Abstract
Alcohol consumption leads to myocardial contractile dysfunction possibly due to the toxicity of ethanol and its major metabolite acetaldehyde. This study was designed to examine the influence of mitochondrial aldehyde dehydrogenase-2 (ALDH2) knockout (KO) on acute ethanol exposure-induced cardiomyocyte dysfunction. Wild-type (WT) and ALDH2 KO mice were subjected to acute ethanol (3 g/kg, i.p.) challenge and cardiomyocyte contractile function was assessed 24 hrs later using an IonOptix® edge-detection system. Western blot analysis was performed to evaluate ALDH2, protein phosphatase 2A (PP2A), phosphorylation of Akt and glycogen synthase kinase-3β (GSK-3β). ALDH2 KO accentuated ethanol-induced elevation in cardiac acetaldehyde levels. Ethanol exposure depressed cardiomyocyte contractile function including decreased cell shortening amplitude and maximal velocity of shortening/relengthening as well as prolonged relengthening duration and a greater decline in peak shortening in response to increasing stimulus frequency, the effect of which was significantly exaggerated by ALDH2 KO. ALDH2 KO also unmasked an ethanol-induced prolongation of shortening duration. In addition, short-term in vitro incubation of ethanol-induced cardiomyocyte mechanical defects were exacerbated by the ALDH inhibitor cyanamide. Ethanol treatment dampened phosphorylation of Akt and GSK-3β associated with up-regulated PP2A, which was accentuated by ALDH2 KO. ALDH2 KO aggravated ethanol-induced decrease in mitochondrial membrane potential. These results suggested that ALDH2 deficiency led to worsened ethanol-induced cardiomyocyte function, possibly due to upregulated expression of protein phosphatase, depressed Akt activation and subsequently impaired mitochondrial function. These findings depict a critical role of ALDH2 in the pathogenesis of alcoholic cardiomyopathy.
Keywords: Ethanol, ALDH2, Cardiomyocyte, Contractile function, Akt, Protein phosphatase
INTRODUCTION
The cardiovascular effects of alcohol consumption are complex and depend on the dose, duration as well as pattern of alcohol consumption. Evidence from case control and cohort studies suggest light-to-moderate alcohol consumption is associated with a reduced risk of coronary-artery related events such as myocardial infarction. However, long-term consumption of large amounts of alcohol, is associated with the development of a cardiomyopathy, as well as increased incidence of arrhythmias and sudden death [1–3]. Several scenarios have been postulated for the pathogenesis of alcoholic cardiomyopathy including direct and indirect toxicity from ethanol and its metabolite acetaldehyde [4, 5], modifications of lipoprotein and apolipoprotein particles as well as accumulation of reactive oxygen species and fatty acid ethyl esters [6]. Others have suggested that ethanol-induced changes occur with the presence of certain permissive factors, such as oxidative stress and ethanol metabolism into more reactive molecules [7]. Although these theories have offered some explanations toward understanding of alcohol-induced tissue damage, specific pathogenic molecular mechanisms remain unknown.
As the very first metabolic product of ethanol, acetaldehyde is formed mainly through oxidation of ethanol via alcohol dehydrogenase (ADH). Subsequently, acetaldehyde is oxidized to acetic acid through aldehyde dehydrogenase (ALDH). Recent evidence has demonstrated a role of acetaldehyde in the pathogenesis of ethanol-induced tissue injuries especially alcoholic cardiomyopathy [7]. Acetaldehyde is approximately 10 times more toxic than ethanol on the basis of its 50% lethal dose (LD50) value [8]. Data from our group and others have depicted that acetaldehyde interrupts excitation-contraction coupling and sarco(endo)plasmic reticulum (SR) Ca2+ handling in cardiomyocytes [9–11]. Moreover, evidence from both human alcoholics and experimental animals has further revealed that acetaldehyde may react with nucleophilic groups forming stable and unstable proteins adducts, both of which detrimental to cellular physiological functions [12]. The speculation of “acetaldehyde toxicity” in alcoholism received further support from our observation that overexpression of ADH in the hearts accentuated alcohol-induced myocardial morphometric and functional defects [13, 14]. To the contrary, overexpression of the mitochondrial isoform of ALDH, ALDH2, offers myocardial protection against alcohol-induced tissue and cellular injury both in vitro and in vivo [15, 16]. It may thus be speculated that genetic mutation or polymorphism in ALDH2 may predispose an individual to a higher risk of alcoholic cardiomyopathy following alcohol intake as blood acetaldehyde levels are approximately tenfold higher in humans carrying defective ALDH2 than healthy individuals [17]. Nonetheless, this notion has not been validated by epidemiological data since individuals with defective ALDH2 are often intolerable to alcohol intake. Mutation in ALDH2 accounts for low ALDH2 activity and the flushing response to alcohol in 30% to 50% of Asian and African American populations in an autosomal-dominant manner [18]. Up-to-date, little information is available with regards to the pathophysiological consequence of ALDH2 deficiency on cardiac pump function following binge drinking. To better understand the role of ALDH2 in the pathogenesis of alcoholic cardiomyopathy, the present study was designed to evaluate the impact of ALDH2 knockout on acute ethanol exposure-induced cardiac contractile depression and the possible mechanism(s) of action involved.
MATERIALS AND METHODS
Experimental animals and acute ethanol challenge
All animal procedures described in the current study were approved by the University of Wyoming Institutional Animal Care and Use Committee and were in accordance with the NIH standards. In brief, adult male wild-type (WT) C57 BL/6 and ALDH2 knockout (KO) mice (4–5 month-old) were used. Generation and characterization of the ALDH2 knockout (KO) mice using gene targeting in embryonic stem cells were described in detail previously by our groups [19, 20]. For acute ethanol challenge, mice were injected intraperitoneally with ethanol (3 g/kg) [21]. The ethanol-untreated mice received equal volume of saline. Six hours after ethanol or saline challenge, blood samples were taken from the tail vein and immediately deproteinized with 6.25% trichloroacetic acid solution. Mice were killed 24 hours after ethanol challenge for cardiac tissue or cardiomyocyte collection. Plasma and hearts were stored in sealed vials at −80°C until use. For ethanol and acetaldehyde determination, a 2 ml aliquot of the headspace gas from each vial was removed through the septum on the cap with a gas-tight syringe and transferred to a 200 μl loop injection system. A volume of 100 μl plasma from each sample was put into an autosampler vial. Six μl of n-propanol and 194 μl H2O were then added to the vial. Following a 20-min incubation at 50°C, 50 μl aliquot of headspace gas was removed. Plasma and heart samples were transferred to a HP 5890 gas chromatograph (Hewlett-Packard, Palo Alto, CA) equipped with a flame ionization detector. Ethanol and acetaldehyde were separated on a 9-m VOCOL capillary column with film of 1.8 μm thickness and an inner diameter of 0.32 mm. The temperature was held at 30°C, and the carrier gas was helium at a flow rate of 1.8 ml/min. Quantitation was achieved by calibrating the gas chromatograph peak areas against those from headspace samples of standards, over a similar concentration range as the tissue samples in the same buffer [22].
Murine cardiomyocyte isolation and in vitro drug treatment
Individual cardiomyocytes were isolated as described [23]. After ketamine/xylazine sedation, hearts were removed and perfused with Krebs-Henseleit bicarbonate (KHB) buffer containing (in mM): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES and 11.1 glucose. Hearts were digested with 10 mg/ml Liberase (Roche Diagnostics, Indianapolis, IN) for 20 min. Left ventricles were removed and minced before being filtered. Myocyte yield was ~ 75% which was not affected by acute ethanol exposure or ALDH KO. Only rod-shaped myocytes with clear edges were selected for mechanical study. To assess the impact of ALDH2 inhibition on cardiomyocyte contractile function in response to ethanol exposure, freshly isolated cardiomyocytes from WT mice were incubated with ethanol (240 mg/dl) at 37°C for 2 hrs in the absence or presence of the ALDH inhibitor cyanamide (25 μM) [14] prior to mechanical function assessment.
Cell shortening/relengthening
Mechanical properties of the cardiomyocytes were evaluated utilizing a SoftEdge MyoCam® system (IonOptix Corporation, Milton, MA) as described [15]. Briefly, cardiomyocytes were visualized under an inverted microscope (Olympus, IX-70, Olympus Optical Co, Tokyo, Japan) and were stimulated with a voltage frequency of 0.5 Hz. The myocyte being observed was shown on a computer monitor using an IonOptix MyoCam camera. An IonOptix SoftEdge software was utilized to capture cell shortening and relengthening changes. Cell shortening and relengthening were assessed using the following indices: peak shortening (PS) - indicative of peak ventricular contractility, time-to-PS (TPS) - indicative of systolic duration, and time-to-90% relengthening (TR90) - indicative of diastolic duration, maximal velocities of shortening (+dL/dt) and relengthening (−dL/dt) - indicatives of maximal velocities of ventricular pressure rise/fall. In the case of altering stimulus frequency (0.1 – 5.0 Hz), the steady-state contraction of myocyte was achieved (usually after the first five to six beats) before PS amplitude was recorded.
Western blot analysis
The total protein was prepared as described [24]. Proteins were separated on SDS-PAGE gels, transferred to nitrocellulose membranes, and incubated overnight at 4°C with primary antibodies [anti-ALDH2 (1:1,000), anti-Akt (1:1,000), anti-phosphorylated Akt (pAkt, Thr308, 1:1,000), anti-glycogen synthase kinase-3β (GSK-3β, 1:1,000), anti-phosphorylated GSK-3β (pGSK-3β, Ser9, 1:1,000) and anti-PP2A (1:1,000)]. After washing blots to remove excessive primary antibody binding, blots were incubated for 1 hr with horseradish peroxidase (HRP)–conjugated secondary antibody (1:5,000). Antibody binding was detected using enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ), and film was scanned and the intensity of immunoblot bands was detected with a Bio-Rad Calibrated Densitometer (Model: GS-800).
Measurement of mitochondrial membrane potential
Cardiomyocytes were suspended in HEPES-saline buffer and mitochondrial membrane potential (ΔΨm) was detected as described [25]. Briefly, following a 10-min pre-incubation with 5 μM JC-1 at 37°C, cells were rinsed twice using the HS buffer free of JC-1. Fluorescence of each sample was read at excitation wavelength of 490 nm and emission wavelength of 530 nm and 590 nm using a spectrofluorimeter (Spectra MaxGeminiXS, Atlanta, GA) at an interval of 10 sec. Results in fluorescence intensity were expressed as 590-to-530-nm emission ratio. The mitochondrial uncoupler sodium azide (NaN3, 10 mM) was used as a positive control.
Statistical analysis
Data were shown as Mean ± SEM. Difference was calculated by repeated measures analysis of variance (ANOVA). When an overall significance was determined a Tukey’s post hoc analysis was incorporated. A p value less than 0.05 was considered significant.
RESULTS
General feature of WT and ALDH2 KO mice with or without acute ethanol challenge
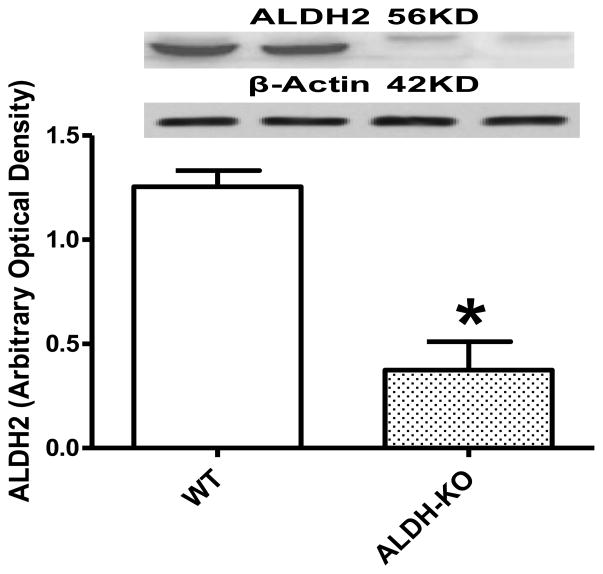
Neither ALDH2 knockout nor acute ethanol challenge affected body and organ weights as well as organ size. Blood alcohol levels were undetectable in non-ethanol-treated WT and ALDH2 KO mice. In contrast, acute ethanol exposure elicited a comparable elevation in blood alcohol levels in WT and ALDH2 KO mice. Interestingly, cardiac acetaldehyde levels were both elevated in WT and ALDH2 KO mice following ethanol exposure, with a more pronounced increase in ALDH2 KO mice than WT mice, validating this transgenic model of reduced acetaldehyde detoxification (Table 1). This is consistent with the significantly reduced ALDH2 protein expression in the hearts (Fig. 1).
Table 1.
General features of WT and ALDH2 KO mice with or without acute ethanol challenge.
| Mouse group | WT | WT+EtOH | ALDH-KO | ALDH-KO+EtOH |
|---|---|---|---|---|
| Body Weight (g) | 24.2 ± 0.3 | 23.9 ± 0.5 | 23.5 ± 0.5 | 24.1 ± 0.5 |
| Heart Weight (mg) | 120 ± 4 | 119 ± 3 | 122 ± 4 | 119 ± 5 |
| Heart Weight/Body Weight Ratio (mg/g) | 4.96 ± 0.22 | 4.98 ± 0.08 | 5.09 ± 0.12 | 4.94 ± 0.13 |
| Liver Weight (g) | 1.34 ± 0.04 | 1.38 ± 0.04 | 1.32 ± 0.03 | 1.37 ± 0.05 |
| Liver Weight/Body Weight Ration (mg/g) | 55.4 ± 1.46 | 57.6 ± 0.98 | 55.91 ± 1.12 | 57.04 ± 1.18 |
| Kidney Weight (mg) | 355 ± 7 | 354 ± 15 | 338 ± 13 | 368 ± 23 |
| Kidney Weight/Body Weight Ratio (mg/g) | 14.68 ± 0.13 | 14.78 ± 0.35 | 14.36 ± 0.35 | 15.25 ± 0.64 |
| Cardiac Acetaldehyde (nmol/mg) | 2.47 ± 0.23 | 55.59 ± 5.52* | 2.83 ± 0.26 | 71.16 ± 6.06*# |
| Blood Alcohol (mM) | Undetectable | 0.114 ± 0.008* | Undetectable | 0.116 ± 0.009* |
WT=wild type; KO=ALDH2 Knockout; EtOH=ethanol. Mean ± SEM, n = 5 mice per group,
p < 0.05 vs. WT group,
p < 0.05 vs. WT+EtOH group.
Fig. 1.
ALDH2 protein expression in myocardium from wild-type (WT) and ALDH2 knockout (ALDH-KO) transgenic mice. Mean ± SEM, n = 4 mice per group, *p < 0.05 vs. WT group.
Effect of ethanol exposure on cardiomyocyte mechanics in WT and ALDH2 KO mice
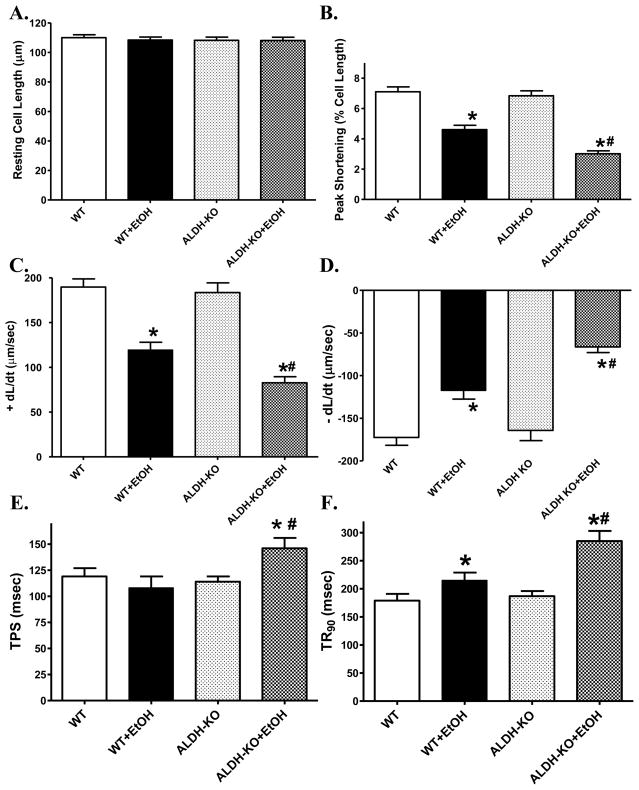
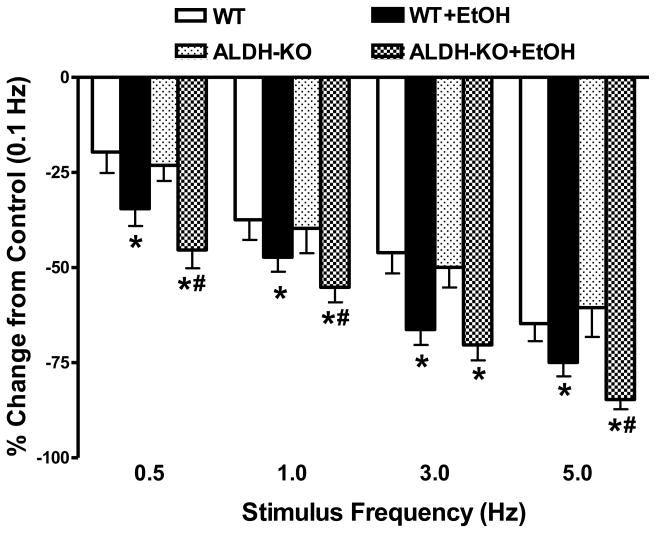
Acute ethanol treatment significantly depressed peak shortening amplitude and maximal velocity of shortening/relengthening (± dL/dt), as well as prolonged duration of relengthening (TR90) without affecting duration of shortening (TPS) in cardiomyocytes from WT mice. Although ALDH2 knockout itself failed to affect any of these mechanical indices measured, it significantly accentuated the ethanol-induced cardiomyocyte mechanical abnormalities. In addition, ALDH2 knockout unmasked an ethanol-induced prolongation of TPS (Fig. 2). To assess the potential contribution of sarcoplasmic reticulum (SR) in the ethanol and/or ALDH2 knockout-elicited cardiac contractile response, cardiomyocytes from WT and ALDH2 KO mice with or without ethanol challenge were paced at higher stimulating frequencies to examine the SR Ca2+ handling capacity. The cells were initially stimulated to contract at 0.5 Hz for 5 min to ensure steady state prior to raising the stimulating frequency to 5.0 Hz. As illustrated in Fig. 3, a comparable negative staircase in PS with the increased stimulus frequency was displayed between WT and ALDH2 KO mice in the absence of acute ethanol exposure. Ethanol challenge significantly augmented the high stimulus frequency-elicited depression in peak shortening amplitude, the effect of which was augmented by ALDH2 knockout with the exception of the stimulus frequency at 3.0 Hz.
Fig. 2.
Effect of acute ethanol (EtOH) exposure on cell shortening and relengthening mechanics in cardiomyocytes from wild-type (WT) and ALDH2 knockout (ALDH-KO) mice. (A): Resting cell length; (B): Peak shortening amplitude (as a percentage of resting cell length); (C): Maximal velocity of shortening (+ dL/dt); (D): Maximal velocity of relengthening (−dL/dt); (E): Time-to- peak shortening (TPS); and (F): Time-to-90% relengthening (TR90). Mean ± SEM, n = 132–133 myocytes per group, *p < 0.05 vs. WT group, # p < 0.05 vs. WT+EtOH group.
Fig. 3.
Effect of increasing stimulates frequency (0.1 – 5.0 Hz) on peak shortening amplitude in cardiomyocytes from wild-type (WT) and ALDH2 knockout (ALDH-KO) transgenic mice following acute ethanol (EtOH) exposure. Change in peak shortening amplitude was normalized to the peak shortening amplitude obtained at 0.1 Hz from the same cell. Mean ± SEM, Cell number from each group was presented in parentheses, *p < 0.05 vs. WT group; #p < 0.05 vs. WT+EtOH group.
Effect of ALDH inhibition on ethanol-induced changes in cardiomyocyte mechanical property
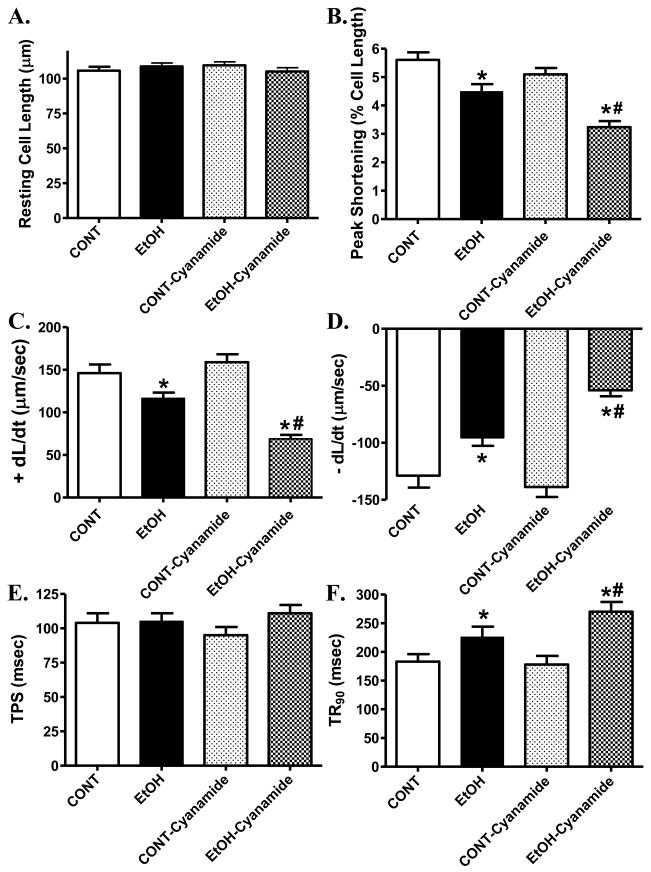
To further evaluate the effect of ALDH2 deficiency on ethanol-induced cardiac contractile defects, freshly isolated cardiomyocytes from WT mice were treated with ethanol (240 mg/dl) for 2 hrs in the absence or presence of the ALDH inhibitor cyanamide (25 μM) [14]. Somewhat similar to our previous finding [26], ethanol incubation significantly decreased PS and ± dL/dt as well as prolonged TR90 without affecting resting cell length and TPS. Interestingly, cyanamide accentuated ethanol-induced cardiomyocyte mechanical defects without eliciting any effect itself (Fig. 4). These data provided direct evidence for a pivotal role of the ALDH enzymatic metabolism in the ethanol-induced cardiac contractile dysfunction.
Fig. 4.
Contractile properties of cardiomyocytes isolated from WT mice incubated for 2 hrs with ethanol (EtOH, 240 mg/dl) in the absence or presence of the ALDH enzyme inhibitor cyanamide (25 μM). (A): Resting cell length; (B): Peak shortening (normalized to cell length); (C): Maximal velocity of shortening (+ dL/dt); (D): Maximal velocity of relengthening (− dL/dt); (E): Time-to- PS (TPS); and (F): Time-to-90% relengthening (TR90). Mean ± SEM, n = 59 – 60 myocytes per group, * p < 0.05 vs. control (CONT) group, # p < 0.05 vs. EtOH group.
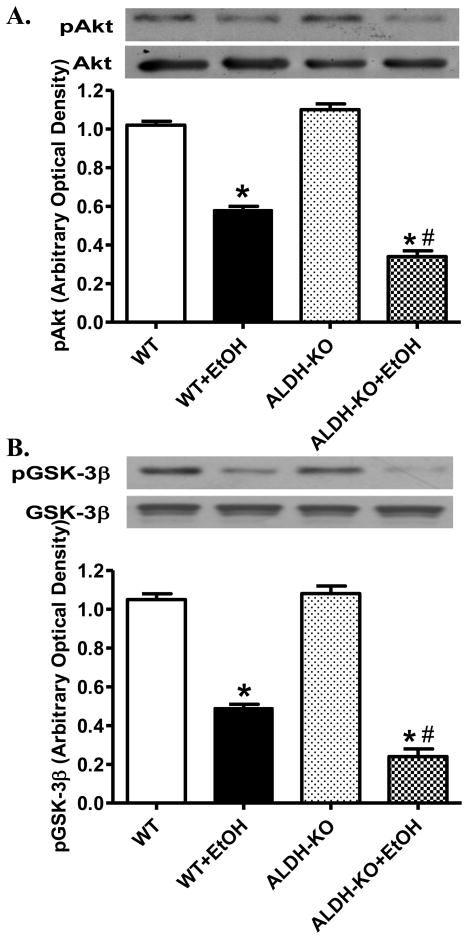
Effect of ALDH2 knockout on ethanol-induced change in Akt and GSK-3β signaling
Our earlier study revealed an essential role of the Akt-GSK signaling cascade in the maintenance of cardiomyocyte survival and function, especially in the setting of alcohol intake [13]. To evaluate if these signaling molecules contributes to ethanol and/or ALDH2 knockout-elicited cardiac mechanical response, expression of total and phosphorylated Akt and GSK-3β was evaluated. Our results indicated that ethanol treatment markedly decreased phosphorylation of both Akt and GSK-3β in WT mice, the effect of which was exacerbated by ALDH2 knockout (Fig. 5). Neither acute ethanol exposure nor ALDH knockout affected the expression of non-phosphorylated Akt and GSK3β. These data suggest that ALDH2 may possess a role in compensating the loss of activation in Akt-GSK3β axis in response to acute ethanol treatment.
Fig. 5.
Effect of acute ethanol (EtOH) exposure on phosphorylation of Akt and GSK3β (normalized to respective non-phosphorylated protein expression) in myocardium from wild-type (WT) and ALDH2 knockout (ALDH-KO) transgenic mice. Inset: Representative gel blots depicting total and phosphorylated Akt and GSK3β expression. Mean ± SEM, n = 3–4 mice per group, *p < 0.05 vs. WT group; #p < 0.05 vs. WT+EtOH group.
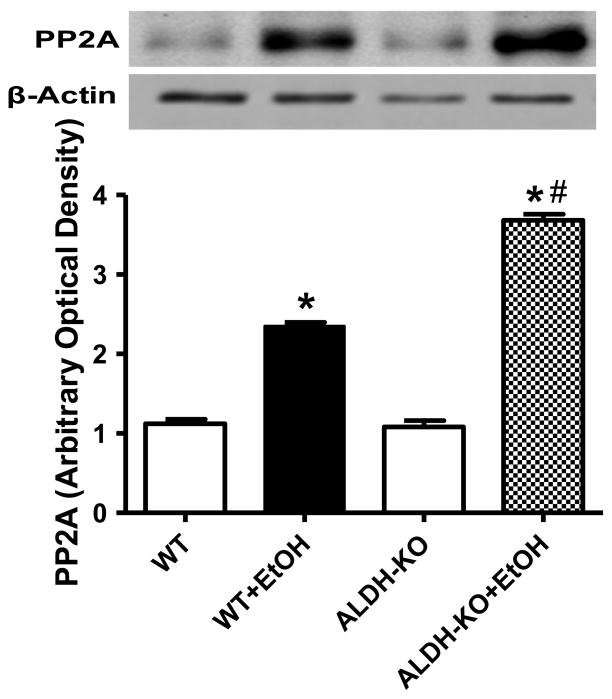
Effect of ALDH2 on ethanol-induced changes in protein phosphatases
Recent evidence revealed that Akt is dephosphorylated by protein phosphatase 2A (PP2A) [27–29]. To explore the possible mechanism(s) of action behind ethanol-induced reduction in Akt phosphorylation, expression of PP2A was examined in myocardium from WT and ALDH KO mice with or without acute ethanol exposure. Our result shown in Fig. 6 depicted that acute ethanol exposure significantly upregulated the expression of PP2A in WT mice although with a more pronounced effect in ALDH KO mice, indicating a likely role of upregulated PP2A protein in the ethanol-induced loss of Akt phosphorylation.
Fig 6.
Effect of acute ethanol (EtOH) exposure on protein phosphatases 2A (PP2A) expression in myocardium from wild-type (WT) and ALDH2 knockout (ALDH-KO) transgenic mice. Inset: Representative gel blots depicting PP2A and β-actin (loading control) expression. Mean ± SEM, n = 3–4 mice per group, *p < 0.05 vs. WT group, #p < 0.05 vs. WT+EtOH group.
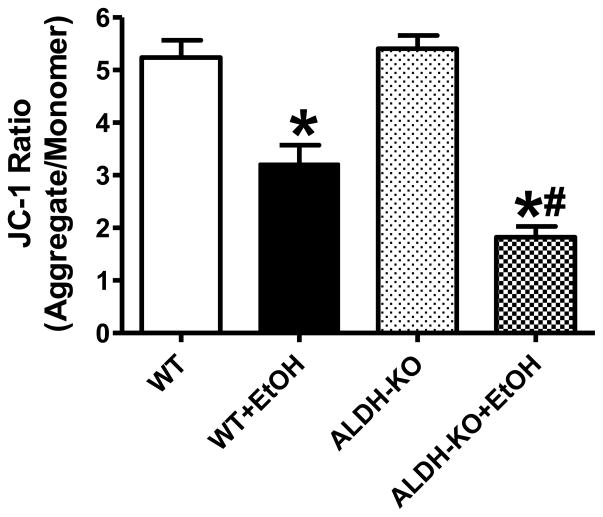
Effect of ALDH2 on ethanol-induced cardiomyocyte mitochondrial damage
Given that mitochondrial function is essential to cardiomyocyte viability and function [5, 30], the cationic lipophilic probe JC-1 was employed to monitor the mitochondrial membrane potential (ΔΨm) following acute ethanol challenge. Dynamic change of ΔΨm was displayed by the ratio between red (aggregated JC-1) and green (monomeric form of JC-1) fluorescence. Quantitative analysis exhibited a significant reduction in the red-to-green fluorescence ratio following acute ethanol treatment in WT mice, indicating a fall in ΔΨm and overt mitochondrial damage. Interestingly, the ethanol exposure-induced fall in ΔΨm was accentuated by ALDH knockout. ALDH2 KO itself did not exert any significant effect on ΔΨm (Fig. 7).
Fig. 7.
Effect of acute ethanol (EtOH) exposure on mitochondrial membrane potential (MMP) in cardiomyocytes isolated from wild-type (WT) and ALDH2 knockout (ALDH-KO) transgenic mice. Mean ± SEM, n = 5 mice per group, *p < 0.05 vs. WT group, #p < 0.05 vs. WT+EtOH group.
DISCUSSION
The salient findings from our current study indicated that ALDH2 deficiency augments cardiac acetaldehyde levels, exacerbates cardiac contractile dysfunction and mitochondrial damage following acute ethanol challenge. These myocardial functional and mitochondrial changes following acute ethanol challenge may be underscored by ALDH2 knockout-induced amplification of ethanol-elicited inhibition of Akt-GSK3β activation. Moreover, loss of ALDH2 enzyme in the knockout mice accentuates ethanol-induced up-regulation of protein phosphatase (PP2A), which may contribute to dampened Akt phosphorylation. In addition, the ALDH enzymatic inhibitor cyanamide mimicked ALDH2 knockout-elicited mechanical response by exaggerating ethanol-induced cardiomyocyte contractile dysfunction. These results provided some compelling evidence for an important role of ALDH2 enzyme in the pathogenesis of alcoholic cardiomyopathy.
The relationship between cardiovascular mortality and alcohol consumption displays a U-shaped curve from a number of epidemiological studies [31]. Binge drinking, even in otherwise light drinkers, often leads to myocardial abnormalities and mortality [32]. It has been shown that the ethanol-induced cardiac damage becomes evident when alcohol intake exceeds 90 to 100 g/d in human [3], which is transpired to a dosage of ~1.5 g/kg for an adult weighing 70 kg. Therefore the single dosage of ethanol used in our study (3 g/kg) closely resembles a state of heavy ethanol consumption given that rodents display near doubled LD50 of ethanol compared with human. Not surprisingly, this model of “binge-like” ethanol challenge has been widely employed to examine acute ethanol toxicity-associated tissue and organ damage [33–35]. Myopathic alteration following acute ethanol challenge is mainly characterized by arrhythmia, compromised myocardial contractility and increased cardiac mortality [36–38]. Data from our present study revealed that acute ethanol challenge compromised cardiomyocyte contractile function as evidenced by depressed cell shortening, maximal velocity of shortening/relengthening and prolonged duration of relengthening (TR90). Several theories have been postulated for the ethanol-induced myocardial contractile dysfunction, including toxicity of alcohol, fatty acid ethyl esters, as well as accumulation of reactive oxygen species and reduced antioxidant defense [4]. Evidence from our previous studies suggested that acetaldehyde interrupts cardiac excitation-contraction coupling and SR Ca2+ release [11, 39]. This is supported by the observation that ALDH2 deficiency, which is accompanied by elevated cardiac acetaldehyde levels, exaggerated ethanol exposure-induced cardiomyocyte mechanical dysfunction. Likewise, the ALDH inhibitor cyanamide also exacerbated ethanol-elicited cardiomyocyte contractile dysfunction in vitro. Mitochondria are essential to SR Ca2+ storage and cardiac contractility. Evidence from earlier studies insinuated that mitochondria serve as a major target for ethanol damage [40]. Alcohol consumption has been demonstrated to directly disrupt mitochondrial function [41], consistent with our present finding of decreased mitochondrial membrane potential following acute ethanol challenge. Interestingly, the ethanol exposure-induced drop in mitochondrial membrane potential was deteriorated by ALDH2 knockout, coordinated with worsened cardiomyocyte mechanical dysfunction and SR capacity decline (manifested by the more pronounced negative staircase in the peak shortening-frequency response). These findings revealed that ALDH2 plays a pivotal role in cardiac acetaldehyde exposure and the severity of myocardial contractile dysfunction following ethanol exposure, probably through its regulatory effect on mitochondrial function. Furthermore, our very recent findings revealed that ADH transgene accentuated ethanol-induced mitochondrial dysfunction including decreased mitochondrial membrane potential, accumulation of mitochondrial O2− anion and activation of mitochondrial apoptosis pathway [34], which favor the pivotal role of mitochondria in ethanol/acetaldehyde-induced myocardial dysfunction.
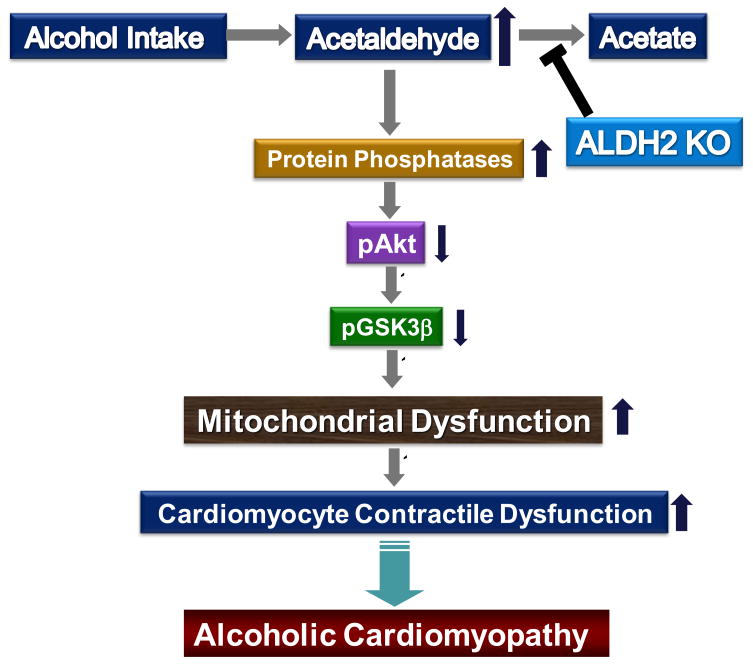
In the heart, Akt contributes to both physiological and pathological myocardial growth, survival and contractile function [42]. Our earlier study revealed that ethanol directly regulates cardiomyocyte survival through Akt phosphorylation [13], the effect of which is antagonized by the antioxidant metallothionein [43]. Data from our study also depicted lessened phosphorylation of GSK-3β, which has been shown to impinge on many aspects of cell signaling including protein synthesis, cell proliferation and apoptosis. The proapoptotic role of GSK-3β is shown to be negatively regulated by Akt [44] and inactivation of GSK-3β by Akt phosphorylation [45]. Overexpression of GSK-3β induces cell apoptosis [46]. To the contrary, activation of Akt and phosphorylation GSK-3β exert cardioprotective effect against myocardial I/R injury through a mitochondria-mediated process [47]. Through phosphorylation of GSK-3β on the end effector, Akt preserves mitochondrial integrity and cardiac function [48]. Taken together, the available data favor that activation of the Akt/GSK-3β signaling pathway is particularly important to mitochondria protection against various environmental stress including alcohol abuse. However, the mechanism responsible for regulation of Akt in alcoholic hearts remains poorly understood. Recent evidence has delineated that Akt is dephosphorylated by protein phosphatase 2A [27, 28]. PP2A activation may concomitantly dephosphorylate and activate GSK-3β directly or indirectly by dephosphorylating Akt [49]. Our present observation provided evidence that acute ethanol exposure may elicit a concerted reduction in Akt and GSK-3β phosphorylation associated with a prominent increase in PP2A expression. More importantly, our data revealed that the ethanol-induced responses in the phosphorylation of Akt and GSK-3β as well as PP2A expression were accentuated in ALDH2 KO heart. These results favor the notion that loss of ALDH2 enzyme deteriorates Akt activity possibly through the upregulation of protein phosphatases following acute ethanol exposure (Fig. 8).
Fig. 8.
Scheme depicting the possible mechanism of action underlying ALDH2 knockout- induced exacerbation of ethanol exposure-induced change in Akt, GSK3β signaling molecules, mitochondrial function and ultimately development of alcoholic cardiomyopathy. The “T”-type arrow denotes inhibitory action.
Excessive alcohol intake leads to an abrupt rise in acetaldehyde levels in both circulation and hearts [22, 50]. As the major metabolite of ethanol, acetaldehyde impairs cardiac contractile function [11], contributes to oxidative damage and lipid peroxidation [7]. The rationale for the high circulating acetaldehyde levels in individuals with alcohol intake is believed to be due to the inability for the ALDH enzyme to detoxify acetaldehyde [51]. Unfortunately, direct approach that can be used to examine acetaldehyde toxicity is impeded by the fact that direct intake of the toxin is unsuitable for human study. On the other hand, effort using ALDH enzymatic inhibitors to alter acetaldehyde levels has been proven to be nonspecific, ineffective, toxic and difficult to manage [51]. To overcome these obstacles, the ALDH2 knockout mice provide a unique model to retard acetaldehyde metabolism, resulting in high acetaldehyde concentration following ethanol ingestion. Our findings demonstrated that ALDH2 deficiency enhances cardiac acetaldehyde levels and exacerbates ethanol exposure-induced cardiac mechanical dysfunction. The loss of ALDH2 enzyme results in a greater upregulation of protein phosphatase in response to acute ethanol challenge, leading to a more pronounced inhibition in the Akt-GSK3β signaling cascade and impaired mitochondrial function. Taken together, these data have depicted a seemingly important role of the ALDH2 enzyme in the pathogenesis and management of alcoholic cardiomyopathy.
Acknowledgments
The work was supported by NIH/NIAAA 1R01 AA013412 (to JR), AHA Postdoctoral Fellowship (09POST2250477 to HM) and National Science Foundation of China (#30700263 to HM; #30728023 to JR). EAB was supported by Wyoming EPSCoR and the Wyoming NASA Space Grant Consortium.
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11(5):453–62. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- 2.Marmot MG. Alcohol and coronary heart disease. Int J Epidemiol. 2001;30(4):724–9. doi: 10.1093/ije/30.4.724. [DOI] [PubMed] [Google Scholar]
- 3.Spies CD, Sander M, Stangl K, Fernandez-Sola J, Preedy VR, Rubin E, et al. Effects of alcohol on the heart. Curr Opin Crit Care. 2001;7(5):337–43. doi: 10.1097/00075198-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PJ, Patel VB, Preedy VR. Alcohol and the myocardium. Novartis Found Symp. 1998;216:35–45. doi: 10.1002/9780470515549.ch4. discussion -50. [DOI] [PubMed] [Google Scholar]
- 5.Szabo G, Hoek JB, Darley-Usmar V, Hajnoczky G, Knudsen T, Mochly-Rosen D, et al. RSA 2004: combined basic research satellite symposium - session three: alcohol and mitochondrial metabolism: at the crossroads of life and death. Alcohol Clin Exp Res. 2005;29(9):1749–52. doi: 10.1097/01.alc.0000179318.48376.cd. [DOI] [PubMed] [Google Scholar]
- 6.Hannuksela ML, Liisanantti MK, Savolainen MJ. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit Rev Clin Lab Sci. 2002;39(3):225–83. doi: 10.1080/10408360290795529. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Li SY, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;32(3):175–86. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Brien JF, Loomis CW. Pharmacology of acetaldehyde. Can J Physiol Pharmacol. 1983;61(1):1–22. doi: 10.1139/y83-001. [DOI] [PubMed] [Google Scholar]
- 9.Ren J, Brown RA. Influence of chronic alcohol ingestion on acetaldehyde-induced depression of rat cardiac contractile function. Alcohol Alcohol. 2000;35(6):554–60. doi: 10.1093/alcalc/35.6.554. [DOI] [PubMed] [Google Scholar]
- 10.Ren J, Wold LE, Epstein PN. Diabetes enhances acetaldehyde-induced depression of cardiac myocyte contraction. Biochem Biophys Res Commun. 2000;269(3):697–703. doi: 10.1006/bbrc.2000.2353. [DOI] [PubMed] [Google Scholar]
- 11.Brown RA, Jefferson L, Sudan N, Lloyd TC, Ren J. Acetaldehyde depresses myocardial contraction and cardiac myocyte shortening in spontaneously hypertensive rats: role of intracellular Ca2+ Cell Mol Biol (Noisy-le-grand) 1999;45(4):453–65. [PubMed] [Google Scholar]
- 12.Niemela O. Acetaldehyde adducts in circulation. Novartis Found Symp. 2007;285:183–92. doi: 10.1002/9780470511848.ch13. discussion 93–7. [DOI] [PubMed] [Google Scholar]
- 13.Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44(6):992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, et al. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;282(4):H1216–22. doi: 10.1152/ajpheart.00780.2001. [DOI] [PubMed] [Google Scholar]
- 15.Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119(14):1941–9. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, et al. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40(2):283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura FT, Fukunaga T, Kajiura H, Umeno K, Takakura H, Ono T, et al. Effects of aldehyde dehydrogenase-2 genotype on cardiovascular and endocrine responses to alcohol in young Japanese subjects. Auton Neurosci. 2002;102(1–2):60–70. doi: 10.1016/s1566-0702(02)00206-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang RS, Nakajima T, Kawamoto T, Honma T. Effects of aldehyde dehydrogenase-2 genetic polymorphisms on metabolism of structurally different aldehydes in human liver. Drug Metab Dispos. 2002;30(1):69–73. doi: 10.1124/dmd.30.1.69. [DOI] [PubMed] [Google Scholar]
- 19.Yu HS, Oyama T, Isse T, Kitakawa K, Ogawa M, Pham TT, et al. Characteristics of aldehyde dehydrogenase 2 (Aldh2) knockout mice. Toxicol Mech Methods. 2009;19(9):535–40. doi: 10.3109/15376510903401708. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa K, Kawamoto T, Kunugita N, Tsukiyama T, Okamoto K, Yoshida A, et al. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation of methoxyacetaldehyde; in vitro analysis with liver subcellular fraction derived from human and Aldh2 gene targeting mouse. FEBS Lett. 2000;476(3):306–11. doi: 10.1016/s0014-5793(00)01710-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Dong F, Li Q, Borgerding AJ, Klein AL, Ren J. Cardiac overexpression of catalase antagonizes ADH-associated contractile depression and stress signaling after acute ethanol exposure in murine myocytes. J Appl Physiol. 2005;99(6):2246–54. doi: 10.1152/japplphysiol.00750.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hintz KK, Relling DP, Saari JT, Borgerding AJ, Duan J, Ren BH, et al. Cardiac overexpression of alcohol dehydrogenase exacerbates cardiac contractile dysfunction, lipid peroxidation, and protein damage after chronic ethanol ingestion. Alcohol Clin Exp Res. 2003;27(7):1090–8. doi: 10.1097/01.ALC.0000075823.73536.DD. [DOI] [PubMed] [Google Scholar]
- 23.Ren J. Interaction between high-fat diet and alcohol dehydrogenase on ethanol-elicited cardiac depression in murine myocytes. Obesity (Silver Spring) 2007;15(12):2932–41. doi: 10.1038/oby.2007.350. [DOI] [PubMed] [Google Scholar]
- 24.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system research challenges and opportunities. J Am Coll Cardiol. 2005;45(12):1916–24. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 25.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, et al. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486 ( Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren J, Brown RA. Hypertension augments ethanol-induced depression of cell shortening and intracellular Ca(2+) transients in adult rat ventricular myocytes. Biochem Biophys Res Commun. 1999;261(1):202–8. doi: 10.1006/bbrc.1999.0944. [DOI] [PubMed] [Google Scholar]
- 27.Tremblay ML, Giguere V. Phosphatases at the heart of FoxO metabolic control. Cell Metab. 2008;7(2):101–3. doi: 10.1016/j.cmet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104(51):20517–22. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuluaga S, Alvarez-Barrientos A, Gutierrez-Uzquiza A, Benito M, Nebreda AR, Porras A. Negative regulation of Akt activity by p38alpha MAP kinase in cardiomyocytes involves membrane localization of PP2A through interaction with caveolin-1. Cell Signal. 2007;19(1):62–74. doi: 10.1016/j.cellsig.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Hajnoczky G, Buzas CJ, Pacher P, Hoek JB, Rubin E. Alcohol and mitochondria in cardiac apoptosis: mechanisms and visualization. Alcohol Clin Exp Res. 2005;29(5):693–701. doi: 10.1097/01.alc.0000163493.45344.7a. [DOI] [PubMed] [Google Scholar]
- 31.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology. 1990;1(5):342–8. doi: 10.1097/00001648-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 32.O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50(11):1009–14. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 33.Cubero I, Navarro M, Carvajal F, Lerma-Cabrera JM, Thiele TE. Ethanol-Induced Increase of Agouti-Related Protein (AgRP) Immunoreactivity in the Arcuate Nucleus of the Hypothalamus of C57BL/6J, but not 129/SvJ, Inbred Mice. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo R, Ren J. Alcohol dehydrogenase accentuates ethanol-induced myocardial dysfunction and mitochondrial damage in mice: role of mitochondrial death pathway. PLoS One. 2010;5(1):e8757. doi: 10.1371/journal.pone.0008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker JE, Jr, Odden AR, Jeyaseelan S, Zhang P, Bagby GJ, Nelson S, et al. Ethanol exposure impairs LPS-induced pulmonary LIX expression: alveolar epithelial cell dysfunction as a consequence of acute intoxication. Alcohol Clin Exp Res. 2009;33(2):357–65. doi: 10.1111/j.1530-0277.2008.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trejbal K, Mitro P. ECG changes in alcoholic intoxication. Vnitr Lek. 2008;54(4):410–4. [PubMed] [Google Scholar]
- 37.Ma H, Li J, Gao F, Ren J. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol. 2009;54(23):2187–96. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 38.Panos RJ, Sutton FJ, Young-Hyman P, Peters R. Sudden death associated with alcohol consumption. Pacing Clin Electrophysiol. 1988;11(4):423–4. doi: 10.1111/j.1540-8159.1988.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 39.Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2(6):497–506. doi: 10.1177/1753944708095137. [DOI] [PubMed] [Google Scholar]
- 40.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122(7):2049–63. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoppet M, Maisch B. Alcohol and the heart. Herz. 2001;26(5):345–52. doi: 10.1007/pl00002037. [DOI] [PubMed] [Google Scholar]
- 42.Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: emerging roles in health and disease. Circ Res. 2008;102(2):157–63. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Ren J. Cardiac overexpression of metallothionein rescues chronic alcohol intake-induced cardiomyocyte dysfunction: role of Akt, mammalian target of rapamycin and ribosomal p70s6 kinase. Alcohol Alcohol. 2006;41(6):585–92. doi: 10.1093/alcalc/agl080. [DOI] [PubMed] [Google Scholar]
- 44.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87(6):1427–35. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, Lin YS. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci. 2007;120(Pt 16):2935–43. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- 46.Hall JL, Chatham JC, Eldar-Finkelman H, Gibbons GH. Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cell apoptosis. Role of GSK3beta. Diabetes. 2001;50(5):1171–9. doi: 10.2337/diabetes.50.5.1171. [DOI] [PubMed] [Google Scholar]
- 47.Song JQ, Teng X, Cai Y, Tang CS, Qi YF. Activation of Akt/GSK-3beta signaling pathway is involved in intermedin(1–53) protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis. 2009 doi: 10.1007/s10495-009-0382-2. [DOI] [PubMed] [Google Scholar]
- 48.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113(11):1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z. Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2009;297(2):H569–75. doi: 10.1152/ajpheart.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espinet C, Argiles JM. Ethanol and acetaldehyde concentrations in the rat foeto-maternal system after an acute ethanol administration given to the mother. Arch Int Physiol Biochim. 1984;92(5):339–44. doi: 10.3109/13813458409080609. [DOI] [PubMed] [Google Scholar]
- 51.Ren J. Acetaldehyde and alcoholic cardiomyopathy: lessons from the ADH and ALDH2 transgenic models. Novartis Found Symp. 2007;285:69–76. doi: 10.1002/9780470511848.ch5. discussion -9, 198–9. [DOI] [PubMed] [Google Scholar]