Abstract
An important mechanism by which vertebrate olfactory sensory neurons rapidly adapt to odorants is feedback modulation of the Ca2+ permeable cyclic nucleotide–gated (CNG) transduction channels. Extensive heterologous studies of homomeric CNGA2 channels have led to a molecular model of channel modulation based on the binding of calcium-calmodulin to a site on the cytoplasmic amino terminus of CNGA2. Native rat olfactory CNG channels, however, are heteromeric complexes of three homologous but distinct subunits. Notably, in heteromeric channels, we found no role for CNGA2 in feedback modulation. Instead, an IQ-type calmodulin-binding site on CNGB1b and a similar but previously unidentified site on CNGA4 are necessary and sufficient. These sites seem to confer binding of Ca2+-free calmodulin (apocalmodulin), which is then poised to trigger inhibition of native channels in the presence of Ca2+.
Vertebrate olfactory sensory neurons (OSNs) convert chemical stimulation by odorant into an electrical signal. In OSNs, odorant binding to G-protein-coupled receptors stimulates an increase in intracellular 3′,5′-cyclic AMP (cAMP) concentration. This, in turn, leads to the opening of Ca2+-permeable CNG ion channels and the triggering of action potentials. The olfactory signal transduction pathway can be exquisitely sensitive. It can also rapidly and repeatedly adjust its sensitivity (or adapt) to stimulation (for review, see ref. 1). Rapid adaptation in OSNs is Ca2+ dependent and is considered to be primarily an effect of modulation of cAMP sensitivity in CNG channels2,3. The currently accepted hypothesis for the mechanism of channel modulation is drawn from extensive studies of heterologously expressed homomeric CNGA2 channels, which show that calmodulin, when complexed with Ca2+ (Ca2+-CaM), binds to an autoexcitatory domain of CNGA2, resulting in a reduced steady-state cAMP sensitivity4–8 (for review, see ref. 9). We have since found, however, that this hypothesis is unsatisfactory, both mechanistically and kinetically, with respect to native channels and adaptation of OSNs10. For example, the binding of Ca2+-CaM to homomeric CNGA2 channels is strongly biased toward closed rather than open channels10. This is conspicuous because modulation of closed channels would be of little use during odorant stimulation of an OSN. Furthermore, homomeric CNGA2 channel modulation by Ca2+-CaM occurs too slowly (by two orders of magnitude) to account for adaptation in OSNs10. Taken together, these findings raise anew the question of how, mechanistically, olfactory CNG channels are modulated by Ca2+-CaM to affect adaptation.
Native olfactory CNG channels comprise three homologous subunits, CNGA2, CNGA4 and CNGB1b10–15. Our previous findings indicate subunits CNGA4 and CNGB1b are crucial for the rapid modulation by Ca2+-CaM of native channels in the open state10, as well as for adaptation14. Here we focus on the native heteromeric configuration of the CNG channels of rat OSNs, addressing the possible combined contributions of CNGA2, CNGA4 and CNGB1b to the molecular mechanism underlying Ca2+-dependent adaptation.
RESULTS
Native channels preassociate with a Ca2+-responsive factor
We began by examining the interaction of calmodulin with native olfactory CNG channels of rat OSNs. We recorded CNG currents in excised, inside-out membrane patches from dendritic knobs of these cells, while maintaining 50 μM Ca2+ on the inside (cytoplasmic face) of the patches. Immediately after patch excision, application of a high concentration of 150 μM cAMP in 50 μM Ca2+ yielded a maximal current, Imax, of ~100 pA (Fig. 1a). When the cAMP concentration was changed to 7.5 μM, which is near the known concentration for half-maximal activation (EC50) for the channel under these Ca2+ conditions11,16, the current decreased unexpectedly to essentially zero. For as long as the patch was held in 50 μM Ca2+, 7.5 μM cAMP was ineffective. When we repeated the solution changes on the same patch after extensive washing (10 min) with 10 mM EGTA, the same saturated CNG current at 150 μM cAMP decreased to a lower but still substantial level, as had been expected for 7.5 μM cAMP (I7.5). The current then decreased to near zero after addition of 500 nM calmodulin. A cAMP dose–response analysis confirmed that the average EC50 value for the native channel, measured at 50 μM Ca2+, was initially 27.3 μM cAMP but shifted to 8.7 μM cAMP after extensive washing in 10 mM EGTA (Fig. 1b). This degree of shift in the EC50 was consistent with the action of Ca2+-CaM on native channels that was reported previously10,14.
Figure 1.

Ca2+-dependent inhibition of native rat olfactory CNG channels. Recordings from inside-out membrane patches excised from dendritic knobs of OSNs. (a) After excision of a patch, almost no current is activated by 7.5 μM cAMP in 50 μM Ca2+ (black trace). This Ca2+-dependent insensitivity to 7.5 μM cAMP disappeared after 10 min of washing in 10 mM EGTA, and was fully restored by application of 500 nM calmodulin in 50 μM Ca2+ (red trace). Holding potential was −40 mV. (b) Dose-response relationship of steady-state CNG current in 50 μM Ca2+ obtained from unwashed and washed patches. Mean ± s.d. (three patches). Smooth curves were drawn from the Hill equation, with EC50 values and Hill coefficients of 8.1 μM and 1.8 (washed) and 27.3 μM and 1.9 (unwashed).
These data indicate that native olfactory CNG channels may be pre-associated with a Ca2+-responsive factor, probably calmodulin. This is also in accordance with earlier studies of native channels from rat17, catfish18 and frog OSNs19, although these studies were less clear on the molecular identification of the factor. Heterologous studies of homomeric CNGA2 channels, however, provide a prevailing yet contradictory view of how Ca2+-CaM-dependent modulation of native CNG channels occurs; calmodulin binds to the CNGA2 subunit only when it is complexed with Ca2+ and it does not preassociate with the homomeric channel4–8. We reconciled these contradictory results by examining whether calmodulin preassociates with the native heteromeric configuration of CNG channels to facilitate rapid Ca2+-dependent modulation of cAMP sensitivity.
Heteromeric channels bind calmodulin in 0 Ca2+
The findings just described raised the possibility that, at the low cytoplasmic Ca2+ concentration of the resting OSN, calmodulin may already be part of the heteromeric native channel complex and thus would be poised to modulate the channel when Ca2+ is present. To test this, we explored the prediction that there should be some Ca2+ concentration below which channels maintain high cAMP sensitivity even when bound with calmodulin. We expressed heteromeric channels in HEK 293 cells10–15 and then exposed them (in excised patches) to calmodulin in different concentrations of Ca2+ while monitoring the sensitivity state of the channels to 7.5 μM cAMP. We applied 100 nM calmodulin for 30 s in 30, 10, 3, 1 or 0.1 μM Ca2+ and monitored potential modulation by binding of Ca2+-CaM as a decrease of the 7.5 μM cAMP–dependent current10 (Fig. 2a). With decreasing Ca2+ concentration, the current declined less rapidly, until in 0.1 μM Ca2+ there was essentially no modulation. Thus a Ca2+ concentration >0.1 μM is necessary for Ca2+-CaM modulation of these channels.
Figure 2.
Calmodulin preassociates with CNGA2-A4-B1b channels. (a) Ca2+ dependence of channel modulation by 100 nM calmodulin. Data are from the same patch and are representative of data from four patches. (b–d) Apocalmodulin can associate with CNGA2-A4-B1b channels without having an effect on cAMP sensitivity. (b) Exposure of channels to 500 nM calmodulin for 30 s in 0.1 μM Ca2+ (red trace) or in 10 mM EGTA/0 Ca2+ (black trace) does not cause modulation but does induce a Ca2+-dependent sensitivity that persists beyond a 3-s calmodulin-free wash (shaded box). Data are from the same patch and are representative of data from 16 patches. Current decline (inset) after calmodulin-free wash periods (shaded box) of 2 s (blue trace) and 3 s (red trace). Data are from the same patch and are representative of data from ten patches. (c) The fraction of channels that are sensitive to Ca2+-dependent current decline is a function of the duration of pre-exposure to apocalmodulin. No apocalmodulin exposure (black trace) and 10-s (blue trace) and 1-s (red trace) pre-exposures to apocalmodulin are shown. Data are from the same patch and are representative of data from seven patches. (d) A double point mutation affecting the Ca2+-CaM binding site in CNGA2 (F68A V75A; A2mut) does not perturb preassociation of apocalmodulin to CNGA2-A4-B1b channels or channel modulation by Ca2+-CaM. Data are from the same patch and are representative of data from six patches. All records were made at −40 mV. Currents were normalized to the maximal amplitudes, Imax, of each trace. Currents at 7.5 μM cAMP (I7.5) were used to test CaM effects.
There remained, however, the question of whether calmodulin associates with the channel at or below a Ca2+ concentration that does not support channel inhibition. To answer this, we again exposed channels in a patch to calmodulin in different Ca2+ concentrations (Fig. 2b). For each condition, the patch and superfusate were then cleared for 3 s of any free calmodulin. Finally, we challenged the channels with 50 μM Ca2+, to test whether a disposition to Ca2+-dependent modulation had been acquired from pre-exposure to 500 nM calmodulin. Regardless of whether calmodulin was applied in 0.1 μM Ca2+, or even in 0 Ca2+, 50 μM Ca2+ induced an identical current decline, indicating that calmodulin did indeed bind (preassociate with) the channels in Ca2+ concentrations ≤0.1 μM. As compared with inhibition by calmodulin in 1 μM Ca2+, however, the degree of the current decline in low Ca2+after preassociation of calmodulin was roughly 50% less (Fig. 2b). We reasoned that this might reflect the dissociation of some of the calmodulin during the interposed step that served to clear all free calmodulin from the vicinity of the channels. To test this interpretation, we assessed whether the duration of this interposed calmodulin-free step influenced the final degree of Ca2+-dependent modulation. We recorded the extent of current decline in 0.1 μM Ca2+ after two different time periods of clearance in calmodulin-free solution. After a 2-s clearance step, the current was reduced by 54%, whereas in the same patch, a longer clearance step of 3 s attenuated current decline to 35% (Fig. 2b, inset). Thus, exposing a patch to calmodulin-free solution resulted in loss of preassociated calmodulin. In the intact OSN, calmodulin is estimated to be present at a concentration of 1–10 μM20, and it shows a robust and uniform pattern of distribution in the cilia by antibody staining (data not shown). Therefore, as was the case during exposure to 500 nM CaM and 0.1 μM Ca2+, in the intact OSN at rest a significant fraction of the channels will be associated with calmodulin.
These results are consistent with the hypothesis that at Ca2+ concentrations ≤0.1 μM, CNGA2-A4-B1b channels bind calmodulin without any effect on cAMP sensitivity. To further test this hypothesis, we examined whether the steady-state decline of the cAMP-dependent current varies as a function of the duration of calmodulin pre-exposure. This was indeed the case. Steady-state current decline was reduced from 36% to 17% when the duration of pre-exposure to calmodulin was reduced from 10 s to 1 s (Fig. 2c). Thus, the pre-exposure time determined the fraction of CNG channels that were sensitive to Ca2+. This further supports the idea that in Ca2+ at or below 0.1 μM, calmodulin associates with CNGA2-A4-B1b channels.
These data qualitatively show that calmodulin binds to CNGA2-A4-B1b channels in the presence of 0.1 μM Ca2+ or less without changing cAMP sensitivity—a process termed preassociation of apocalmodulin. In the prevailing model for olfactory adaptation, however, no association of apocalmodulin at basal Ca2+ concentrations has been described4–8. On the contrary, the calmodulin binding site identified in the N terminus of CNGA2 (ref. 5) is a classic basic amphiphilic α-helix (Baa) motif21, with high affinity for Ca2+-CaM and no affinity for apocalmodulin5–7.
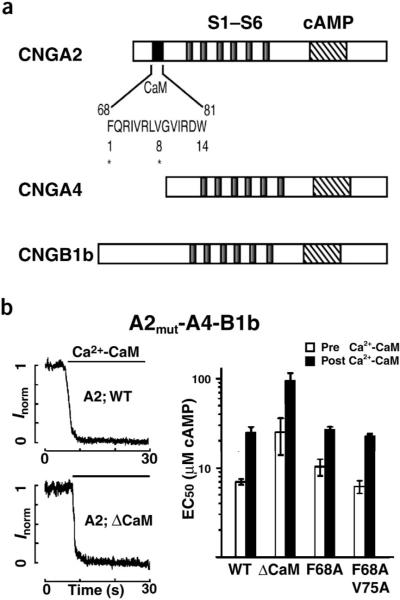
To test whether, in the context of the native heteromeric configuration of the channel, the Baa motif of CNGA2 participates either in the binding of apocalmodulin or in subsequent channel modulation by Ca2+-CaM, we repeated the preassociation experiment with heteromeric channels containing a mutant CNGA2 subunit, CNGA2(F68A V75A) (CNGA2mut). This mutation abolishes both the binding of Ca2+-CaM to CNGA2 in vitro and the modulation by Ca2+-CaM of homomeric CNGA2 channels7. CNGA2mut-A4-B1b channels showed preassociation and inhibition kinetics that were similar to heteromeric channels containing a wild-type CNGA2 (Fig. 2d). Thus, integrity of the Baa motif in CNGA2 is not required either for interaction with apocalmodulin or for modulation by Ca2+-CaM in heteromeric channels.
The Baa motif of CNGA2 is irrelevant to Ca2+-CaM modulation
To test for any relevance of the CNGA2 Baa CaM binding site in the heteromeric channel complex, we expressed a CNGA2 mutant that lacked this site completely5–7 (A2ΔCaM; corresponding to del 86 in ref. 5) together with CNGA4 and CNGB1b as heteromeric channels in HEK 293 cells (Fig. 3). In excised inside-out membrane patches, the kinetics of modulation by Ca2+-CaM (50 μM Ca2+ and 500 nM calmodulin) of CNGA2ΔCaM-A4-B1b channels was largely similar to those for wild-type CNGA2-A4-B1b channels (Fig. 3b). The same was also found with two other CNGA2 mutants when they were expressed with CNGA4 and CNGB1b; these mutants contained either a single-residue substitution, F68A, or the double substitution F68A V75A (data not shown and Fig. 3b). These substitutions abolish both the in vitro binding of Ca2+-CaM to CNGA2 and the modulation by Ca2+-CaM of homomeric CNGA2 channels7. Notably, the Baa motif in the N terminus of CNGA2 was necessary for the high cAMP efficacy of heteromeric channels in the absence of Ca2+ (Fig. 3b; compare open bars), as was shown previously for homomeric CNGA2 channels9,15. We found, however, that the integrity and even the presence of the Baa motif in CNGA2 were not required for Ca2+-CaM modulation of heteromeric channels (Fig. 3b). This result indicates that in heteromeric channels, Ca2+-CaM modulation and the autoexcitatory mechanism mediated by the N terminus of CNGA2 are unrelated.
Figure 3.
Ca2+-CaM modulation of CNGA2-A4-B1b channels does not involve the N-terminal Baa motif of CNGA2. (a) Schematic representation of the three rat olfactory CNG channel subunits, showing transmembrane regions S1–S6 (gray) and binding sites for calmodulin (black) and cAMP (crosshatching). Asterisks in the calmodulin-binding site identify amino acids targeted for mutagenesis; numbers are signature residues of the 1-8-14-type Baa Ca2+-CaM-binding sites. (b) Left, current decline owing to channel inhibition by 500 nM calmodulin in 50 μM Ca2+ (black bar) in inside-out patches of HEK 293 cells expressing (upper) CNGA2-A4-B1b or (lower) CNGA2mut-A4-B1b channels that lack the 1-8-14-type Ca2+-CaM binding site (deletion of residues 61–90). All records were obtained at +40 mV with 50 μM Ca2+ and 10 μM cAMP for all subunit compositions, except for those with A2ΔCaM, where 30 μM was used. Currents were normalized to the amplitude of the CaM effect. Right, concentrations for half-maximal channel activation (EC50; mean ± s.d. of five cells) without calmodulin (open bars) and with fully developed Ca2+-CaM modulation (black bars) of CNGA2-A4-B1b channels and CNGA2mut-A4-B1b channels.
IQ-type apocalmodulin binding sites control modulation
In the absence of a role for CNGA2, how does calmodulin associate with the native heteromeric configuration of the channels, and by what mechanism does calmodulin facilitate Ca2+-dependent feedback modulation of cAMP sensitivity? To answer these questions, we began by examining CNGB1b. The gene encoding CNGB1b is expressed as several splice variants (ref. 15 and references therein). Variant CNGB1a is expressed in retinal rod photoreceptors and has two previously identified calmodulin binding sites (CaM1 and CaM2), both of which are retained in CNGB1b (Fig. 4a). In addition, CNGB1b has a unique 74 amino acids at the beginning of its N terminus. Otherwise, CNGB1a and CNGB1b are identical11,13. CaM1, termed an unconventional calmodulin-binding site, is located in the N terminus of CNGB1 subunits and confers a weak ability of Ca2+-CaM to alter cGMP sensitivity of native heteromeric rod CNGA1-B1a channels22,23. CaM2, a Baa motif, resides in the C terminus of CNGB1 subunits and has not been ascribed any function22,23. Expression of CNGA2 and CNGA4 with a CNGB1b mutant lacking either CaM2 (B1bΔCaM2) or the unique 74-residue sequence (B1bΔ1–74) did not alter the modulatory effect of Ca2+-CaM, whereas a mutant that lacked CaM1 (B1bΔCaM1) led to a loss of all sensitivity to Ca2+-CaM in the heteromeric channels (Fig. 4b). Between residues 183 and 193 of CNGB1b (LQELVKMEKER), CaM1 resembles a generalized IQ-type calmodulin-binding motif ({I,L,V}QxxxRxxxx{R,K})23, which is understood to mediate binding of apocalmodulin24–26. A mutation affecting a single key residue, L183E, in this site specifically eliminated Ca2+-CaM modulation of heteromeric CNG channels, while at the same time maintaining the high cAMP sensitivity in the absence of Ca2+ (Fig. 4b). Thus the integrity of a binding site for apocalmodulin in CNGB1b is necessary for Ca2+-CaM modulation of native channels but not for high sensitivity to cAMP.
Figure 4.
Calmodulin-binding sites in CNGB1b and CNGA4 that mediate Ca2+-CaM modulation of CNGA2-A4-B1b channels. (a) Schematic representation of the three rat olfactory CNG channel subunits, showing transmembrane regions S1–S6 (gray) and binding sites for calmodulin (black) and cAMP (crosshatching). Sequence represents the IQ-type apocalmodulin-binding site in the N terminus and 1-8-14-type Ca2+-CaM-binding site in the C terminus of CNGB1b. Numbers are signature residues of the 1-8-14-type motif in CNGB1b; asterisk in CaM1 identifies leucine, which was targeted for mutagenesis. (b) Left, CNGA2-A4-B1b channels become insensitive to Ca2+-CaM after deletion of the IQ-type CaM1 site of the CNGB1b subunit or an L183E substitution in CaM1. Deletion of the CaM2 site or the N-terminal 74 CNGB1b-specific residues does not compromise calmodulin sensitivity. Right, concentrations for half-maximal channel activation (EC50; mean ± s.d. of five cells) without calmodulin (open bars) and with fully developed Ca2+-CaM modulation (black bars) of CNGA2-A4-B1b channels and CNGA2-A4-B1bmut channels. (c) Schematic representation of the three rat olfactory CNG channel subunits, showing transmembrane regions S1–S6 (gray) and binding sites for calmodulin (black) and cAMP (crosshatching). Sequence represents the IQ-type apocalmodulin binding site in the C-linker of CNGA4; asterisk identifies leucine 292, which was targeted for mutagenesis (d) Left, channel inhibition is lost when CNGA2-A4-B1b channels contain a mutant CNGA4 subunit in which a L292E exchange disables the IQ-like calmodulin-binding site in the C-linker. Right, CNGA2-A4mut-B1b channels examined as in b. All records were obtained at +40 mV with 50 μM Ca2+ and 10 μM cAMP for all subunit compositions. Currents were normalized to the amplitude of the CaM effect.
Because CNGA4 is also necessary for rapid binding and modulation by Ca2+-CaM of native channels10, we scanned it for calmodulin-binding sites27. We found in CNGA4, between the sixth transmembrane domain (S6) and the cAMP-binding site in the cytoplasmic C terminus, an IQ-type motif (LQHVNKRLERR) that is very similar in sequence to the CaM1 site in CNGB1a and CNGB1b (Fig. 4c). This region, termed the C-linker, is thought to be an integral part of the gating machinery of CNG channels (for review, see ref. 15). As for L183E of CNGB1b, a single amino acid substitution (L292E) in this IQ site of CNGA4 also rendered heteromeric channels completely insensitive to inhibition by Ca2+-CaM, while maintaining the high cAMP sensitivity in the absence of Ca2+ (Fig. 4d; compare open bars). Heteromeric channels with mutant IQ motifs in both CNGA4 and CNGB1b, but with an intact Baa motif in CNGA2 (CNGA2-A4(L292E)-B1b(L183E)), also did not respond at all to Ca2+-CaM (Fig. 4d), supporting our conclusion that CNGA2 has no function in native channel modulation by Ca2+-CaM.
DISCUSSION
Although the Baa motif in CNGA2 does not mediate the effect of Ca2+-CaM in the native heteromeric channels, it is clear that it has an autoexcitatory function (it enhances the open probability; Fig. 3b), as it does in homomeric CNGA2 channels. Because modulation of heteromeric channels by Ca2+-CaM persists after deletion of this site in CNGA2, however, we consider that the accepted mechanism for channel modulation, in which Ca2+-CaM acts by simply interfering with this autoexcitatory domain4–8, is incorrect. Thus, the autoexcitatory site of CNGA2 seems to exemplify a number of Baa motifs that, although characterized in vitro as Ca2+-CaM binding sites, have in vivo functions that are unrelated to calmodulin7,28,29. The amphiphilic helix of CNGA2 causes high cAMP sensitivity in properly assembled heteromeric channels, probably by interacting with other parts of the channel subunits. Calmodulin binding in CNGA2 homomers, however, is probably an artifact arising from homomeric subunit assembly.
Our results indicate that two IQ-type apocalmodulin binding sites, located on CNGA4 and CNGB1b, act together in heteromeric olfactory CNG channels to mediate feedback modulation of cAMP sensitivity by Ca2+. The preassociation of apocalmodulin with the channel ensures rapid feedback modulation because of the proximity of the Ca2+ sensor to the Ca2+ source. The speed of feedback is further maximized by the fact that the modulation rate is independent of channel open probability10.
Our experiments indicate that the steady-state amount of apocalmodulin that is bound to channels before odorant stimulation depends on the free concentration of both calmodulin and Ca2+ in the sensory cilia. The total calmodulin concentration is estimated to be 1–10 μM20 and the resting Ca2+ concentration is near 0.1 μM30. Our results indicate that under these conditions a significant fraction of the sites on the channel complex are occupied by apocalmodulin.
The stoichiometry of the native olfactory channel complex is now thought to be 2 CNGA2:1 CNGA4:1 CNGB1b (J. Zheng and W.N. Zagotta, Biophys. J. Abstr. 84, 138A, 2003). Recent NMR studies indicate that the CaM1 site on CNGB1b forms a 2:1 complex with Ca2+-CaM and that both the N- and C-terminal lobes of the calmodulin molecule in that complex are involved in binding to the two targets31. This indicates that each calmodulin molecule may be able to link two distinct CaM1-like binding sites, possibly the two sites on CNGA4 and CNGB1b. In olfactory CNG channels, CNGA4 and CNGB1b increase cAMP sensitivity, so that cAMP concentrations of a few micromolar are enough to open the heteromeric channels11,13. Apocalmodulin seems to function as a cAMP sensitivity switch for the channel, blocking the effects of either CNGA4 or CNGB1b, or both, when triggered by Ca2+. Permanently associated apocalmodulin and dimerization of CNGA4 and CNGB1b by Ca2+-CaM seem to underlie the Ca2+-dependent reduction in cAMP sensitivity of native olfactory CNG channels and may represent the molecular mechanism of transduction-channel modulation during rapid adaptation in vertebrate OSNs.
METHODS
Recording from channels of OSNs
OSNs were isolated from 4- to 6-week-old Sprague-Dawley rats as described4, except for the addition (after trypsin digestion) of a 15-min incubation at 22–25 °C with DNase I (Roche 104 159) in a solution containing 140 mM NaCl, 2 mM MgCl2, 10 mM HEPES and 2 mM EGTA (pH 7.4; adjusted with NaOH). After trituration of the tissue, cells were plated on concanavalin A–coated coverslips and allowed to settle for 30 min before transfer to the recording chamber with a bath solution containing 140 mM NaCl, 20 mM HEPES, 25 mM glucose, 2 mM CaCl2 and 1 mM MgCl2 (pH 7.4; adjusted with NaOH). OSNs are bipolar cells, with single dendrites terminating at their apical ends in knob-like structures from which emanate 10–20 sensory cilia. Cilia are often lost during tissue dissociation, and patches for inside-out recordings were taken from the dendritic knobs. Pipette solution and 0 Ca2+ superfusion solution were the bath solution without added divalent cations and with 10 mM EGTA (pH 7.4). Superfusion solutions with 50 μM Ca2+ were 30 mM NaCl, 110 mM methanesulfonic acid, 20 mM HEPES, 25 mM glucose, 2.5 mM Na-NTA (nitrilotriacetic acid) and 0.78 mM CaCl2 (pH 7.4; adjusted with NaOH). Recordings were made at chloride equilibrium potential (−40 mV) to suppress Ca2+-activated chloride currents.
Recording from heterologously expressed channels
Transient expression of CNG channels was driven by insertion of subunit cDNAs into pCIS (Genentech). Cells were recorded from approximately 2 d after Ca2+-phosphate-mediated transfection of subconfluent cells in 35-mm dishes. Cells in medium were incubated for 16–20 h with 0.125 ml of a 0.5-ml precipitate (5 μg channel plasmids at a 1:1:1 ratio, plus 0.2 μg of a GFP- and TAg-expressing plasmid). Cells were washed once with PBS, and the medium was replaced 16–20 h before recording with medium supplemented with 3 mM sodium butyrate. Coexpression of CNGA2, CNGA4 and CNGB1b produces heteromeric channels that have functional properties similar to those of native channels, and therefore probably the same subunit composition10–15. For inside-out recording, the pipette solution contained 140 mM NaCl, 5 mM KCl, 25 mM glucose, 20 mM HEPES, 10 mM EGTA (pH 7.4; adjusted with NaOH); the bath solution was the pipette solution without EGTA plus 2 mM CaCl2 and 1 mM MgCl2. For 0.1 μM buffered Ca2+, the pipette solution was supplemented with 2.5 mM EGTA and 1.53 mM CaCl2. Test solutions of 1, 3, 10 or 30 μM Ca2+ were pipette solution with 2.5 mM HEDTA and 1.09, 1.39, 2.22 or 2.34 mM CaCl2,respectively. Ca2+ solutions of 50 or 200 μM Ca2+ were the pipette solution with 2.5 mM Na-NTA and 0.78 or 1.76 mM CaCl2, respectively. Before recording, we washed the inside-out patch extensively (usually 10 min) in 10 mM EGTA until the current elicited with 7.5 μM cAMP, in either 50 μM Ca2+ or 200 μM Ca2+(ref. 17), was stable and approximately half the current in 150 μM cAMP. Maximal cAMP-induced current (Imax) was recorded at −40 mV with 150 μM cAMP in 140 mM NaCl, 5 mM KCl, 25 mM glucose, 20 mM HEPES, 2.5 mM Na-NTA, 0.78 mM CaCl2 (pH 7.4; adjusted with NaOH). Calmodulin (0.1 or 0.5 μM) and Ca2+ (indicated free concentrations) were applied with 7.5 μM cAMP in test solutions. Currents were normalized to Imax (Fig. 2) or to the maximal amplitude of the CaM response, ΔICaM, according to Inorm(t) = ICaM(t)/ΔICaM (Figs. 3 and 4) where ICaM(t) is the calmodulin-sensitive part of the current. All plots of Inorm(t) thus run from unity (before addition of calmodulin) to zero (at steady-state channel modulation).
Mutagenesis
Site-directed mutagenesis was carried out by standard procedures and constructs were sequenced before functional assay.
ACKNOWLEDGMENTS
We thank J. Reisert and V. Bhandawat for many discussions; J. Kehoe, B. Barbour, N. Pardigon, J. Lynch, S. Hattar, R. Kuruvilla and P. Bauer for comments; P. Ascher for critically reading the manuscript and V. Kefelov for suggesting the experiment for Figure 2c. J.B. dedicates this paper to Norman Davidson. This work was supported by the Deutsche Forschungsgemeinschaft under grant SPP 1025 (S.F.) and by the Howard Hughes Medical Institute (J.B. & K.-W.Y.).
Footnotes
COMPETING INTERESTS STATEMENT The authors declare they have no competing financial interests.
References
- 1.Frings S. Chemoelectrical signal transduction in olfactory sensory neurons of air-breathing vertebrates. Cell Mol. Life Sci. 2001;58:510–609. doi: 10.1007/PL00000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- 3.Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- 4.Chen TY, Yau KW. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Chen TY, Ahamed B, Li J, Yau KW. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 6.Varnum MD, Zagotta WN. Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- 7.Grunwald ME, Zhong H, Lai J, Yau KW. Molecular determinants of the modulation of cyclic nucleotide-activated channels by calmodulin. Proc. Natl. Acad. Sci. USA. 1999;96:13444–13449. doi: 10.1073/pnas.96.23.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Varnum MD, Zagotta WN. Disruption of an intersubunit interaction underlies Ca2+-calmodulin modulation of cyclic nucleotide-gated channels. J. Neurosci. 2003;23:8167–8175. doi: 10.1523/JNEUROSCI.23-22-08167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trudeau MC, Zagotta WN. Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels. J. Biol. Chem. 2003;278:18705–18708. doi: 10.1074/jbc.R300001200. [DOI] [PubMed] [Google Scholar]
- 10.Bradley J, Reuter D, Frings S. Facilitation of calmodulin-mediated odor adaptation by cAMP-gated channel subunits. Science. 2001;294:2176–2178. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 11.Bönigk W, et al. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 1999;19:5332–5347. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley J, Frings S, Yau KW, Reed R. Nomenclature for ion channel subunits. Science. 2001;294:2095–2096. doi: 10.1126/science.294.5549.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sautter A, Zong X, Hofmann F, Biel M. An isoform of the rod photoreceptor cyclic nucleotide-gated channel β subunit expressed in olfactory neurons. Proc. Natl. Acad. Sci. USA. 1998;95:4696–4701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munger SD, et al. Central role of the CNGA4 channel subunit in Ca2+-calmodulin-dependent odor adaptation. Science. 2001;294:2172–2175. doi: 10.1126/science.1063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 16.Frings S, Lynch JW, Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. J. Gen. Physiol. 1992;100:45–67. doi: 10.1085/jgp.100.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch JW, Lindemann B. Cyclic nucleotide-gated channels of rat olfactory receptor cells: divalent cations control the sensitivity to cAMP. J. Gen. Physiol. 1994;103:87–106. doi: 10.1085/jgp.103.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer RH, Siegelbaum SA. Intracellular Ca2+ regulates the sensitivity of cyclic nucleotide-gated channels in olfactory receptor neurons. Neuron. 1992;9:897–906. doi: 10.1016/0896-6273(92)90242-6. [DOI] [PubMed] [Google Scholar]
- 19.Kleene SJ. Both external and internal calcium reduce the sensitivity of the olfactory cyclic-nucleotide-gated channel to CAMP. J. Neurophysiol. 1999;81:2675–2682. doi: 10.1152/jn.1999.81.6.2675. [DOI] [PubMed] [Google Scholar]
- 20.Anholt RR, Rivers AM. Olfactory transduction: cross-talk between second-messenger systems. Biochemistry. 1990;29:4049–4054. doi: 10.1021/bi00469a004. [DOI] [PubMed] [Google Scholar]
- 21.O'Neil KT, DeGrado WF. How calmodulin binds its targets: sequence independent recognition of amphiphilic α-helices. Trends. Biochem. Sci. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- 22.Grunwald ME, Yu WP, Yu HH, Yau KW. Identification of a domain on the beta-subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium-calmodulin. J. Biol. Chem. 1998;273:9148–9157. doi: 10.1074/jbc.273.15.9148. [DOI] [PubMed] [Google Scholar]
- 23.Weitz D, et al. Calmodulin controls the rod photoreceptor CNG channel through an unconventional binding site in the N-terminus of the beta-subunit. EMBO J. 1998;17:2273–2284. doi: 10.1093/emboj/17.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Letters. 2002;513:107–113. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- 25.Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- 26.Zuhlke RD, Pitt GS, Tsien RW, Reuter H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the α1C subunit. J. Biol. Chem. 2000;275:21121–21129. doi: 10.1074/jbc.M002986200. [DOI] [PubMed] [Google Scholar]
- 27.Yap KL, et al. Calmodulin target database. J. Struct. Funct. Genomics. 2000;1:8–14. doi: 10.1023/a:1011320027914. [DOI] [PubMed] [Google Scholar]
- 28.Peng C, Rich ED, Thor CA, Varnum MD. Functionally important calmodulin binding sites in both N- and C-terminal regions of the cone photoreceptor cyclic nucleotide-gated channel CNGB3 subunit. J. Biol. Chem. 2003;278:24617–24623. doi: 10.1074/jbc.M301699200. [DOI] [PubMed] [Google Scholar]
- 29.Sencer S, et al. Coupling of RYR1 and L-type calcium channels via calmodulin binding domains. J. Biol. Chem. 2001;276:38237–38241. doi: 10.1074/jbc.C100416200. [DOI] [PubMed] [Google Scholar]
- 30.Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J. Neurosci. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orsale M, et al. Two distinct calcium-calmodulin interactions with N-terminal regions of the olfactory and rod cyclic nucleotide-gated channels characterized by NMR spectroscopy. FEBS Letters. 2003;548:11–16. doi: 10.1016/s0014-5793(03)00716-6. [DOI] [PubMed] [Google Scholar]