Summary
Physical activity elicits physiological responses in skeletal muscle that result in a number of health benefits, in particular in disease states, such as type 2 diabetes. An acute bout of exercise/muscle contraction improves glucose homeostasis by increasing skeletal muscle glucose uptake, while chronic exercise training induces alterations in the expression of metabolic genes, such as those involved in muscle fiber type, mitochondrial biogenesis, or glucose transporter 4 (GLUT4) protein levels. A primary goal of exercise research is to elucidate the mechanisms that regulate these important metabolic and transcriptional events in skeletal muscle. In this review, we briefly summarize the current literature describing the molecular signals underlying skeletal muscle responses to acute and chronic exercise. The search for possible exercise/contraction-stimulated signaling proteins involved in glucose transport, muscle fiber type, and mitochondrial biogenesis is ongoing. Further research is needed because full elucidation of exercise-mediated signaling pathways would represent a significant step toward the development of new pharmacological targets for the treatment of metabolic diseases such as type 2 diabetes.
Keywords: skeletal muscle, AMPK, glucose uptake, training adaptation, exercise
INTRODUCTION
Throughout the world, diabetes afflicts over 180 million people, and epidemiological estimates from the World Health Organization project that this number will reach 366 million (4.4% of the world population) by 2030. Thus, diabetes is rapidly being recognized as a public health threat that is rising to epidemic proportions. While the rate of diabetes is on the increase, it has long been recognized that physical activity has important health benefits for people with type 2 diabetes. In the acute state, exercise positively moderates glucose homeostasis by enhancing glucose transport and insulin action in contracting skeletal muscle, the major tissue responsible for total body glucose disposal (1). Chronic physical activity (i.e. exercise training) increases glucose transporter 4 (GLUT4) protein levels, mitochondrial enzyme content, and alters fiber type in skeletal muscle (2, 3), thus providing an additional mechanism for exercise-mediated improvements in insulin sensitivity. Collectively, these effects help to explain the strong epidemiological evidence that regular physical activity prevents or delays the onset of type 2 diabetes (4, 5).
Despite the physiological importance of exercise in regulating skeletal muscle metabolism, the molecular mechanisms that underlie these important phenomena are only partly understood. Elucidating exercise-stimulated and insulin-independent signals that mediate glucose transport have already led to the identification of new targets for the treatment of diabetes (e.g., AMP-activated protein kinase (AMPK)). Determining the comprehensive mechanism will undoubtedly provide more targets for treatment, as well as provide fundamental knowledge of this complex physiological process. In this manuscript, we will briefly review the current literature on this important area of exercise and diabetes research.
ACUTE EFFECTS OF EXERCISE: REGULATION OF SKELETAL MUSCLE GLUCOSE TRANSPORT
Insulin and exercise are the two most physiologically relevant stimulators of skeletal muscle glucose transport (6, 7). In individuals with type 2 diabetes, the insulin-dependent regulation of skeletal muscle glucose transport is impaired. Importantly, insulin independent mechanisms, including exercise/contraction-mediated mechanisms for regulating glucose uptake remain intact (8, 9). Both insulin and exercise/muscle contraction increase skeletal muscle glucose uptake by translocation of glucose transporters from an intracellular location to the plasma membrane and t-tubules. GLUT4 is the predominant glucose transporter isoform expressed in skeletal muscle. Our laboratory and others have worked to elucidate the signaling mechanisms leading to exercise-stimulated GLUT4 translocation (6, 7). Early studies have demonstrated that there are distinct proximal signaling mechanisms responsible for the stimulation of GLUT4 translocation and glucose transport by insulin and exercise. Insulin signaling involves the rapid phosphorylation of the insulin receptor, insulin receptor substrate-1/2 (IRS-1/2) on tyrosine residues, and the activation of phosphatidylinositol 3-kinase (PI3-K) (10, 11). In contrast, exercise and muscle contraction have no effect on insulin receptor and IRS-1 phosphorylation or on PI3-K activity (11, 12), and muscle-specific knockout of the insulin receptor does not impair contraction-stimulated glucose transport (13). Clearly, these data demonstrate that the initiating signals that lead to GLUT4 translocation by insulin and exercise in skeletal muscle are distinct.
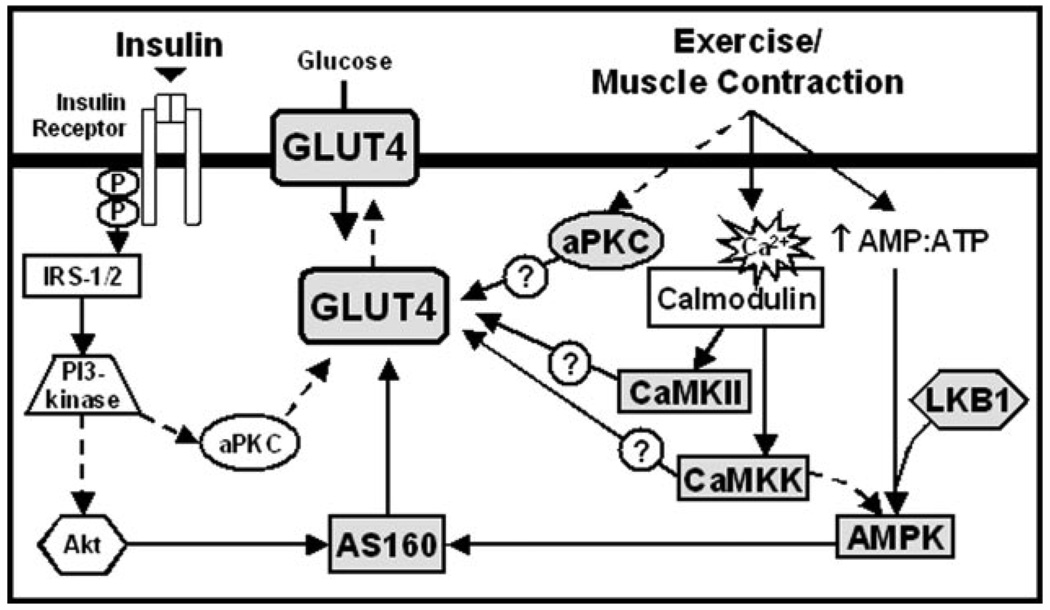
Muscle contraction is a multifactorial process involving changes in cellular energy status (i.e. increased AMP:ATP), increases in intracellular Ca2+ levels, activation of protein kinase C (PKC), and so forth. Not surprisingly, this has led many investigators to speculate that these processes activate one or more intracellular signaling pathways that coordinately act to increase plasma membrane GLUT4 transporters and glucose uptake in response to physical activity. In the following sections, we will discuss the signaling proteins that have been implicated in this complex process, including AMPK, LKB1, Ca2+/calmodulin-dependent protein kinases (CaMKs), PKCs, and AS160 (Fig. 1).
Figure 1.
Proposed model for the signaling pathways mediating insulin and contraction-induced skeletal muscle glucose transport. Insulin and contraction-mediated glucose transport occurs by translocation of glucose transporter 4 (GLUT4) from intracellular locations to the plasma membrane. Insulin binding leads to phosphorylation of the insulin receptor with subsequent activation of insulin receptor substrate 1/2 (IRS-1/2) and phosphatidylinositol 3-kinase (PI3-kinase). Downstream of PI3-kinase the protein kinases, Akt, which then regulates activation of Akt Substrate of 160 kD (AS160), and atypical protein kinase C (aPKC), have been identified to mediate insulin stimulated GLUT4 translocation. Contraction stimulated glucose uptake is mediated by multiple signaling pathways including aPKC, Ca2+/calmodulin-dependent protein kinase II (CaMKII), Ca2+/calmodulin-dependent protein kinase kinase (CaMKK), LKB1, and AMP-activated protein kinase (AMPK).
AMP-activated Protein Kinase
The AMPK and its primary upstream kinase, LKB1, are the most widely studied proteins implicated in muscle glucose transport in response to changes in the cellular energy status. AMPK is a heterotrimeric protein that is activated by a complex mechanism involving an increase in the AMP:ATP ratio and allosteric modification and phosphorylation by one or more upstream kinases, including LKB1 (14, 15). Studies using the AMP-analog, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), have demonstrated that activation of AMPK is positively correlated with an increase in muscle glucose uptake (16). However, data obtained from mouse models of attenuated AMPK activity demonstrate that inhibition of AMPK has little or no effect on contraction-induced glucose uptake (17–19). Furthermore, muscle-specific ablation of LKB1 only partially inhibits contraction-stimulated glucose transport (20, 21). Collectively, these data suggest that various signals are involved in the regulation of contraction-stimulated glucose transport in skeletal muscle.
Ca2+/Calmodulin-dependent Protein Kinases
Increases in intracellular Ca2+ levels are a fundamental part of muscle contraction, and recent studies have implicated Ca2+/calmodulin signaling and Ca2+/calmodulin-dependent protein kinases as critical molecules underlying Ca2+ - and contraction-stimulated skeletal muscle glucose transport. Incubation of rat epitrochlearis muscles with the Ca2+/calmodulin competitive inhibitor, KN-93, decreases skeletal muscle glucose transport in response to contraction and to stimulation with the sarcoplasmic reticulum Ca2+ store releasing agent, caffeine (22). In the same study, KN-93 significantly inhibited contraction-induced CaMKII phosphorylation in the absence of AMPK inhibition, suggesting that CaMKs regulate glucose uptake independent of AMPK signaling. These results are consistent with a recent study from our lab, demonstrating that CaMK kinase α-dependent signaling can stimulate muscle glucose uptake without changes in AMPK activity (23). In contrast, a recent study using isolated mouse muscles has shown that inhibition of CaMK signaling with KN-93 or the CaMK kinase inhibitor, STO-609, inhibits contraction-induced skeletal muscle glucose uptake via an AMPK-dependent signaling pathway (24). Thus, the role of AMPK in the regulation of Ca2+/calmodulin-mediated increases in skeletal muscle glucose uptake is still being debated.
Protein Kinase C
PKC is another molecule which is activated by muscle contraction and has been implicated in the regulation of contraction-stimulated muscle glucose transport (25, 26). In mammalian cells, 12 different PKC isoforms have been identified and classified into three subfamilies based on amino acid similarity and mode of activation (27). The conventional PKCs (cPKCs, α, β1, β2, and γ isoforms) are dependent on Ca2+ and diacyl-glycerol for activation, the novel PKCs (nPKCs, δ, ε, θ, and η isoforms) are dependent on only diacylglycerol for activation, and the atypical PKCs (aPKCs, ζ and λ isoforms) are activated independently of both Ca2+ and diacylglycerol. Pharmacological inhibition of cPKCs and nPKCs blunts contraction-stimulated skeletal muscle glucose uptake in skeletal muscle (25, 26), suggesting that PKCs are important in this process. However, recent studies assessing isoform-specific PKC activation have failed to demonstrate an increase in cPKC or nPKC activity by exercise/contraction (28–30). This controversy might be explained by a certain degree of nonspecificity of the pharmacological compounds. In contrast to the findings on cPKCs and nPKCs, our laboratory and others have recently shown that aPKCs are activated by exercise (29, 31, 32). In addition, in 3T3-L1 adipocytes and L6 myotubes, overexpression of wild type and constitutively active aPKCs stimulates or potentiates the effects of insulin on glucose transport, while kinase-inactive aPKCs inhibit the effect of insulin on glucose transport (33–35). Other data in support of a role for aPKCs in the regulation of glucose transport comes from a study where PKCζ and λ were hypothesized to be downstream of AMPK (31). This study, performed in L6 myotubes and isolated rat muscles, suggests that the effects of AMPK on glucose transport are mediated through the sequential activation of the extracellular signal-regulated kinase (ERK), proline-rich tyrosine kinase-2, phospholipase D, and aPKCs (31). These results are not entirely consistent with our previous data which show insulin- and contraction-stimulated glucose transport to be independent of ERK signaling (36, 37). In future studies, it will be important to further elucidate a putative AMPK-aPKC interaction.
Akt Substrate of 160 kDa (AS160)
The Akt substrate of 160 kDa (AS160) is a recently discovered protein that regulates insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes (38), L6 myotubes (39), and rat skeletal muscle (40). It is phosphorylated on six different phospho-Akt-substrate (PAS) sites in response to both insulin and contraction in skeletal muscle (40–42). Recent evidence from our lab has shown that AMPK phosphorylates AS160 (PAS) in response to AICAR and contraction in skeletal muscle (41), and that mutation of four PAS sites significantly inhibits both insulin and contraction-induced glucose uptake (43). In addition, exciting new data from our lab demonstrates that mutation of the calmodulin-binding domain on AS160 significantly inhibits contraction- but not insulin-stimulated glucose uptake (44). Thus, both phosphorylation and calmodulin-binding on AS160 appear to play a role in the regulation of contraction-stimulated glucose uptake. Collectively, these results suggest that AS160 may serve as a point of convergence for both insulin- and contraction-dependent signaling in the regulation of glucose uptake.
Other Putative Mediators of Skeletal Muscle Glucose Uptake
Investigators have speculated on the possible involvement of other signals in the regulation of contraction-stimulated skeletal muscle glucose transport, including bradykinin, reactive oxygen species, and nitric oxide. Although, there is currently no evidence in support of a role for bradykinin in muscle glucose transport (45), several studies have provided data supporting a role for reactive oxygen species (46) and nitric oxide (47–50). However, further investigations have suggested that nitric oxide stimulates glucose transport via a mechanism distinct from contraction and insulin-dependent signaling pathways (48, 49, 51).
ADAPTATIONS OF SKELETAL MUSCLE TO EXERCISE TRAINING
Regular physical activity leads to a number of adaptations in skeletal muscle that allow the muscle to more efficiently utilize substrates for ATP production and thus become more resistant to fatigue. In the following sections, we will summarize the current literature on three major adaptations to exercise training: 1) muscle fiber type transformations as defined by the expression of specific contractile proteins (myosin heavy chain isoforms), 2) increases in mitochondrial activity and content, and 3) increases in GLUT4 protein expression.
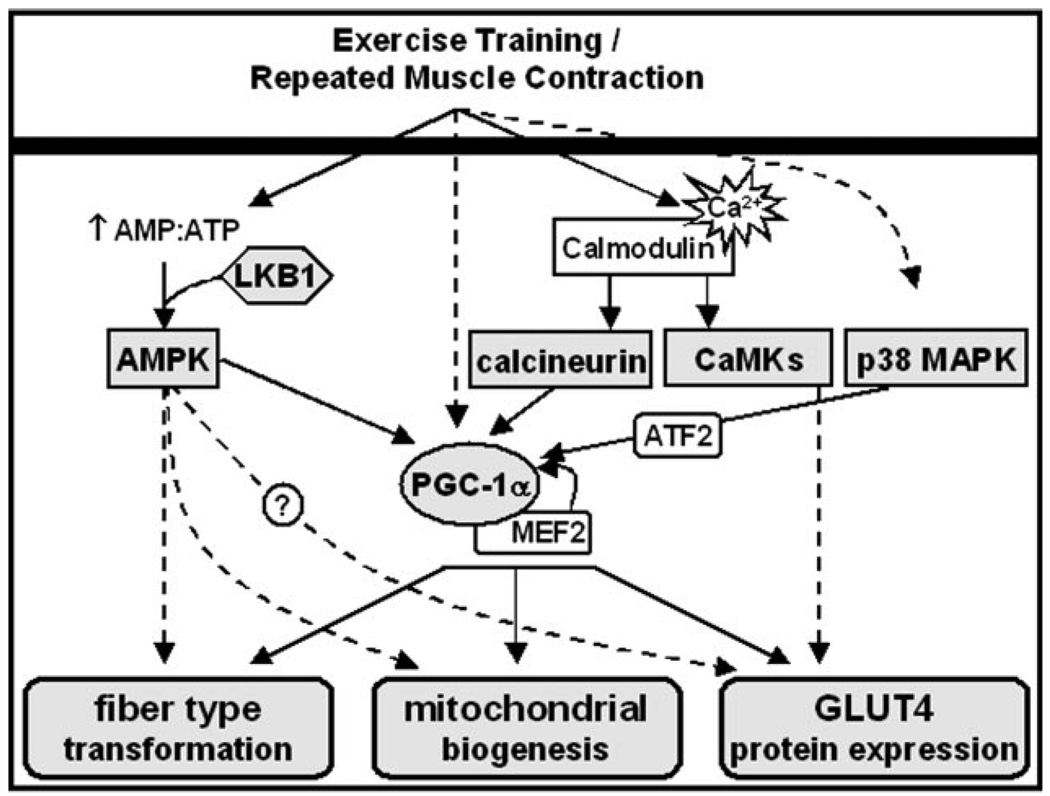
Muscle fiber types have traditionally been classified according to their expression of myosin heavy chain isoforms as fast twitch fibers (type IIb, IIx, and IIa) and slow twitch fibers (type I). Type IIb and type IIx fibers mainly depend on glycolytic, and type IIa and type I fibers on oxidative pathways for ATP production (52–54). There is an association between fiber type and mitochondrial content, with type IIb fibers tending to have the lowest and type I fibers the highest abundance of mitochondria. Endurance exercise has been shown to induce an increase in mitochondrial content and activity within the same fiber type but also a change in myosin heavy chain isoform expression, thus provoking a fiber type transformation from type IIb to IIx and IIa, and in rare cases also to type I muscle fibers (54). It is important to understand that mitochondrial biogenesis and fiber type transformation can occur independent from each other, suggesting distinct signaling mechanisms for both types of adaptive responses. In addition to increases in slow muscle fiber types and mitochondria, GLUT4 protein expression also increases in response to exercise training, facilitating glucose uptake into the trained muscle (6). GLUT4 protein is expressed to the highest level in slow, oxidative fiber types (55). Thus, muscle fiber type, mitochondrial content, and the abundance of GLUT4 are associated within the muscle cell but appear to be regulated independently. Interestingly, individuals with insulin resistance or type 2 diabetes have a distinct muscle phenotype with decreased slow, oxidative muscle fibers (56, 57), and decreased GLUT4 expression within the slow muscle fibers (58). Skeletal muscle mitochondrial dysfunction has also been linked with insulin resistance and type 2 diabetes (59), although more studies are needed to directly connect mitochondrial deficits with impaired muscle glucose metabolism. Taken together, these findings imply that the discussed aspects of muscle fiber plasticity play an important role in the pathogenesis of diabetes, and that the benefits of exercise training for people with diabetes may stem from the aforementioned training-induced skeletal muscle adaptations. In the following sections, we will give a brief overview of the signaling molecules which are considered to play a key role in muscle fiber type transformation, mitochondrial biogenesis, and increased GLUT4 expression in response to exercise training (Fig. 2).
Figure 2.
Proposed model for the signaling pathways mediating fiber type transformation, mitochondrial biogenesis, and GLUT4 protein expression with skeletal muscle adaptations to endurance training. Exercise training leads to skeletal muscle fiber type transformation, mitochondrial biogenesis, and increased glucose transporter 4 (GLUT4) protein expression, and multiple signaling pathways have been suggested to be involved in these adaptations. Changes in the cellular energy status (AMP:ATP) stimulate AMP-activated protein kinase in the presence of the AMPK kinase, LKB1. AMPK may be involved in fiber type transformation, mitochondrial biogenesis, and GLUT4 biogenesis through increasing peroxisome-proliferator-activated receptor-γ coactivator 1α (PGC-1α) expression and probably also independent of PGC-1α. Exercise training-induced increases in PGC-1α are potentiated by a positive feedback loop through myocyte-enhancing factor 2 (MEF2) and are involved in fiber type transformation, mitochondrial biogenesis, and increased GLUT4 expression. Increases in intracellular Ca2+ levels lead to activation of the Ca2+/calmodulin-dependent phosphatase, calcineurin, as well as Ca2+/calmodulin-dependent protein kinases (CaMKs). While calcineurin is involved in a number of skeletal muscle adaptations, acting primarily through PGC-1α, a role of CaMKs has so far pointed toward increasing GLUT4 protein expression. Contraction-induced activation of p38 mitogen activated protein kinase (p38 MAPK) increases PGC-1α expression through activating transcription factor 2 (ATF2) and may therefore also be involved in skeletal muscle adaptations to exercise training.
AMPK
The AMPK has been implicated in the regulation of muscle fiber type (60–62), mitochondrial biogenesis (63), and GLUT4 biogenesis in skeletal muscle (64), making AMPK a key protein of interest in the study of exercise-mediated muscle adaptations. In young rats, administration of the AMPK activator, AICAR, for 14 days (1 mg/g body wt, subcutaneous) significantly increased the number of type IIx fibers in extensor digitorum longus (61). In addition, recent work from our lab using AMPKγ1 (R70Q) transgenic mice, a mouse model of constitutively active AMPK activity (65), demonstrated that chronic elevations in AMPK activity resulted in significant increases in the ratio of type IIa/x fibers in triceps muscles (62). Collectively, these results suggest a role for AMPK in exercise-induced muscle fiber type transformation. However, exercise training of AMPKα2 (D157A) transgenic mice, mice with chronic inhibition AMPKα2 activity (17), only showed a partial impairment in the exercise-induced increase in type IIa/x fibers (62). Thus, in addition to AMPK signaling, other pathways must be involved in training-induced muscle fiber type transformations.
Activation of AMPK with the AMP-analog AICAR or the creatine analog, β-guanidinopropionic acid, increases the activity and content of mitochondrial proteins (63, 66, 67), and this effect is abolished in AMPKα2 kinase dead (67) and knockout mice (68). Consistent with these findings, transgenic mice with increased AMPK activity show increases in mitochondrial markers (62). Furthermore, chronic AICAR injections induce peroxisome-proliferator-activated receptor-γ coactivator 1α (PGC-1α) RNA expression in rat skeletal muscle (61, 69), suggesting a possible link between AMPK activation and PGC-1α signaling. Surprisingly, in response to exercise training, AMPKα2 knockout and AMPKα2 kinase inactive mice show no defect in mitochondrial increases (62, 68). Thus, despite its capacity to induce mitochondrial biogenesis, AMPK is not essential for exercise training-induced increases in mitochondria.
Additional studies using AICAR demonstrated that chronic activation of AMPK (5 days) increased muscle GLUT4 expression ~50–200% (64), suggesting that AMPK may regulate muscle GLUT4 biogenesis. However in two recent studies, AMPKγ1 (R70Q) transgenic mice that exhibit chronic increases in AMPK activity, did not have increased muscle GLUT4 mRNA and protein expression (62, 65). Thus, the involvement of AMPK in AICAR- or exercise-stimulated increases in GLUT4 protein levels is currently controversial.
Peroxisome-proliferator-activated Receptor-γ Coactivator 1α
PGC-1α is a potent transcriptional coactivator that interacts with a variety of transcription factors (e.g. MEF2, ERRα, NRF-1, NRF-2) to regulate glucose and fatty acid metabolism, mitochondrial biogenesis, and muscle fiber type transformation from type II to type I fibers (70–74). Both short-term exercise and endurance training stimulate PGC-1α expression in myocytes (75), and this is thought to occur via a positive feedback mechanism involving increased expression of MEF2. In addition, in studies using in vivo gene transfection, mutations of the PGC-1α promoter at MEF2 binding sites or a cAMP response element (CRE) show that contraction-induced PGC-1α promoter activity in skeletal muscle is dependent on its MEF2 and CRE sequence elements (76). Research over the last several years has attempted to identify upstream signaling molecules involved in the activation of PGC-1α including AMPK, the Ca2+/calmodulin-dependent protein phosphatase calcineurin, CaMKs, and p38 mitogen activated protein kinase (p38 MAPK) (69, 73, 77, 78). In a recent study, AMPK was shown to induce increases in the binding of PGC-1α to its promoter, via direct phosphorylation of PGC-1α on amino acids Thr177 and Ser538 (79). Thus, a number of signaling pathways activate the transcriptional coactivator PGC-1α to regulate muscle fiber types, mitochondria, and glucose metabolism. This suggests a pivotal role of PGC-1α in skeletal muscle adaptations to exercise.
Calcineurin
The Ca2+/calmodulin-dependent protein phosphatase, calcineurin, is known as the master regulator of fast to slow twitch fiber type changes (80–82). In transgenic mice expressing a constitutively active form of calcineurin, there is a substantial increase in the number of slow twitch type I muscle fibers (83). Conversely, inhibition of calcineurin activity by treatment with cyclosporin (5 mg/kg, daily for 6 weeks) promotes slow-to-fast fiber type transformation (80). In C2C12 myocytes, calcineurin significantly increases the ability of PGC-1α to activate slow fiber promoters, suggesting a cooperation between the calcineurin pathway and PGC-1α (73).
Reports on the role of calcineurin in mitochondrial biogenesis have been controversial (84). Transgenic mice expressing constitutively active calcineurin show increased PGC-1 expression (85), and in cultured cardiac myocytes, constitutively active calcineurin upregulates a large number of genes involved in mitochondrial energy metabolism (86). Furthermore, transplant patients maintained on the calcineurin inhibitor cyclosporin A develop a loss of skeletal muscle oxidative capacity (87). Despite these data suggesting an important role of calcineurin in mitochondrial biogenesis, other studies have shown that the phosphatase cannot fully account for exercise training-induced adaptations in skeletal muscle. Cyclosporin treatment (500 ng/ml for 14 days) does not prevent the upregulation of mitochondrial markers in Ca2+ ionophore-treated myotubes (88)or in trained rats (20 mg/kg for 7 days (89) or 50 mg/kg for 10 days (90)).
A role for Ca2+-dependent signaling in skeletal muscle GLUT4 expression first emerged from experiments in L6 cells using the sarcoplasmic reticulum Ca2+ mobilizing agent, caffeine (91). In this study, intermittent caffeine treatment (5 mM, 3 h/day, 5 days) induced increases in cytosolic Ca2+ levels, and significantly increased GLUT4 protein levels (~50%), suggesting that Ca2+ signaling regulates muscle GLUT4 expression. In addition, transgenic mice overexpressing a constitutively active form of calcineurin have increased skeletal muscle GLUT4 protein, suggesting that calcineurin can induce GLUT4 biogenesis (85). However, cyclosporin treatment of rats (20 mg/kg, daily for 4 weeks) did not impair exercise training-induced increases in GLUT4 protein and mRNA expression despite complete inhibition of calcineurin (92). Thus, a physiological role of calcineurin in GLUT4 upregulation following exercise training is still being debated.
Ca2+/Calmodulin-dependent Protein Kinases
Similar to the findings from constitutively active calcineurin transgenic mice, transgenic mice expressing a constitutively active form of CaMKIV exhibit an abundance of slow twitch type I muscle fibers (77). However, the physiological relevance of these findings is questionable, since recent findings have established that CaMKIV protein is not expressed in mouse skeletal muscle (93). Future studies will be needed to investigate the potential relevance of other CaMK family members in skeletal muscle fiber type adaptations to exercise.
A role for CaMKs in muscle mitochondrial biogenesis first emerged from studies in L6 cells (a rat muscle cell culture model) using the Ca2+ ionophores, A-23187 (94) and ionomycin (95), and the sarcoplasmic reticulum Ca2+ mobilizing agent, caffeine (95, 96). These studies demonstrated that intermittent or sustained increases in cytosolic Ca2+ levels significantly increased mitochondrial enzymes, including COX-I, δ-aminole-vulinate (ALAS), citrate synthase, and cytochrome c, and that treatment with the Ca 2+/calmodulin competitive inhibitor, KN-93 (10 µM) completely blocked caffeine-induced increases in ALAS, COXI, cytochrome c, and citrate synthase protein expression. In addition, transgenic mice expressing a constitutively active form of CaMKIV exhibited significant increases in muscle mitochondrial mass, along with increases in cytochrome b, CPT-1, and PGC-1 expression (77). However, whole body CaMKIV knockout mice do not exhibit impaired muscle mitochondrial biogenesis in response to contractile activity, and, as mentioned earlier, CaMKIV protein is actually not detectable in mouse skeletal muscle (93). Thus, the potential significance of the findings in CaMKIV transgenic mice lies in a possible homology with other members of the CaMK family that are expressed in skeletal muscle.
As described earlier, increased cytosolic Ca2+ levels by caffeine treatment induce the expression of GLUT4 protein (91). In addition, pretreatment of cells with the Ca2+/calmodulin competitive inhibitor, KN-93, completely prevents the caffeine-induced increase in GLUT4 protein. These findings implicate an important role of CaMKs in elevated GLUT4 protein expression following increases in intracellular Ca2+.
p38 MAPK
The p38 mitogen activated protein kinase (p38 MAPK) is activated by various stimuli including contraction, insulin, environmental stress, and proinflammatory cytokines (97–99). p38 MAPK has been suggested to play a functional role in myogenic cell differentiation (100), and our lab and others have shown increased p38 MAPK activation following various muscle contraction or running exercise protocols in rodents and humans (101, 102). Studies in C2C12 myocytes show p38 MAPK as an activator of the PGC-1α promoter, and this activation is mediated by the transcription factor ATF2 (78). In transgenic mice, muscle specific activation of p38 MAPK leads to enhanced PGC-1α gene expression and increased mitochondrial proteins (78). An acute bout of exercise in mice (3 h of voluntary wheel running) or rats (2 h of swimming) increases p38 MAPK and ATF2 phosphorylation, leading to PGC-1α activation (78, 103). Since studies have so far focused on acute effects, it will be an interesting area of future research to determine the role of p38 MAPK in exercise training-induced adaptations in skeletal muscle.
SUMMARY
Exercise is of critical importance for people with insulin resistance or diabetes. Our current understanding is that one of the many benefits of an acute bout of exercise is an insulin-independent increase in the glucose uptake capacities of skeletal muscle. Important chronic adaptations to exercise training are the increase of mitochondria and thus oxidative capacities in skeletal muscle, the transformation of muscle fiber types, and the increase in GLUT4 protein expression.
Contractile activity and insulin are the most potent and physiologically relevant stimuli of glucose transport in skeletal muscle. While significant progress has been made in elucidating the insulin signaling pathway leading to GLUT4 translocation, identification of the signals mediating contraction-stimulated glucose transport has proved challenging. A growing body of data suggests that multiple signaling cascades mediate the metabolic effects of contraction. While the proximal signals leading to contraction- and insulin-stimulated glucose transport are clearly distinct, emerging studies have shown a reconnection or convergence of these signals at AS160.
Exercise training induces an increase of oxidative capacity, fiber type changes, and elevated GLUT4 protein levels in skeletal muscle; adaptations which are of critical importance to lower free fatty acids, improve glucose uptake, and decrease the risk of insulin resistance and diabetes. Again, multiple signaling pathways appear to act synergistically to mediate adaptive responses to exercise training. In particular, AMPK and calcineurin have evolved as major candidates for mediating exercise-training adaptations. PGC-1α may be a point of convergence for both pathways. While considerable progress has been made in decoding molecular mechanisms around these molecules, more research will be needed to test their physiological role in skeletal muscle adaptations to exercise training.
ACKNOWLEDGEMENTS
Work in the authors’ laboratory was supported by grants to L.J. Goodyear (National Institutes of Health R01AR45670 and R01DK068626) and a Diabetes Endocrinology Research Grant at the Joslin Diabetes Center (National Institutes of Health DK36836). C.A. Witczak was supported by fellowships from the National Institutes of Health (F32AR051663) and the American Diabetes Association (mentor-based to L.J.G.). K.S.C. Röckl was supported by a fellowship within the Postdoc-Program of the German Academic Exchange Service, DAAD.
REFERENCES
- 1.DeFronzo RA, Ferrannini E, Sato Y, Felig P. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J. Clin. Invest. 1981;68:1468–1474. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holloszy JO. Biochemical adaptations in muscle: effects of exercise on mitochondrial oxygen uptake and respiratory activity in skeletal muscle. J. Biol. Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 3.Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J. Appl. Physiol. 1972;33:312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of Type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 6.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu. Rev. Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am. J. Physiol. 1997;273:E1039–E1051. doi: 10.1152/ajpendo.1997.273.6.E1039. [DOI] [PubMed] [Google Scholar]
- 8.Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action in skeletal muscle from patients with NIDDM. Mol. Cell Biochem. 1998;182:153–160. [PubMed] [Google Scholar]
- 9.Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48:1192–1197. doi: 10.2337/diabetes.48.5.1192. [DOI] [PubMed] [Google Scholar]
- 10.Folli F, Saad MJA, Backer JM, Kahn CR, Saad MJ. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J. Biol. Chem. 1992;267:22171–22177. [PubMed] [Google Scholar]
- 11.Goodyear LJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. Effects of contractile activity on tyrosine phosphoproteins and phosphatidylinositol 3-kinase activity in rat skeletal muscle. Am. J. Physiol. 1995;268:E987–E995. doi: 10.1152/ajpendo.1995.268.5.E987. [DOI] [PubMed] [Google Scholar]
- 12.Treadway JL, James DE, Burcel E, Ruderman NB. Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am. J. Physiol. 1989;256:E138–E144. doi: 10.1152/ajpendo.1989.256.1.E138. [DOI] [PubMed] [Google Scholar]
- 13.Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J. Clin. Invest. 1999;104:1257–1264. doi: 10.1172/JCI7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 15.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem. Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 16.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 17.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase α2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J. Biol. Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J. Biol. Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 19.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 20.Hirshman MF, Koh HJ, Goodyear LJ. LKB1 in muscle is critical for exercise capacity and partially regulates glucose transport. Diabetes. 2006;55 Suppl. 1:A13. [Google Scholar]
- 21.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame HD, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- 23.Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- 24.Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- 25.Cleland PJ, Appleby GJ, Rattigan S, Clark MG. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J. Biol. Chem. 1989;264:17704–17711. [PubMed] [Google Scholar]
- 26.Henriksen EJ, Sleeper MD, Zierath JR, Holloszy JO. Polymyxin B inhibits stimulation of glucose transport in muscle by hypoxia or contractions. Am. J. Physiol. 1989;256:E662–E667. doi: 10.1152/ajpendo.1989.256.5.E662. [DOI] [PubMed] [Google Scholar]
- 27.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 28.Heled Y, Shapiro Y, Shani Y, Moran DS, Langzam L, Barash V, Sampson SR, Meyerovitch J. Physical exercise enhances hepatic insulin signaling and inhibits phosphoenolpyruvate carboxykinase activity in diabetes-prone Psammomys obesus. Metabolism. 2004;53:836–841. doi: 10.1016/j.metabol.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Rose AJ, Michell BJ, Kemp BE, Hargreaves M. Effect of exercise on protein kinase C activity and localization in human skeletal muscle. J. Physiol. 2004;561:861–870. doi: 10.1113/jphysiol.2004.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrini S, Henriksson J, Zierath JR, Widegren U. Exercise-induced protein kinase C isoform-specific activation in human skeletal muscle. Diabetes. 2004;53:21–24. doi: 10.2337/diabetes.53.1.21. [DOI] [PubMed] [Google Scholar]
- 31.Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Jr, Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise - and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J. Biol. Chem. 2002;277:23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- 32.Aschenbach WG, Ho RC, Sakamoto K, Fujii N, Li Y, Kim YB, Hirshman MF, Goodyear LJ. Regulation of dishevelled and β-catenin in rat skeletal muscle: an alternative exercise-induced GSK-3β signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2006;291:E152–E158. doi: 10.1152/ajpendo.00180.2005. [DOI] [PubMed] [Google Scholar]
- 33.Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J. Biol. Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 34.Bandyopadhyay G, Standaert ML, Kikkawa U, Ono Y, Moscat J, Farese RV. Effects of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinases C-zeta and C-lambda. Biochem. J. 1999;337:461–470. [PMC free article] [PubMed] [Google Scholar]
- 35.Condorelli G, Vigliotta G, Trencia A, Maitan MA, Caruso M, Miele C, Oriente F, Santopietro S, Formisano P, Beguinot F. Protein kinase C (PKC)-alpha activation inhibits PKC-zeta and mediates the action of PED/PEA-15 on glucose transport in the L6 skeletal muscle cells. Diabetes. 2001;50:1244–1252. doi: 10.2337/diabetes.50.6.1244. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T, Hirshman MF, Dufresne SD, Goodyear LJ. Skeletal muscle contractile activity in vitro stimulates mitogenactivated protein kinase signaling. Am. J. Physiol. 1999;277:C701–C707. doi: 10.1152/ajpcell.1999.277.4.C701. [DOI] [PubMed] [Google Scholar]
- 37.Wojtaszewski JF, Lynge J, Jakobsen AB, Goodyear LJ, Richter EA. Differential regulation of MAP kinase by contraction and insulin in skeletal muscle: metabolic implications. Am. J. Physiol. 1999;277:E724–E732. doi: 10.1152/ajpendo.1999.277.4.E724. [DOI] [PubMed] [Google Scholar]
- 38.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 39.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 2005;20:271–284. doi: 10.1152/physiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 40.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- 41.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 42.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 43.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J. Biol. Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 44.Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. The calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes. 56:2854–2862. doi: 10.2337/db07-0681. [DOI] [PubMed] [Google Scholar]
- 45.Constable SH, Favier RJ, Uhl J, Holloszy JO. Bradykinin does not mediate activation of glucose transport by muscle contraction. J. Appl. Physiol. 1986;61:881–884. doi: 10.1152/jappl.1986.61.3.881. [DOI] [PubMed] [Google Scholar]
- 46.Sandstrom ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J. Physiol. 2006;575:251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J. Appl. Physiol. 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- 48.Etgen GJ, Jr, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes. 1997;46:1915–1919. doi: 10.2337/diab.46.11.1915. [DOI] [PubMed] [Google Scholar]
- 49.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001;50:241–247. doi: 10.2337/diabetes.50.2.241. [DOI] [PubMed] [Google Scholar]
- 50.Young ME, Radda GK, Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem. J. 1997;322:223–228. doi: 10.1042/bj3220223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rottman JN, Bracy D, Malabanan C, Yue Z, Clanton J, Wasserman DH. Contrasting effects of exercise and NOS inhibition on tissue-specific fatty acid and glucose uptake in mice. Am. J. Physiol. Endocrinol. Metab. 2002;283:E116–E123. doi: 10.1152/ajpendo.00545.2001. [DOI] [PubMed] [Google Scholar]
- 52.Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev. Physiol. Biochem. Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- 53.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J. Appl. Physiol. 1994;77:493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- 54.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 55.Kong X, Manchester J, Salmons S, Lawrence JC., Jr Glucose transporters in single skeletal muscle fibers. Relationship to hexokinase and regulation by contractile activity. J. Biol. Chem. 1994;269:12963–12967. [PubMed] [Google Scholar]
- 56.Marin P, Andersson B, Krotkiewski M, Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care. 1994;17:382–386. doi: 10.2337/diacare.17.5.382. [DOI] [PubMed] [Google Scholar]
- 57.Nyholm B, Qu Z, Kaal A, Pedersen SB, Gravholt CH, Andersen JL, Saltin B, Schmitz O. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes. 1997;46:1822–1828. doi: 10.2337/diab.46.11.1822. [DOI] [PubMed] [Google Scholar]
- 58.Gaster M, Staehr P, Beck-Nielsen H, Schroder HD, Handberg A. Glut4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001;50:1324–1329. doi: 10.2337/diabetes.50.6.1324. [DOI] [PubMed] [Google Scholar]
- 59.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 60.Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem. J. 2002;363:167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- 62.Röckl KSC, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 63.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 64.Holmes BE, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT4, hexokinase, and glycogen in muscle. J. Appl. Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 65.Barre L, Richardson C, Hirshman MF, Brozinick J, Fiering S, Kemp BE, Goodyear LJ, Witters LA. Genetic model for the chronic activation of skeletal muscle AMP-activated protein kinase leads to glycogen accumulation. Am. J. Physiol. Endocrinol. Metab. 2007;292:E802–E811. doi: 10.1152/ajpendo.00369.2006. [DOI] [PubMed] [Google Scholar]
- 66.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 67.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of α2-AMPK in basal, training- and AICAR-induced GLUT4, hexokinase II and mitochondrial protein expression in mouse muscle. Am. J. Physiol. Endocrinol. Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- 69.Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem. Biophys. Res. Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 70.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 71.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 72.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 74.Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc. Natl. Acad. Sci. USA. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 76.Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1alpha promoter activity in skeletal muscles of living mice. Am. J. Physiol. Cell Physiol. 2004;287:C790–C796. doi: 10.1152/ajpcell.00425.2003. [DOI] [PubMed] [Google Scholar]
- 77.Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 78.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 79.Jäger S, Handschin C, St. Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin- dependent pathway. EMBO J. 2001;20:6414–6423. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chakkalakal JV, Stocksley MA, Harrison MA, Angus LM, Deschenes-Furry J, St. Pierre S, Megeney LA, Chin ER, Michel RN, Jasmin BJ. Expression of utrophin A mRNA correlates with the oxidative capacity of skeletal muscle fiber types and is regulated by calcineurin/NFAT signaling. Proc. Natl. Acad. Sci. USA. 2003;100:7791–7796. doi: 10.1073/pnas.0932671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- 84.Chin ER. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc. Nutr. Soc. 2004;63:279–286. doi: 10.1079/PNS2004335. [DOI] [PubMed] [Google Scholar]
- 85.Ryder JW, Bassel-Duby R, Olson EN, Zierath JR. Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J. Biol. Chem. 2003;278:44298–44304. doi: 10.1074/jbc.M304510200. [DOI] [PubMed] [Google Scholar]
- 86.Schaeffer PJ, Wende AR, Magee CJ, Neilson JR, Leone TC, Chen F, Kelly DP. Calcineurin and calcium/calmodulindependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle. J. Biol. Chem. 2004;279:39593–39603. doi: 10.1074/jbc.M403649200. [DOI] [PubMed] [Google Scholar]
- 87.Goy JJ, Stauffer JC, Deruaz JP, Gillard D, Kaufmann U, Kuntzer T, Kappenberger L. Myopathy as possible sideeffect of cyclosporin. Lancet. 1989;1:1446–1447. doi: 10.1016/s0140-6736(89)90147-5. [DOI] [PubMed] [Google Scholar]
- 88.Meissner JD, Gros G, Scheibe RJ, Scholz M, Kubis HP. Calcineurin regulates slow myosin, but not fast myosin or metabolic enzymes, during fast-to-slow transformation in rabbit skeletal muscle cell culture. J. Physiol. 2001;533:215–226. doi: 10.1111/j.1469-7793.2001.0215b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia-Roves PM, Huss J, Holloszy JO. Role of calcineurin in exercise-induced mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1172–E1179. doi: 10.1152/ajpendo.00633.2005. [DOI] [PubMed] [Google Scholar]
- 90.Terada S, Nakagawa H, Nakamura Y, Muraoka I. Calcineurin is not involved in some mitochondrial enzyme adaptations to endurance exercise training in rat skeletal muscle. Eur. J. Appl. Physiol. 2003;90:210–217. doi: 10.1007/s00421-003-0858-7. [DOI] [PubMed] [Google Scholar]
- 91.Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca(2+) Am. J. Physiol. Endocrinol. Metab. 2002;282:E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Roves PM, Jones TE, Otani K, Han DH, Holloszy JO. Calcineurin does not mediate exercise-induced increase in muscle GLUT4. Diabetes. 2005;54:624–628. doi: 10.2337/diabetes.54.3.624. [DOI] [PubMed] [Google Scholar]
- 93.Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am. J. Physiol. Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- 94.Freyssenet D, Di Carlo M, Hood DA. Calcium-dependent regulation of cytochrome c gene expression in skeletal muscle cells. Identification of a protein kinase c-dependent pathway. J. Biol. Chem. 1999;274:9305–9311. doi: 10.1074/jbc.274.14.9305. [DOI] [PubMed] [Google Scholar]
- 95.Ojuka EO, Jones TE, Han DH, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1040–E1045. doi: 10.1152/ajpendo.00242.2002. [DOI] [PubMed] [Google Scholar]
- 96.Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J. 2003;17:675–681. doi: 10.1096/fj.02-0951com. [DOI] [PubMed] [Google Scholar]
- 97.Somwar R, Perreault M, Kapur S, Taha C, Sweeney G, Ramlal T, Kim DY, Keen J, Cote CH, Klip A, Marette A. Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: potential role in the stimulation of glucose transport. Diabetes. 2000;49:1794–1800. doi: 10.2337/diabetes.49.11.1794. [DOI] [PubMed] [Google Scholar]
- 98.Tsakiridis T, Taha C, Grinstein S, Klip A. Insulin activates a p21-activated kinase in muscle cells via phosphatidylinositol 3-kinase. J. Biol. Chem. 1996;271:19664–19667. doi: 10.1074/jbc.271.33.19664. [DOI] [PubMed] [Google Scholar]
- 99.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 100.Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- 101.Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J. Appl. Physiol. 2001;90:1936–1942. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- 102.Boppart MD, Asp S, Wojtaszewski JF, Fielding RA, Mohr T, Goodyear LJ. Marathon running transiently increases c-Jun NH2-terminal kinase and p38 activities in human skeletal muscle. J. Physiol. (Lond) 2000;526:663–669. doi: 10.1111/j.1469-7793.2000.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]