDEs that is insensitive to IBMX). Collectively, these data show that functional PDE8A protein is expressed in cardiomyocytes.
Given the pivotal role of cAMP in modulating EC coupling, we investigated the possible functional role of PDE8A in EC coupling in ventricular myocytes. To do this, we recorded action potential-evoked [Ca2+]i in WT and PDE8A−/− ventricular myocytes loaded with the Ca2+ indicator fluo-4 (Fig. 1D and 1E). The amplitude of the [Ca2+]i transient was similar in WT and PDE8A−/− myocytes, indicating that under non stimulated basal conditions loss of PDE8A activity has little affect on this process.
Next, we examined whether PDE8A modulates βAR signaling in ventricular myocytes. [Ca2+]i transients were recorded in WT and PDE8A−/− cells before and after the application of the βAR agonist isoproterenol (ISO; 1 to100 nmol/L). As expected, ISO increased the amplitude of the evoked [Ca2+]i transient in WT myocytes. Note, however, that ISO increased the [Ca2+]i transient to a larger extent in PDE8A−/− than in WT myocytes (Fig. 1D). As shown in Fig. 1E, the difference in the transient fold increase over the basal becomes statistically significant at 100 nmol/L ISO. Diastolic [Ca2+]i, assessed using the ratiometric indicator fura-2 (Supplementary Fig. 3), was similar in WT and PDE8A−/− myocytes. This rules out the possibility that the larger systolic [Ca2+]i levels seen during βAR signaling in PDE8A−/− myocytes than in WT cells were due differences in resting [Ca2+]i between these cells. Rather, the data indicate that PDE8A is an important acute regulator of [Ca2+]i during βAR signaling in ventricular myocytes. In agreement with this hypothesis, we found similar levels of cAMP in WT and PDE8A−/− myocytes in the basal state, but the increase in cAMP after ISO was higher in the PDE8A−/− myocytes, (Supplementary Fig. 4). However this was not accompanied by a different level of global PKA activation, as assessed indirectly by measurement of PKA substrate phosphorylation (Supplementary Fig. 5). This result likely suggests that PDE8A does not regulate all compartment of cAMP in these cells.
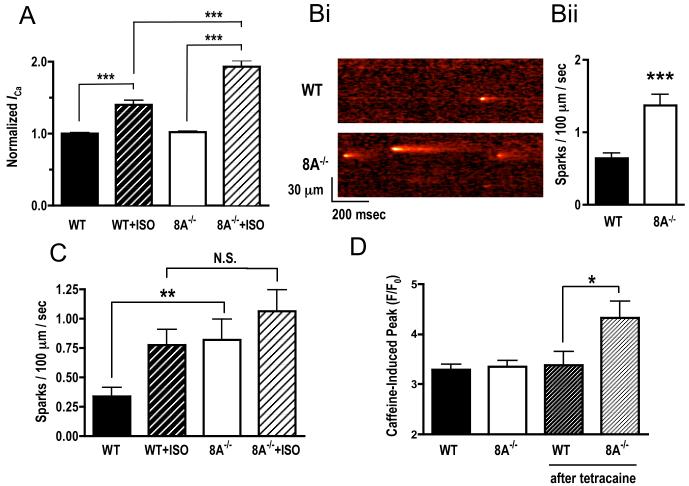
We also investigated some of the possible mechanisms underlying the larger [Ca2+]i transients seen in PDE8A−/− myocytes during EC coupling. L-type Ca2+ channels are known to be activated by PKA during βAR signaling [14]. Thus, we recorded ICa in WT and PDE8A−/− myocytes before and after ISO (Fig. 2A). ICa was evoked by the application of 200 ms voltage steps to voltages ranging from −30 to +60 mV from a holding potential of −40 mV. Under control conditions (i.e. without ISO), ICa was similar in WT and PDE8A−/− myocytes at all voltages examined (Fig.2A). Consistent with the [Ca2+]i data, application of ISO increased ICa to a larger extent in PDE8A−/− than in WT myocytes. Thus, the data suggest that the presence of PDE8A modulates [Ca2+]i, at least in part, by regulating ICa during βAR signaling.
Figure 2.
Effects of lack of PDE8A on ISO-stimulated ICa and SR leak. (A) Average normalized ICa in WT and 8A−/− myocytes before and after 100 nmol/L ISO stimulation (n=6 cells from 2 hearts for each group; ***P<0.005). (B) Confocal line-scan images showing spontaneous Ca2+ sparks in WT and 8A−/− cells and statistic on calculated spark frequency (n=42 to 56 cells from 5 hearts for each group; ***P<0.005 WT vs. 8A−/−). (C) Average spark frequency before and after 2 minute exposure to 100 nmol/L Isoproterenol (ISO) (n=20 to 24 cells from 5 hearts for each group; **P<0.01 WT basal vs. 8A−/− basal, *P<0.05 WT basal vs. WT ISO). (D) SR loading measured by caffeine induced Ca2+ peak in resting cells and after 5 minutes of 100 μmol/L Tetracaine exposure (n=12 to 16 cells from 3 hearts for each group; *P<0.05 WT+Tetracaine vs. 8A−/− +Tetracaine).
To determine whether PDE8A might also modulate SR Ca2+ release in ventricular myocytes, we recorded spontaneous Ca2+ sparks in WT and PDE8A−/− ventricular myocytes. Fig. 2Bi shows representative Ca2+ sparks recording in these cells. Interestingly, Ca2+ spark frequency was higher in PDE8A−/− than in WT cells (Fig. 2Bii). However, the amplitude and duration were similar in WT and PDE8A−/− myocytes (Supplementary Fig 6). As reported in many other studies, application of 100nmol/L ISO dramatically increased sparks frequency in WT cells. However in PDE8A−/− myocytes this increase was not significantly greater (Fig. 2C). Thus, absence of PDE8A appears to affect basal RyR activity in ventricular myocytes but not ISO stimulated activity, likely due to the spontaneous rates already being increased in the PDE8A−/− cells.
Because Ca2+ spark activity can be modulated by luminal Ca2+, we directly examined the SR Ca2+ load in WT and PDE8A−/− myocytes (Fig. 2D). We found that SR Ca2+ load was similar in WT and PDE8A−/− myocytes as might be expected from the fact that spark amplitude was not different. When we re-measured SR Ca2+ load after WT and PDE8A cells were exposed to 100μmol/L tetracaine to block Ca2+ sparks (i.e. leak), SR Ca2+ load increased to a larger extent in PDE8A−/− than WT myocytes (Fig. 2D). This indirectly suggests that PDE8A−/− myocytes might have a higher rate of SR refilling, which would compensates for the Ca2+ leak through the RyR in the form of Ca2+ sparks, and maintain a normal level of SR Ca2+ loading.
In summary, the present study introduces a novel PDE, PDE8A, as a key modulator of cAMP signaling in mouse cardiac myocytes. Removal of this enzyme causes leaky RyR channels and potentiates cellular responses to β-adrenergic stimulation in the form of increased L-type ICa and Ca2+ transients. We are currently investigating the molecular mechanism behind these observations.
Supplementary Material
Acknowledgments
SOURCES OF FUNDINGS
This work was supported by NIH grant R01 GM083926-02 to J.A.B., NIH grant R01 HL085686 to L.F.S., and the Fondation Leducq.
Footnotes
DISCLOSURE
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 3.Osadchii OE. Myocardial phosphodiesterases and regulation of cardiac contractility in health and cardiac disease. Cardiovasc Drugs Ther. 2007;21(3):171–94. doi: 10.1007/s10557-007-6014-6. [DOI] [PubMed] [Google Scholar]
- 4.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99(8):816–28. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 5.Zaccolo M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol. 2006;85(7):693–7. doi: 10.1016/j.ejcb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279(1):H429–36. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 7.Vasta V, Shimizu-Albergine M, Beavo JA. Modulation of Leydig cell function by cyclic nucleotide phosphodiesterase 8A. Proc Natl Acad Sci U S A. 2006;103(52):19925–30. doi: 10.1073/pnas.0609483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544(Pt 1):57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, et al. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76(2):606–17. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingworth S, Peet J, Chandler WK, Baylor SM. Calcium sparks in intact skeletal muscle fibers of the frog. J Gen Physiol. 2001;118(6):653–78. doi: 10.1085/jgp.118.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santana LF, Kranias EG, Lederer WJ. Calcium sparks and excitation-contraction coupling in phospholamban- deficient mouse ventricular myocytes. J Physiol. 1997;503(Pt 1):21–9. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher DA, Smith JF, Pillar JS, St Denis SH, Cheng JB. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J Biol Chem. 1998;273(25):15559–64. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- 13.Soderling SH, Bayuga SJ, Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A. 1998;95(15):8991–6. doi: 10.1073/pnas.95.15.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Heyden MA, Wijnhoven TJ, Opthof T. Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res. 2005;65(1):28–39. doi: 10.1016/j.cardiores.2004.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.