1. Summary
The mechanisms by which the immune system responds to an infection or disease depend on a complex interplay between the elements of innate and adaptive immunity. While most of the focus so far has been on the innate instruction of the adaptive immune responses, considerable evidence now suggests an equally important adaptive control of the innate immunity. Several studies yield new insights into how the adaptive immunity by initiating an antigen–specific response can compensate, suppress and activate innate responses at the site of tissue antigen. Here we discuss recent advances in our understanding of the adaptive control of immune effector functions in various pathological and physiological conditions.
Keywords: Adaptive immunity, innate immunity, T cells, NK cells, inflammation, activation, effector response
2. Introduction
Organisms fight an infection or disease with the help of an intricate system of immunity classified as innate and adaptive in nature. Innate immunity is an evolutionarily conserved defence mechanism capable of fighting a diverse threat of viral, prokaryotic and eukaryotic parasites and pathogens in plants and invertebrate animals. Adaptive immunity evolved at the time of differentiation of vertebrates between the jawless hagfish and lampreys by a gene conversion mechanism giving rise to variable lymphocyte receptors [1] and subsequent genomic invasion of a retroposon encoding site-specific recombinases [2-4]. As a consequence of hypervariability, rearrangement of receptor gene segments and clonal selection, unlimited numbers of receptors are generated to allow the adaptive immune cells to specifically recognize pathogens and tissue insult. Adaptive immunity added to the immune system a specific recognition of pathogenic proteins, carbohydrates, lipids, and nucleic acids in its effort to mount an immune attack against the ever growing pool of diseases and defy their immune evasion strategies. In addition, adaptive immunity provided a tight control of innate immunity not only by regulating innate inflammation but also by activating innate effectors, when they were specifically needed, and offered a long-term immunological memory of insult.
In some cases, the adaptive immune elements restrain innate immune responses. In others, the combination of innate and adaptive immunity maximizes host defense while minimizing collateral damage to the host tissues: innate immunity generates help signals in the damaged tissues; adaptive immunity provides specific responses to the insult that directly attack the pathogenic process or recruit other powerful innate effector cells that, though not specific by themselves, can act specifically by their selective recruitment. Besides this linear progression from innate to adaptive immunity by innate signals following tissue insult triggering adaptive immune cells to respond to the pathogen or disease, several studies now demonstrate an adaptive control of the effector mechanisms of innate immunity as a feedback step necessary to fine-tune the mechanisms of host defence by specifically recruiting, activating or blocking innate effectors. We discuss here these studies that suggest an intricate control of innate immune reactivity by adaptive immunity.
3. Adaptive Compensation of Innate Immunity
The adaptive immunity possesses a capacity to compensate for conditions of innate deficiency. A recent study investigated the relative roles that innate and adaptive responses play in containing commensal microbiota in the intestine of mammals [5]. They discovered a flexible continuum between innate and adaptive immunity. Clean germ-free Myd88-/-Ticam-/- mice are deficient in signalling through Toll-like receptors (TLRs) and IL-1 / IL-18 receptors. Nos2-/-Cybb-/- mice are deficient in phagocyte oxidative burst. Following intragastric exposure to Escherichia coli bacteria, these mice spontaneously developed a vigorous CD4+ T cell dependent antibody response. Such a spontaneous activation of B cells compensating for the defects in innate immune clearance of bacteria suggests that the functional demarcation between innate and adaptive immunity is flexible. Similar compensatory host defence mechanisms have been described in TLR signalling deficient IRAK-4-/- children [6,7]. Thus, in the absence of innate immune signalling and cognate antigenic stimuli, adaptive immune cells can still function in innate–type responses. The innate immunity no doubt is a powerful initiator and regulator of adaptive immunity, but in its absence, there seems to exist alternate signalling pathways to allow adaptive immune cells to induce innate–like immune phenomena. The alternate signalling pathways responsible for the activation of adaptive immune cells need to be defined.
Rapid non-cognate activation of CD8+ T cells within 15 h of bacterial infection to produce interferon (IFN)-γ in response to cytokines secreted by phagocyte cells seems to be important for immune defence against intracellular pathogens [8]. Indeed, CD44high CD8+ T cells represent the major population of early IFN-γ-producers responding to bacterial and viral products [9]. This is also evident when memory CD8+ T cells respond to intracellular bacterial pathogen Listeria monocytogenes in antigen nonspecific manner by secreting IFN-γ in response to cytokines IL-12 and IL-18 [10].
Thus, the innate and adaptive components play complementary roles in responding to commensal microbiota and intracellular pathogens. The signalling pathways leading to the activation of adaptive immune cells to provide innate–like immune phenomena and the precise rules of adaptive compensation of innate immunity yet remain to be investigated.
4. Adaptive Suppression of Innate Immunity
A tight control of innate immunity is essential to avoid overproduction of inflammatory cytokines during an innate immune response. Recently, CD4+ T cell effector and memory cells were shown to block macrophage inflammasome–mediated cryopyrin and caspase-1 activation, interleukin (IL)-1β release, IL-18 secretion and neutrophil recruitment in an antigen dependent manner and thereby suppress overt inflammation in a murine peritonitis model [11]. Effector CD8+ T cells also suppressed such potentially damaging inflammation to a lesser extent. This blockade of the caspase-1 axis of inflammasome dependent on contact with T cells did not affect the primary inflammatory response and production of inflammatory mediators such as CXCL2, IL-6, IL-12 and tumour necrosis factor (TNF) important for the onset of immunity and tissue healing. In this context, it is also noteworthy that IFN-γ produced during T cell responses to influenza infection in the lung of mice inhibits bacterial clearance from the lung by alveolar macrophages [12]. These studies suggest that the stimulated T cells have the capacity to edit the quality of innate inflammation and its mediators via TNF superfamily ligands and IFN-γ.
Furthermore, in a mouse hepatitis viral infection model, it was observed that even resting T cells are necessary and sufficient to suppress an early innate response by suppressing IFN-γ and TNF-α production [13]. This suppression was dependent on direct contact between T cells and innate immune cells, requiring the major histocompatibility complex molecule, yet independent of antigen specificity. Infection with hepatitis virus, or administration of TLR3 ligand poly-inosinic:polycytidylic acid, which mimics viral double–stranded RNA, produced a cytokine storm in lymphocyte–deficient mice. Consequently, in the absence of T cell control, mice that showed negligible increases in viral load succumbed to the high amounts of inflammatory cytokines secreted during an unrestrained innate immune response. The T cells are thus required to keep the unnecessary early innate inflammation in check.
Another adaptive regulation comes via regulatory T cells (Treg) that, amongst other roles, directly suppress innate immunity–driven inflammation as delineated, for instance, in intestinal innate immune pathology [14,15] and ulcerative colitis [16]. Treg–derived membrane–bound immunosuppressive cytokine, transforming growth factor (TGF)-β was found to directly inhibit NK cell cytotoxicity in NK cell–mediated rejection of tumours [17] or in bone marrow graft rejection [18]. Furthermore, Treg cells blocked IFN-γ production by NK cells by a TGF-β–dependent down regulation of the natural killer group 2D (NKG2D) activating receptor [19]. The presence of Treg has also been shown to reduce the number of NK cells recruited to the site of the tumour [20]. In the absence of Treg in mouse, NK cell numbers in the spleen increase fourfold and in the lymph nodes seven fold, most likely due to IL15Rα–mediated trans-presentation of IL-15 by resident dendritic cells (DC) [21,22]. Treg cells inhibit the expression of IL15Rα by DC in a TGF-β–dependent manner [21]. They also inhibit the effector function of DC [23] and macrophages [24]. Some studies have implicated IL-10 and TGF-β for Treg–mediated suppression of innate immune responses [14,25]. Moreover, it has been reported that tumour–induced granzyme B expression on Treg cells killed NK cells by direct cytotoxicity [26]. However, since cytotoxic cells (CD8+ T cells and NK cells) express cathepsin B [27], it is surprising that NK cells can be killed via the granzyme / perforin pathway. More studies are therefore warranted to understand the mechanism by which Treg cells degranulate and release cytolytic molecules, and how these cytolytic molecules overcome the NK cell resistance to granule–mediated cytotoxicity.
The requirement for adaptive suppression of innate immunity is evident in neonates. The development of the adaptive immune system follows that of the innate immune system in mouse embryos [28]. Consequently, due to insufficient numbers of T cells, neonatal mice are hypersensitive to various TLR stimulation and easily succumb to infection. Neonates exhibit robust inflammatory response due to higher TNF-α and IL-6 production from dendritic cells that are present in larger number in neonatal mice than in adult [29]. B cell–produced IL-10 also is implicated in dampening the neonatal inflammatory response [30]. Also in human infants, who are small for gestational age, the proportion of T cells is lower in peripheral blood [31,32]. Expectedly, increased levels of inflammatory cytokines such as TNF-α, IL-1 and IL-6 have been detected in some human newborn diseases following mild infections [33-37].
Identifying the underlying mechanisms responsible for the suppression of innate immune cells by different populations of resting, effector, memory and regulatory T cells might lead to the discovery of new inhibitory networks. Several intercellular co-inhibitory molecules, such as cytotoxic T lymphocyte antigen-4, programmed cell death-1, and B and T lymphocyte attenuator, have been implicated in maintaining balance in adaptive immune responses and homeostasis of immune cells [38]. It will be worth investigating whether these co-inhibitory molecules have similar roles in adaptive control of innate immunity.
5. Adaptive Activation of Innate Immunity
Not only can adaptive immunity regulate or compensate innate immunity but it can also play a critical role in activating innate immunity at the site and at the time when it is specifically needed. It is well established that various activated cells of the adaptive immunity provide amplification of innate immune responses to effectively combat pathogens. Thus, type 1 helper T (Th1) cells activate macrophages through both cell-cell contact and IFN-γ secretion [39,40]. Th2 cells activate eosinophils and mast cells through IL-5 and IFN-γ release [41,42]. B cells secrete antibodies to activate the cascade of complement proteins, phagocytic opsonization, NK cell cytotoxicity and mast cell degranulation [43-46]. Many such examples have been widely reviewed.
In addition to being an amplifier of innate responses in classical settings, adaptive immunity also is crucial for activating dormant innate immunity. Recently, while analyzing the ability of tumour antigen–specific CD8+ T cells to reject a mastocytoma tumour, it was discovered that these T cells, in addition to their direct effector function, also provide help to NK cells in eliciting their antitumour activity [47]. Adoptive T cell transfers into immunodeficient tumour–bearing mice, and non-invasive bioluminescence imaging of tumours, demonstrated that there was an emergence of antigen–deficient tumour escape variants following an efficient CD8+ T cell–dependent response against antigen–expressing tumour cells. Gene expression and functional analyses of tumour–infiltrating lymphocytes demonstrated that the antigen–specific CD8+ T cells and NK cells showed an activated phenotype at the site of tumour, where antigen–expressing tumour cells were injected. Surprisingly, antigen–escape tumour variants could be lysed by NK cells only if antigen–specific activated CD8+ T cells were present locally in their surroundings. This study showed for the first time that the adaptive immune cells can convert dormant NK cells into a killer effector type at the site of tumour. On the basis of this study, an important T cell-NK cell cooperative interaction emerged that is illustrated in Figure 1. The T cells helped broaden the effector response by activating NK cells in their vicinity, thereby eliminating antigen–deficient tumour cells, leading to more effective clearance of tumour. The outcome of tumour rejection was manifest only when there was a productive interaction of activated T cells and NK cells.
Figure 1. T cell-NK cell cooperativity in tumour rejection.
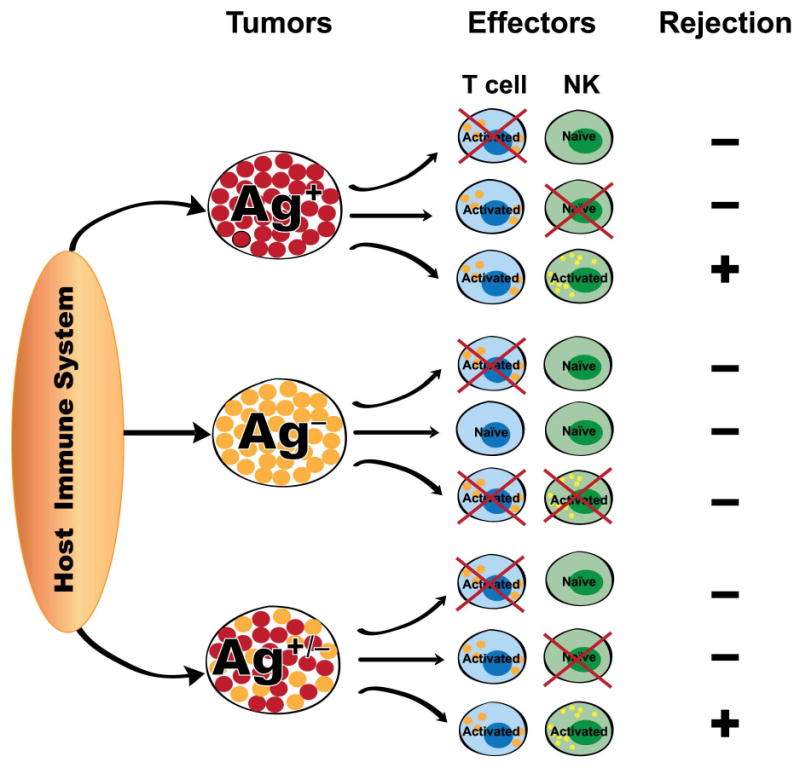
In the antigen (Ag) positive environment, if in the presence of activated T cells, NK cells are activated, complete tumour rejection ensues. If they fail to activate NK cells early enough, antigen escape variants develop and tumour is not rejected. In the antigen negative environment, in the absence of T cell activation, there is no tumour rejection. In the Ag+/- mix tumour environment, again if the activated T cells fail to activate NK cells early enough, there is no tumour rejection. When both the effectors are activated, there is complete tumour rejection.
Furthermore, under conditions of restricted T cell receptor diversity, tumour resistance was affected by the variation in precursor frequencies of tumour–specific CD8+ T cell and NK cell effectors. NK cells contributed to tumour resistance, in part through an NKG2D–mediated mechanism [48]. In another study, photodynamic therapy–induced CD8+ T cell–dependent control of distant late stage tumours was also found to require NK cells [49]. Moreover, even CD4+ T cells were found to work in concert with NK cells for maximal antitumor effect [50]. The molecular mechanisms of this T cell–dependent recruitment/activation of NK cells, however, need to be dissected. Some possible mediators may include T cell–produced IL-2, TNF-α or T cell-NK cell contact. It is known that human CD56bright NK cells that express high affinity IL-2 receptor use endogenous IL-2 produced by the antigen–activated T cells to stimulate IFN-γ production [51].
Further support to the adaptive activation of innate immunity comes from an obesity model of chronic inflammation. The CD8+ T cells infiltrate the epididymal adipose tissue early on during the development of obesity caused by a fat-rich diet in mice, concomitant with a decrease in the numbers of CD4+ T helper and regulatory cells [52]. CD8+ T cell effectors promote the recruitment, differentiation and activation of macrophages in adipose tissue by secreting substantial amounts of humoral factors such as IFN-inducible protein-10, monocyte chemoattractant protein (MCP)-1, MCP-3 and regulation upon activation, normal T cell expressed and secreted protein (RANTES). This is an essential step in the initiation of inflammation characterized by increased expression of the pro-inflammatory cytokines IL-1, IL-6 and TNF-α, as well as of intercellular adhesion molecule-1 and matrix metalloproteinase-2 in adipose tissue, resulting in a systemic insulin resistance and metabolic disorder. The accumulation of CD8+ T cells has also been confirmed in human obese adipose tissue.
An increase in the ratio of adipose tissue CD8+ to CD4+ T cells weeks before macrophages infiltrate fat have also been reported [53,54]. Regulatory T cells in the adipose tissue of lean mice provide anti-inflammatory signals to block adipose tissue inflammation. Obesity alters the delicate balance between Th1 and Th2 stimuli in fat by depleting adipose tissue Th2 and Treg cells, and increasing CD8+ T and Th1 cells. The early appearing CD8+ T cells in the adipose tissue play a leading role in the development of inflammation caused by the infiltrating macrophages. It remains to be investigated if NK cells, Th17, or B cells influence adipose tissue. B cells seem to enter fat even before T cells in obesity [55]. Altogether, these studies highlight the crucial role for adaptive immunity in initiating and propagating obesity–associated chronic inflammation by controlling the recruitment and activation of macrophages in obese adipose tissue. It would be interesting to know what cues in the adipose tissue initiate the activation and infiltration of adaptive immune cells. This also has clinical relevance in atherosclerosis as the role of T cells in regulating the magnitude of the atherogenic proinflammatory response of macrophages and the formation of thrombus has been recently reviewed [56].
The role of adaptive immunity in activating innate immunity is also manifested during herpes simplex virus infection in vaginal mucosa of mice. Following ablation of Treg cells in these mice, there was a delay in the arrival of NK cells, DCs and T cells to the site of infection [57]. Although the signals that activate Treg cells during infection remain unclear, Treg cells, besides limiting the extent of inflammation, seem to control early protective responses to local viral infection by facilitating a timely entry of innate immune cells into infected tissue. It will be relevant to investigate whether fluctuations in Treg cells, such as those seen in the genetically diverse human population, during infection, or with aging, affect immune responses in the same way.
Therefore, in multiple pathological conditions adaptive immune cells become crucial for activating innate immune cells. The conventional unidirectional view of innate instruction of adaptive immunity is thus overly simplistic. A more consistent view in agreement with the new findings would be a two-way bi-directional activation of the two arms of the immune system.
6. Adaptive-Innate Cooperativity in Immunological Memory
Immune memory is believed to be primarily in the hands of memory T cells. However, the memory CD8+ T cells alone are not sufficient to clear the secondary infection [58]. Instead, activation of innate mononuclear phagocytic cells (MPCs) by the memory T cells seems a necessary step for the elimination of pathogen. Upon re-exposure to the pathogen, existing memory T cells release the chemokine CCL3 to activate MPCs. The MPCs release TNF-α, which in turn cause neutrophils and other MPCs to produce radical oxygen intermediates (ROI) to clear the bacteria. Interestingly, these activated innate immune cells during the secondary infection can also clear an unrelated pathogen that is sensitive to ROIs.
These findings uncover two-tier control of the secondary protective response: (i) an antigen–dependent phase in which memory CD8+ T cells are reactivated and control the activation of the innate immune system, and (ii) an antigen–independent phase in which the activated MPCs coordinate innate immunity and promote effector activities against bacteria. In a similar fashion, following influenza infection memory CD4+ T cells also act to markedly enhance early expression of many innate inflammatory cytokines and chemokines via a pathogen–independent but antigen–dependent pathway to further recruit and activate innate effector cells to control viral infection (personal communication, Susan L. Swain, Trudeau Institute, NY).
Innate immune cells have also been shown to possess characteristics of immunological memory. Mice devoid of T cells and B cells demonstrate contact hypersensitivity responses to haptens that persist for four weeks. Transferable hapten–specific memory reside in a Ly49C-I+ NK cell subpopulation localized specifically in the sensitized donor livers [59]. More strikingly, NK cells bearing virus–specific Ly49H receptors reside in lymphoid and non-lymphoid organs for several months after cytomegalovirus infection, and confer protective immunity in naïve animals upon adoptive transfer by a robust secondary expansion following viral challenge [60]. Similarly, macrophages are also capable of undergoing a differentiation program with features of memory; this mechanism involves selective modification of the histone proteins that package genes activated in response to pathogens, to adapt to repeated exposure [61]. It thus appears that many features of adaptive immunity are manifest in the evolutionarily ancient innate immunity.
7. Immunological Constant of Rejection
Based on the gene and protein expression profiling of tissues in mammals including humans in various pathological conditions, common pathways of immune–mediated tissue–specific destruction emerge. These pathways can be synthesized into a unified theory of ‘Immunologic constant of rejection’ as proposed recently [62]. Irrespective of different mechanisms of immune recognition conceptualized by the existing immune–sensing concepts of missing self theory [63], pattern recognition theory [64], danger theory [65], and guard theory [66], or alternative mechanisms yet to be discovered, this theory proposes that adaptive immune responses are necessary to mediate tissue specificity by directing the innate effector cells to the site of infection or disease. This helps explain the observed immune responses in the host against their target cells not only in the context of cancer, but also in the context of chronic infections, well-controlled allo-transplant reactions and autoimmunity. According to this theory, immune responses are a facet of a tissue–specific phenomenon that may or may not result in the successful immune–destruction of target cells, depending on an assortment of genetic factors related to the background of the host or evolving phenotypes of the heterogenous tissue environment in question. In this regard, using various types of immune–responsive and non-responsive human engraft tumours, it was shown that tumour rejection was associated with activation of IFN–stimulated genes and innate immune effectors functions [67]. These gene signatures reproduce those observed in humans during immune–mediated destruction that causes tumour or allograft rejection, graft versus host disease, autoimmunity, clearance of pathogens, or during acute cardiovascular or chronic obstructive pulmonary diseases. Disruption of IFN signalling within lymphocytes may thus be a common mechanism of immune dysfunction in some immunopathologies. Indeed, in peripheral blood lymphocytes from patients with three major cancers: breast cancer, melanoma and gastrointestinal cancer, type-I IFN (IFN-α)–induced signalling was reduced in T and B cells, and type-II (IFN-γ)–induced signalling was reduced in B cells from all three cancer patient groups [68].
8. Concluding remarks
Based on the recent developments in the field, it is clear that the innate and adaptive immune systems form one integrated defence network, where both systems rely on each other for regulation, activation, suppression and compensation of immune response. Even the immune memory functions require a collaboration of the effector components of both innate and adaptive immunity. Accordingly, the conventional boundaries between innate and adaptive immunity are arbitrary. Defects in innate immunity are rare in comparison with defects in adaptive immunity. Few patients survive the lack of innate immune mechanisms. On the other hand, T and B cell–deficient animals seem to be quite resistant to most pathogens while lack self-non-self discrimination, which make them tolerant to xeno-transplantation.
Successful induction and control of effector response thus depends on an intricate balance between both arms of immunity. Adaptive immunity provides not only effector response but also tissue specificity in a cognate manner by directing the innate effector response to the infected or diseased tissue site. The triggers and the magnitude of this immune collaboration may be regulated by the concentration of pathogen-associated and/or danger-associated molecular patterns, or other unknown factors. The cooperativity between adaptive and innate responses optimizes a successful host immune rejection. The dynamics of this powerful immunological ‘orchestra’, where adaptive T cells seemingly function as a “conductor”, will become clear as more meaningful immune interactions and regulatory networks with the conductor are identified.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported [in part] by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
The author thanks Francesco M. Marincola (Clinical Center, NIH), Gonzalo de la Rosa (NCI-Frederick) and Susan L. Swain (Trudeau Institute, NY) for critical reading of the manuscript and helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, et al. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson CB. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity. 1995;3:531–539. doi: 10.1016/1074-7613(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Nayak BP, Rao KV. B cell responses to a peptide epitope. VII. Antigen-dependent modulation of the germinal center reaction. J Immunol. 1998;161:5832–5841. [PubMed] [Google Scholar]
- 4.Hiom K, Melek M, Gellert M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell. 1998;94:463–470. doi: 10.1016/s0092-8674(00)81587-1. [DOI] [PubMed] [Google Scholar]
- 5.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku CL, Picard C, Erdos M, Jeurissen A, Bustamante J, Puel A, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet. 2007;44:16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 9.Kambayashi T, Assarsson E, Lukacher AE, Ljunggren HG, Jensen PE. Memory CD8+ T cells provide an early source of IFN-gamma. J Immunol. 2003;170:2399–2408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- 10.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 12.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 13.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maloy KJ, Antonelli LR, Lefevre M, Powrie F. Cure of innate intestinal immune pathology by CD4+CD25+ regulatory T cells. Immunol Lett. 2005;97:189–192. doi: 10.1016/j.imlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 18.Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, et al. Suppression of natural killer cell- mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallimore AM, Simon AK. Positive and negative influences of regulatory T cells on tumour immunity. Oncogene. 2008;27:5886–5893. doi: 10.1038/onc.2008.269. [DOI] [PubMed] [Google Scholar]
- 21.Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol. 2008;180:4679–4686. doi: 10.4049/jimmunol.180.7.4679. [DOI] [PubMed] [Google Scholar]
- 22.Giroux M, Yurchenko E, St-Pierre J, Piccirillo CA, Perreault C. T regulatory cells control numbers of NK cells and CD8alpha+ immature dendritic cells in the lymph node paracortex. J Immunol. 2007;179:4492–4502. doi: 10.4049/jimmunol.179.7.4492. [DOI] [PubMed] [Google Scholar]
- 23.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 24.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Balaji KN, Schaschke N, Machleidt W, Catalfamo M, Henkart PA. Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J Exp Med. 2002;196:493–503. doi: 10.1084/jem.20011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Liu J, Feng Z, Hu S, Liu Y, Sheng X, et al. Clinical outcomes and experience of 20 pediatric patients treated with extracorporeal membrane oxygenation in Fuwai Hospital. ASAIO J. 2008;54:302–305. doi: 10.1097/MAT.0b013e318172b445. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo-Man R. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J Exp Med. 2007;204:1107–1118. doi: 10.1084/jem.20062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heldrup J, Kalm O, Prellner K. Blood T and B lymphocyte subpopulations in healthy infants and children. Acta Paediatr. 1992;81:125–132. doi: 10.1111/j.1651-2227.1992.tb12187.x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Jauhari P, Singh U, Singla PN. Quantitation of T cells in venous blood of healthy neonates. Indian J Pediatr. 1994;61:711–714. doi: 10.1007/BF02751986. [DOI] [PubMed] [Google Scholar]
- 33.Atici A, Satar M, Cetiner S, Yaman A. Serum tumor necrosis factor-alpha in neonatal sepsis. Am J Perinatol. 1997;14:401–404. doi: 10.1055/s-2007-994168. [DOI] [PubMed] [Google Scholar]
- 34.Blackwell CC, Moscovis SM, Gordon AE, Al Madani OM, Hall ST, Gleeson M, et al. Cytokine responses and sudden infant death syndrome: genetic, developmental, and environmental risk factors. Journal of leukocyte biology. 2005;78:1242–1254. doi: 10.1189/jlb.0505253. [DOI] [PubMed] [Google Scholar]
- 35.Ozdemir A, Oygur N, Gultekin M, Coskun M, Yegin O. Neonatal tumor necrosis factor, interleukin-1 alpha, interleukin-1 beta, and interleukin-6 response to infection. Am J Perinatol. 1994;11:282–285. doi: 10.1055/s-2007-994592. [DOI] [PubMed] [Google Scholar]
- 36.Vege A, Rognum TO, Scott H, Aasen AO, Saugstad OD. SIDS cases have increased levels of interleukin-6 in cerebrospinal fluid. Acta Paediatr. 1995;84:193–196. doi: 10.1111/j.1651-2227.1995.tb13608.x. [DOI] [PubMed] [Google Scholar]
- 37.Vege A, Rognum TO, Aasen AO, Saugstad OD. Are elevated cerebrospinal fluid levels of IL-6 in sudden unexplained deaths, infectious deaths and deaths due to heart/lung disease in infants and children due to hypoxia? Acta Paediatr. 1998;87:819–824. doi: 10.1080/080352598750013563. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 2004;6:759–766. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Stout RD, Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–765. [PubMed] [Google Scholar]
- 40.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 41.Ohnishi T, Sur S, Collins DS, Fish JE, Gleich GJ, Peters SP. Eosinophil survival activity identified as interleukin-5 is associated with eosinophil recruitment and degranulation and lung injury twenty-four hours after segmental antigen lung challenge. J Allergy Clin Immunol. 1993;92:607–615. doi: 10.1016/0091-6749(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 42.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper NR. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- 44.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 45.Leibson PJ. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 46.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 47.Shanker A, Verdeil G, Buferne M, Inderberg-Suso EM, Puthier D, Joly F, et al. CD8 T cell help for innate antitumor immunity. J Immunol. 2007;179:6651–6662. doi: 10.4049/jimmunol.179.10.6651. [DOI] [PubMed] [Google Scholar]
- 48.Shanker A, Buferne M, Schmitt-Verhulst AM. Cooperative action of CD8 T lymphocytes and natural killer cells controls tumour growth under conditions of restricted T-cell receptor diversity. Immunology. 2009;129:41–54. doi: 10.1111/j.1365-2567.2009.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 2007;96:1839–1848. doi: 10.1038/sj.bjc.6603792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 53.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2009 doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narni-Mancinelli E, Campisi L, Bassand D, Cazareth J, Gounon P, Glaichenhaus N, et al. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 60.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol. 2009;130:7–15. doi: 10.1016/j.clim.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang E, Worschech A, Marincola FM. The immunologic constant of rejection. Trends Immunol. 2008;29:256–262. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 64.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 66.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 67.Worschech A, Chen N, Yu YA, Zhang Q, Pos Z, Weibel S, et al. Systemic treatment of xenografts with vaccinia virus GLV-1h68 reveals the immunologic facet of oncolytic therapy. BMC genomics. 2009;10:301. doi: 10.1186/1471-2164-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL, et al. Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci U S A. 2009;106:9010–9015. doi: 10.1073/pnas.0901329106. [DOI] [PMC free article] [PubMed] [Google Scholar]


