Abstract
Seeing begins in the photoreceptors, where light is absorbed and signaled to the nervous system. Throughout the animal kingdom, photoreceptors are diverse in design and purpose. Nonetheless, phototransduction—the mechanism by which absorbed photons are converted into an electrical response—is highly conserved and based almost exclusively on a single class of photoproteins, the opsins. In this Review, we survey the G protein-coupled signaling cascades downstream from opsins in photoreceptors across vertebrate and invertebrate species, noting their similarities as well as differences.
Introduction
The great majority of animals have photoreceptors of one sort or another for detecting food source, mate, predator/prey, orientation, or simply the light/dark cycle dictated by the movement of the sun. Such photoreceptors, whether ocular or extraocular, are generally distinguishable into two types: ciliary and rhabdomeric, depending on whether the proliferation of photosensitive membranes necessary for efficient light absorption is derived from a modified cilium or from microvillar projections of the apical cell surface forming a rhabdom (for review, Arendt, 2003; Lamb et al., 2007). Uniformly, these photoreceptors sense light with a visual pigment composed of a vitamin A-based chromophore and a seven-transmembrane-helix apoprotein, opsin. These pigments are prototypical G protein-coupled receptors (GPCRs), signaling via heterotrimeric G proteins. More than 1000 opsins have now been identified in the animal kingdom, all believed to originate from a common ancestor and separate from the structurally similar bacteriorhodopsin and channelopsins (Arendt, 2003; Terakita, 2005). Most opsins belong to two major groups: c-opsin (“c” for ciliary) and r-opsin (“r” for rhabdomeric), classified according to molecular phylogeny but also matching the corresponding cell types with which the pigments are associated (Figure 1A). There are also minor groups: Go-opsin, peropsin, neuropsin, encephalopsin/teleost multiple tissue (tmt) opsin, and photoisomerase (Terakita, 2005). Go-opsins mediate phototransduction in certain ciliary photoreceptors, whereas photoisomerases serve to regenerate the chromophore. The functions of the remaining groups remain unclear. Ciliary photoreceptors are characteristic of the deuterostome lineage, which includes the vertebrates, whereas rhabdomeric photoreceptors are predominantly found in protostome invertebrates such as flatworms (platy-helminthes), polychaetes, arthropods, and molluscs (Figure 1B) (Arendt, 2003). However, in most phyla, ciliary and rhabdomeric photoreceptors often coexist in the same organism, implying that they arose before the protostome/deuterostome split ~550 million years ago and have evolved independently since then (Arendt, 2003). Not surprisingly, there is an enormous diversity of animal photoreceptors, organized into elaborate eyes, simple eyespots, or just cell clusters, or even as isolated photoreceptive cells.
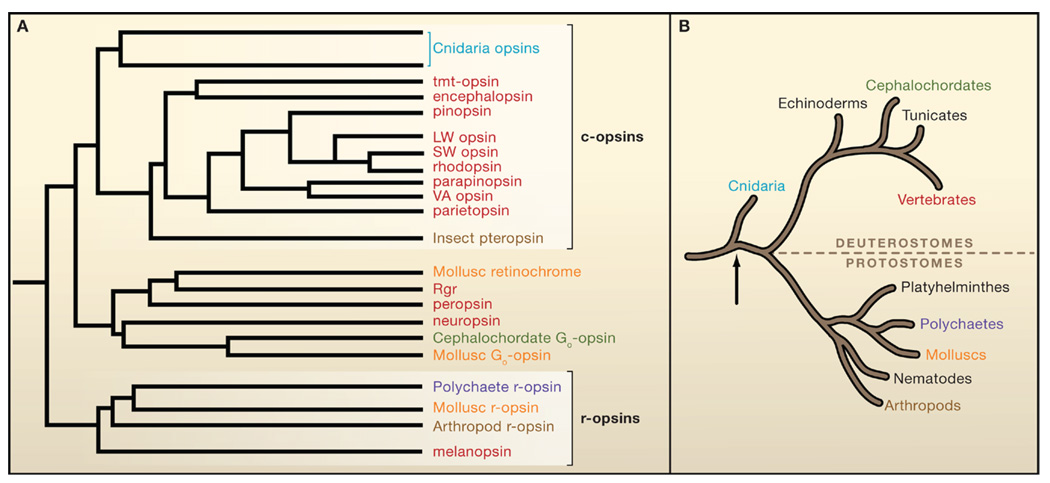
Figure 1. Opsin Phylogenetic Tree.
(A) Schematic phylogenetic tree of the opsin family. Depicted opsins (vertebrate unless otherwise stated) are color-coded with respect to the phyla of origin shown in (B). There are two main groups: c-opsins and r-opsins, together with a miscellaneous group (Go-opsin, etc.) more closely related to c-opsins than r-opsins. Branch lengths are arbitrary. Simplified from that originally derived with a maximum-likelihood algorithm by Suga et al. (2008).
(B) Simplified evolutionary tree of present-day animal phyla. Modern-day bilaterians comprise the protostome invertebrates and the deuterostomes, which include the vertebrate lineage. These two lines diverged ~550 million years ago (Mya), by which time an “Urbilaterian,” a common ancestor, had already evolved with both rhabdomeric- and ciliary-type photoreceptors (Arendt, 2003). Arrow indicates the Cnidarian (prebilaterians)/bilaterian split.
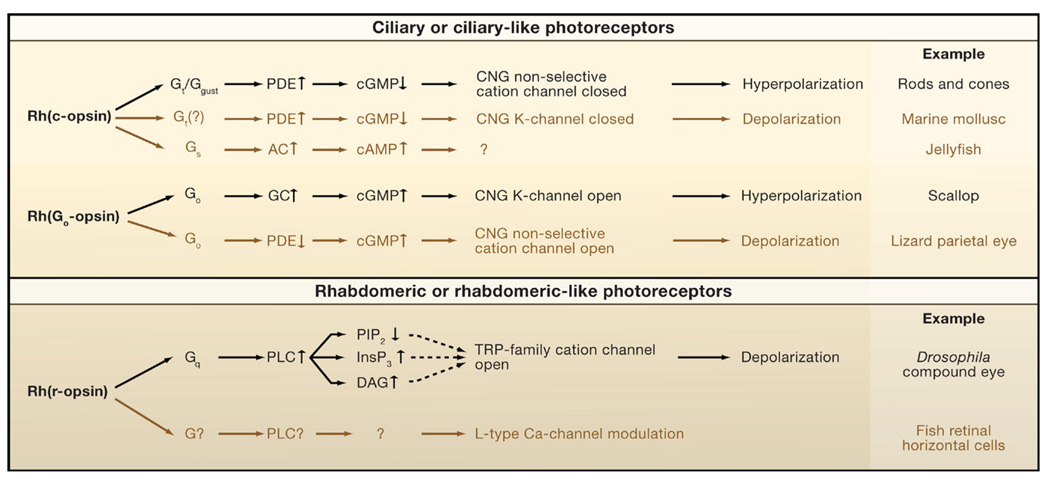
With respect to physiology, the ciliary vertebrate rods and cones hyperpolarize to light, whereas rhabdomeric photoreceptors depolarize to light. However, light-response polarity is not an absolute distinguishing feature between ciliary and rhabdomeric photoreceptors, or between vertebrates and invertebrates. One principle does seem to hold, however; namely, ciliary photoreceptors invariably use a cyclic-nucleotide motif for phototransduction, whereas rhabdomeric photoreceptors invariably use a phospholipase C (PLC) motif (Finn et al., 1997; Xiong et al., 1998; Nasi et al., 2000). Some photoreceptors with no telltale morphological features also exist, such as the newly discovered intrinsically photosensitive retinal ganglion cells in vertebrates (for review, Berson, 2007) and some simple photoreceptors in the neural ganglia of invertebrates (for review, Gotow and Nishi, 2008). Nonetheless, so far as is known, these still appear to conform to one or the other canonical motif.
In this Review, we describe the cyclic-nucleotide and PLC motifs in some detail, based on the well-studied vertebrate rod photoreceptor and Drosophila compound-eye photoreceptor. We also describe some interesting variations in details within each motif found in other photoreceptors.
Vertebrates
Retinal rods and cones underlie our conscious vision. They exist in all vertebrates and are the classic photoreceptors of study. They were long thought to be the only photoreceptors in mammals. Recently, however, this view has dramatically changed with the discovery of a novel class of photoreceptor in the inner retina, in the form of intrinsically photosensitive retinal ganglion cells. These unusual photoreceptors will be described later together with other photoreceptors found in lower vertebrates.
Rods
Rods mediate vision in dim light. Their phototransduction process is extremely well understood—arguably the best understood of all G protein-mediated signaling processes (for historical perspective, Luo et al., 2008b). It serves as a benchmark for understanding G protein-coupled processes generally and for understanding other sensory transduction processes such as in olfaction and taste. Many steps are known down to mathematical detail, and crystal structures have been solved for some components.
Phototransduction Motif
Phototransduction takes place in the cell’s ciliary outer segment, which is tightly packed with membrane discs full of visual pigment (rhodopsin, Rh; “Rh” is also adopted in this Review to denote a pigment generally). The key components of phototransduction and their interactions are shown in Figure 2. Essentially, photoisomerized, active Rh (Rh*) activates the G protein transducin (Gt), which in turn stimulates a phosphodiesterase (PDE) that hydrolyzes specifically cGMP. Both Gt and PDE are peripheral membrane proteins. In darkness, the free cGMP is at a relatively high concentration and, by direct binding, maintains cGMP-gated, nonselective cation channels on the plasma membrane in the open state. These channels, with the unusual property of showing no desensitization to ligand, maintain a steady inward current in darkness (“dark current”) and depolarize the cell sufficiently (dark membrane potential at ~−30 mV) to sustain synaptic-transmitter (glutamate) release. The light-induced, graded decrease in free cGMP closes the cGMP-gated channels, thus hyperpolarizing the cell and reducing or stopping the glutamate release. These cells do not fire action potentials. In rods, Rh, Gt, and PDE have relative concentrations of about 100:10:1.
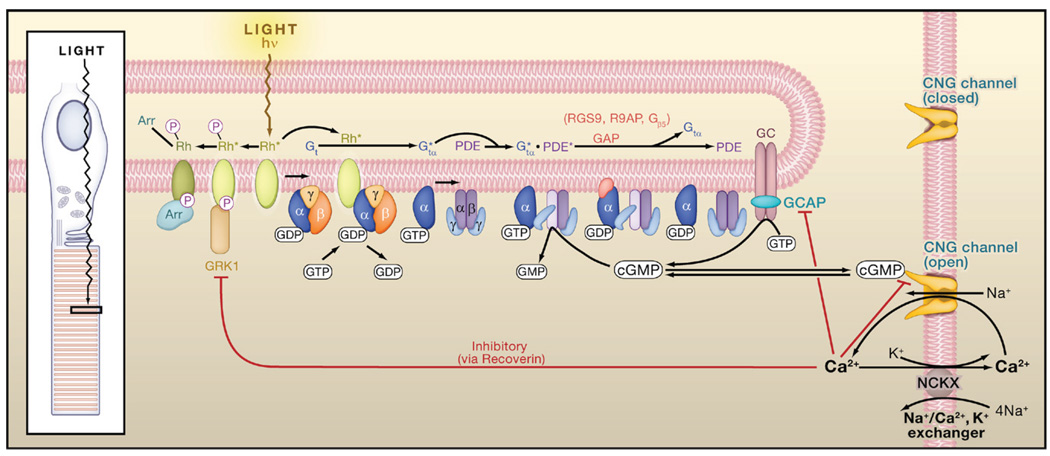
Figure 2. Phototransduction in Vertebrate Rods.
Light converts rhodopsin into an active form, Rh*, which activates heterotrimeric Gt by GTP-GDP exchange. Active Gtα (Gtα*) binds to and activates phosphodiesterase (PDE), which hydrolyzes cyclic GMP (cGMP) to GMP, thereby closing the cyclic-nucleotide-gated (CNG) channels that are open in darkness. hν, photon; Rh*-P, phosphorylated Rh*, which retains partial Rh* activity; Rh*-P-Arr, phosphorylated Rh* with arrestin bound, rendered fully inactive. Red lines ending in a small bar indicate negative-feedback (inhibitory) pathways via Ca2+. The inhibition by Ca2+ on the cGMP gating of the CNG channel is via a Ca2+-binding protein that may be calmodulin. Based on Yau (1994), Luo et al. (2008b), and Pugh et al. (1999). Inset: schematic diagram of the ciliary rod photoreceptor, with a light-sensitive outer segment formed from a highly expanded cilium.
Activation
Rh* activates Gt supposedly through random diffusional encounters between the two proteins in the disc membrane (for review, Arshavsky et al., 2002). These encounters catalyze GDP-GTP exchange on the guanine-nucleotide-binding site of the α subunit of Gt (Gtα), which constitutively binds GDP. The active Gtα (Gtα*.GTP, sometimes referred to here as simply Gtα*) then dissociates from its partnering transducin βγ subunits (Gtβγ ) and binds to a PDE γ subunit (PDEγ) (Figure 2). This binding removes the inhibition by PDEγ on the catalytic PDEαβ subunits, allowing the latter to hydrolyze cGMP. In each PDE complex, there are one PDE (PDEα and PDEβ being tightly associated with each other) and two PDEγ subunits. Thus, one Gtα* presumably stimulates only half of the activity of the PDE tetramer. Also, once Gtα binds GTP, Rh* dissociates from Gt and goes on encountering and activating other Gt’s. It was initially thought that an Rh* activates as many as 103 Gt’s during the single-photon response, which lasts ~1 s at room temperature. However, it now appears that perhaps only ~20 Gtα* molecules are produced by an Rh* during a mouse rod’s single-photon response (Krispel et al., 2006). This is nonetheless a substantial amplification in signaling. Furthermore, the high hydrolytic rate of the active PDE (PDE*) provides additional amplification. Indeed, it is the high Gtα*/Rh* signaling ratio in rod phototransduction that famously contributed to the textbook dogma of high amplification in GPCR signaling. However, it now appears that this high signaling ratio may not be a general characteristic of GPCR signaling (Bhandawat et al., 2005). Most recently, the longstanding belief that Rh exists as a monomer has also been questioned. It may, instead, be a dimer like some other GPCRs and can apparently form paracrystalline arrays (for review, Fotiadis et al., 2006; Müller et al., 2008; Wensel, 2008; Scheerer et al., 2008). This important issue remains to be resolved. Regardless of whether rhodopsin exists as a monomer or dimer, it takes only one absorbed photon, hence one Rh* molecule, to trigger phototransduction (for review, Baylor, 1987; Whorton et al., 2008).
Deactivation
The deactivation of phototransduction is complex (Figure 2) and still under investigation (for review, Burns and Baylor, 2001; Burns and Arshavsky, 2005; Luo et al., 2008a). For complete deactivation, each active component must shut down. Rh*, corresponding to the meta-II state of Rh, decays over a minute into an inactive state (meta-III). Long before this decay, however, Rh* is phosphorylated by a rhodopsin kinase (now called G protein-coupled-receptor-kinase 1, or GRK1), followed rapidly by the binding of another protein, arrestin (Arr), which recognizes phosphorylated Rh* (Rh*~P). Rh*~P still has perhaps some activity, but Rh*~P-Arr loses all activity. There are 6–7 C-terminal phosphorylation sites (serine/threonine residues) on Rh, many or all of which apparently need to be phosphorylated for the normal decay of the response; otherwise, the response decay is slowed (Mendez et al., 2000; Doan et al., 2006). Eventually, Rh, perhaps in the free-opsin state after meta-III decay/hydrolysis or in the regenerated rhodopsin state, loses its bound arrestin and is dephosphorylated, most likely through the action of a generic phosphatase, such as protein phosphatase 2A (Palczewski et al., 1989).
Gtα* deactivates itself by intrinsic GTPase activity, which converts the active Gtα*.GTP to the inactive Gtα.GDP. This GTPase activity, as in some other Gα subunits, is facilitated by a GTPase-activating-protein (GAP) complex (for review, Cowan et al., 2001). In rods, this complex consists of a protein called regulator of G-protein signaling 9 (RGS9), a RGS9-anchoring protein (R9AP), and an orphan G protein β subunit (Gβ5), together with the substrate of Gtα*, PDEγ (Burns and Arshavsky, 2005). The requirement for PDEγ ensures that Gtα*. GTP has found and activated its substrate before deactivation occurs. Upon GTP hydrolysis, the resulting Gtα.GDP dissociates from PDEγ and reassociates with Gtβγ, allowing PDEγ to resume its inhibition of PDEαβ (Figure 2). It is thought that PDEγ never physically dissociates from PDEαβ during PDE activation but is simply sterically displaced. Because the stoichiometry of PDE is 1PDEαβ:2PDEγ, both constituent PDEγ’s presumably have to be restored before the enzyme declines fully to its dark state. Some dark PDE activity does exist, which balances constitutive guanylate cyclase (GC) activity to maintain a steady free cGMP concentration of ~1 µM (for review, Yau, 1994). This dark PDE activity comes from the constitutive “rocking” of PDEγ on PDEαβ (Rieke and Baylor, 1996), causing intermittent PDEαβ activity and producing dark-current noise (Baylor, 1987). Finally, although cytoplasmic free cGMP is ~1 µM, the total cGMP concentration in the rod outer segment is much higher, at ~60 µM, almost all of which is tightly bound to noncatalytic sites on PDE (~30 µM PDE tetramers, with a single noncatalytic binding site on each of PDEα and PDEβ). The bound cGMP does not readily exchange with the free cGMP and is released only when free cGMP decreases to a very low level in bright light, supposedly to modulate the PDEαβ catalytic activity (Cote et al., 1994).
Transducing Ion Channel
The cGMP-gated cation channel is a tetrameric complex composed of A and B subunits, each with a single cGMP-binding site on its cytoplasmic C terminus. These belong to the small family of cyclic-nucleotide-gated (CNG) cation channels (for review, Yau and Baylor, 1989; Finn et al., 1996; Kaupp and Seifert, 2002; Hofmann et al., 2005) and correspond to CNGA1 and CNGB1. The rod-channel complex has an unusual, asymmetrical 3A:1B stoichiometry (e.g., Zhong et al., 2002). The channel has a moderate affinity for cGMP (K1/2 ~50 µM; activation Hill coefficient of 2.5–3.0). With free cGMP at ~1 µM in darkness, only ~1% of the channels, or ~104 out of an overall 106 channels, are open (Yau, 1994). This may seem wasteful because light only closes these channels. However, the low affinity ensures that the cGMP already bound to the open channels in darkness will dissociate rapidly when cytosolic cGMP falls, thus providing a fast response to light (Yau and Baylor, 1989).
Being nonselective to cations, the rod cGMP-gated channel (and CNG cation channels in general) is permeable to monovalent and divalent cations. The divalent cations, Ca2+ and Mg2+, partially block the channel as they permeate through (Yau, 1994), with the resulting fast “flicker” block producing a very small effective single-channel conductance of ~0.1 pS. This small conductance minimizes open-channel noise for a given dark-current amplitude, effectively increasing the signal-to-noise ratio of the light response (Yau and Baylor, 1989).
Ca2+ Feedback
Besides the timely terminations of Rh*, Gtα*, and PDE*, the decline of the light response is speeded by multiple negative-feedback mechanisms mediated by Ca2+ (Figure 2; for review, Koutalos and Yau, 1996; Pugh et al., 1999; Fain et al., 2001). Ca2+ carries ~15% of the dark inward current, the rest being carried largely by Na+ (Yau, 1994). In darkness, this steady Ca2+ influx is balanced by an equal Ca2+ efflux via a Na/Ca,K exchanger (NCKX) on the outer-segment plasma membrane, which couples Na+ influx to Ca2+ and K+ effluxes (4Na+:1Ca2+:1K+ stoichiometry) (for review, Schnetkamp, 2004). In the light, the closure of cGMP-gated channels reduces or stops the Ca2+ influx, but the Ca2+ efflux continues, thus lowering the intracellular free Ca2+ concentration (Yau, 1994). This Ca2+ decrease has three effects. First, the GC activity increases. The reason is that the GC activity requires two guanylate cyclase-activating proteins, GCAP1 and GCAP2, which are Ca2+-binding proteins that are negatively modulated by Ca2+ when it binds to their EF-hands (for review, Palczewski et al., 2004). In darkness, the relatively high free Ca2+ concentration (~600 nM) keeps the GC largely in check. In the light, the Ca2+ decrease disinhibits the GCAPs, thus elevating the GC activity to chase after the light-stimulated PDE activity, producing negative feedback. Second, GRK1 is negatively modulated by Ca2+ through another EF-hand-containing, Ca2+-binding protein called recoverin or S-modulin (for review, Kawamura and Tachibanaki, 2002), so that Rh* phosphorylation (and hence arrestin binding) is moderately slow in dim light but accelerates when Ca2+ progressively decreases in brighter light, reducing the active lifetime of Rh* and thus the amplification (i.e., the Gtα*/Rh* signaling ratio decreases). Third, high Ca2+ (possibly via calmodulin) reduces the affinity of cGMP for the channel, so some channels initially closed by light reopen as Ca2+ falls (for review, Warren and Molday, 2002). Na/Ca exchangers (NCXs), which employ the inward-directed Na+ electrochemical gradient to extrude Ca2+, are found in most cells, but NCKX, first found in rods, has a much more restricted presence. The additional outward K+ movement (driven by the outward-directed K+ electrochemical gradient) provides extra driving force in order to reduce intracellular Ca2+ to a level lower than can be achieved by Na+ alone (Cervetto et al., 1989). The steady Na+ and K+ fluxes through the cGMP-gated channels and the exchanger in darkness require an active Na/K ATPase at the inner segment (adjacent to the outer segment) and cell body to maintain the respective electrochemical gradients. The associated large energy consumption is presumably met by the densely packed mitochondria adjoining the outer segment. In fact, the outer retina has an extremely high oxygen-consumption rate (Braun et al., 1995; Okawa et al., 2008). Some of the details in the modulation of the GC by the GCAPs and in the modulation of GRK1 by recoverin are still being actively studied.
Light Adaptation
The Ca2+ feedback already takes effect during the rod’s response to a dim flash, even during that to a single photon, and contributes to the speedy recovery of the cell after the flash. In steady light, the same feedback leads to “background-light adaptation,” manifested as a reduced sensitivity of the cell (i.e., a smaller response to a criterion test flash) and faster response kinetics (Koutalos and Yau, 1996; Pugh et al., 1999; Fain et al., 2001). At low and intermediate light intensities, the Ca2+ feedback via the GC modulation is dominant; at higher intensities, the feedback via the GRK1 modulation begins to kick in, becoming increasingly important with increasing light. Thus, there is a division of labor. The feedback via the channel modulation is weak and relatively unimportant (Koutalos and Yau, 1996). The ability of rods to adapt to light is nonetheless quite limited, and they become useless for vision when their response saturates in moderately bright light.
The high cGMP flux rate (i.e., continuous hydrolysis and synthesis) under steady light also contributes to light adaptation (Pugh et al., 1999). Thus, with a higher steady PDE activity, the fractional increase in PDE per additional absorbed photon becomes smaller (hence lower sensitivity), and the post-light recovery of cGMP also becomes faster owing to a correspondingly higher GC activity.
Even in complete darkness, the Ca2+ feedback has the critical role of suppressing any excessive fluctuations in the free cGMP concentration, which otherwise, apart from producing noise, could be detrimental to the cell by occasionally opening an excessive number of cGMP-gated channels (Yau, 1994; Burns et al., 2002).
Single-Photon Response and Its Invariance
When dark-adapted, an amphibian rod’s response to a single absorbed photon is ~1 pA in size, or ~3% of the maximum (saturated) light response (Baylor, 1987). As few as ~30 absorbed photons will produce a half-maximal response. These values broadly apply to mammalian rods as well (the mouse-rod single-photon response is ~0.5 pA). Thus, rods are immensely sensitive to light. Indeed, it has long been known that a rod can signal the absorption of a single photon to the postsynaptic cell, and it takes only several absorbed photons in a small area of the retina for a fully dark-adapted human subject to report a light flash (Hecht et al., 1942). Finally, the single-photon response is remarkably constant in amplitude and kinetics (Baylor, 1987). In principle, the lifetime of a single Rh* molecule should be stochastic, with a probability distribution described by a single-exponential decline; consequently, the same should happen to the amplitude and kinetics of the single-photon response, thereby jeopardizing the signal-to-noise ratio. The observed response parameters are actually much tighter in dispersion than expected. This longstanding mystery appears better understood now. Based on recent findings, the constancy in amplitude may be ascribed to the intrinsic averaging due to multiple phosphorylations of Rh* (Doan et al., 2006), and the constancy in response kinetics may be ascribed to the averaging due to multiple Gtα molecules being activated per Rh* (Krispel et al., 2006). After much debate, the decay time course of the response now appears to be dominated by the deactivation of Gtα* (i.e., this is the slowest step), which lasts ~200 ms in mouse (Krispel et al., 2006). It remains to be seen whether this important question is finally settled.
Transduction Noise
Rh is very stable in darkness (half-life ~103 years at room temperature), but spontaneous (thermal) isomerization events triggering transduction are nonetheless occasionally detectable (Baylor, 1987), owing to the high pigment content (~108–109 Rh molecules) in a rod. Such spontaneous events, at ~1 event per minute in a mouse or human rod, produce noise that interferes with light detection especially at threshold (Barlow, 1957). In addition, as mentioned earlier, there is continuous PDE noise in darkness. The actual dark noise experienced by a human subject in classic psychophysical experiments is close to the rate of spontaneous isomerization of Rh. Thus, the PDE noise may be mostly filtered out at subsequent synapses (for review, Field et al., 2005). The same presumably happens to any noise originating from the opening of cGMP-gated channels, which has even faster kinetics than the light response.
After deactivated Rh* dissociates into opsin and the chromophore (i.e., in the bleached state), the free opsin nonetheless retains a constitutive, albeit extremely low, activity (~10−6 of Rh*) (Fain et al., 2001). When the bare opsin level accumulates and becomes substantial (say, ≥1% of total pigment after a strong light), this residual activity can be significant and resembles the presence of a steady light, causing adaptive change by the rod. In other words, Rh bleaching reduces sensitivity far more than would be expected from simply a lower photon catch due to a lower Rh content. This phenomenon is called “bleaching adaptation” (for review, Fain et al., 2001; Lamb and Pugh, 2004). In this sense, the chromophore, 11-cis-retinaldehyde (11-cis-retinal), acts as a negative agonist of opsin in darkness to suppress its constitutive activity.
Pigment Cycle
There have been major advances in our understanding of pigment regeneration in recent years (for review, Lamb and Pugh, 2004; Muniz et al., 2007; Travis et al., 2007). The visual pigment consists of the protein moiety, opsin, and the chromophore, a derivative of vitamin A called 11-cis-retinal, which are covalently linked by a protonated Schiff base. Most vertebrates use the same chromophore (11-cis-retinal, except for some amphibian and aquatic species, which use 11-cis-3 dehydroretinal). Light isomerizes 11-cis-retinal to all-trans-retinal, followed rapidly by several spontaneous conformational changes in opsin that lead to the active state, meta-II (Rh*), within ~1 ms. Meta-II eventually decays to inactive meta-III, followed by the latter’s hydrolysis into opsin and free all-trans-retinal. All-trans-retinal is reduced to all-trans-retinol, exits the cell, and travels (helped by the interphotoreceptor retinoid-binding protein, IRBP, an extracellular carrier protein) to the overlying retinal pigment epithelial cell, where it is reconverted by an elaborate chemical reaction (for review, Rando, 2001; Travis et al., 2007) into 11-cis-retinol, then 11-cis-retinal, and returned to the rod for spontaneous combination with opsin to reform the holopigment (Figure 3A, left). After extensive search, the key enzyme for this reisomerization, a retinyl isomerohydrolase, has been identified (RPE65) (Jin et al., 2005; Moiseyev et al., 2005; Redmond et al., 2005). Because the chromophore is highly hydrophobic, intracellular carrier proteins (CRBP and CRALBP) are involved in its shuttling.
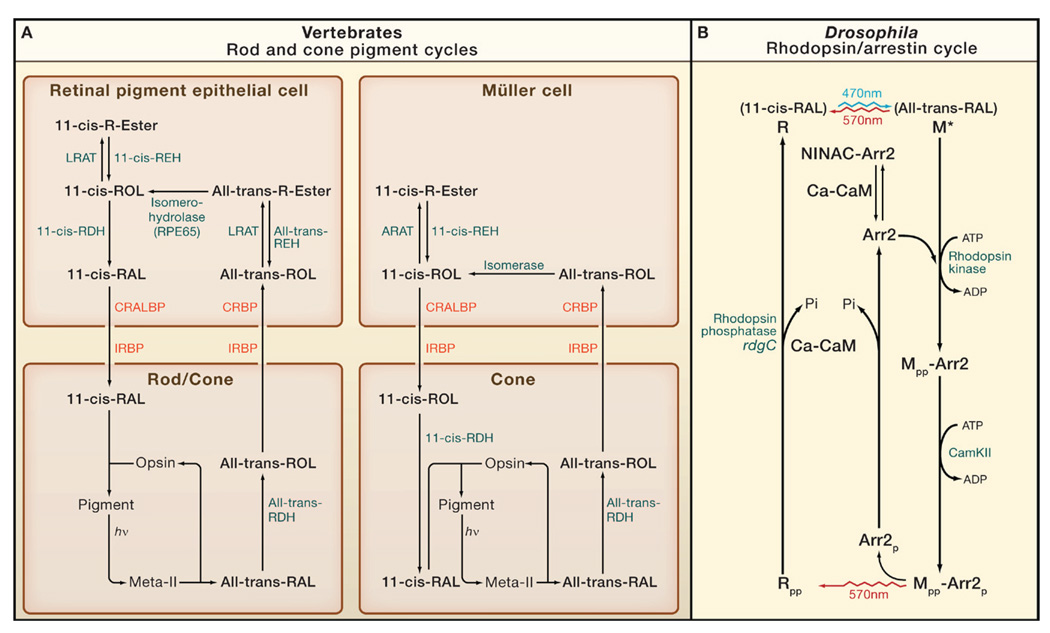
Figure 3. Pigment Cycles in Vertebrate and Drosophila Photoreceptors.
(A) Rods and cones. There are two cycles: one involving the retinal pigment epithelial cell and used by both rods and cones, and the other involving the Müller cell and used exclusively by cones (Travis et al., 2007 and Muniz et al., 2007). hν, photon; RAL, retinal; ROL, retinol; R-ester, retinyl ester; IRBP, interphotoreceptor retinoid-binding protein; CRBP, cellular retinol-binding protein; CRALBP, cellular retinaldehyde-binding protein; RDH, retinol dehydrogenase; REH, retinyl ester hydrolase; LRAT, lecithin:retinol acyl transferase; ARAT, acyl-CoA:retinol acyl transferase. LRAT and RPE65 are absent in Müller cells. The isomerase and ARAT in Müller cells are kinetically coupled and together named isomerosynthase.
(B) Drosophila photoreceptor. Blue light (470 nm) photoisomerizes 11-cis-3-hydroxy-retinal in rhodopsin (R) to all-trans 3-hydroxy-retinal (top), generating active metarhodopsin (M*). Photoregeneration is achieved simply by long-wavelength light (570 nm), which reisomerizes all-trans to 11-cis, thereby reconverting M to R irrespective of whether it is phosphorylated (indicated by “-pp”) or bound to arrestin (Arr2). M* is thermostable and continues to activate Gq until it binds Arr2. M is also phosphorylated by rhodopsin kinase (RK) on C-terminal serines, but this is not required for Arr2 binding or response termination. The Mpp-Arr2 state is a target for clathrin-mediated endocytosis, but this endocytosis is inhibited by the CaMKII-dependent phosphorylation of Arr2. After Mpp is photoreconverted to Rpp, Arr2 is released, as long as it has been phosphorylated (Arr2p). Rpp is dephosphorylated by Ca-CaM-dependent rhodopsin phosphatase (coded by rdgC) to recreate the ground state, R. Prior to Ca2+ influx, Arr2 is prevented from binding to M*, by being bound to NINAC or a NINAC-regulated target. Once channels open to allow Ca2+ influx, Ca-CaM releases Arr2 to allow it to rapidly bind to M* (Liu et al., 2008).
Cones
Cones mediate vision in bright light. They also mediate color vision by virtue of there being more than one spectral cone type in the retina. Qualitatively, cone phototransduction is similar to that in rods (Yau, 1994; Luo et al., 2008a). There are, however, quantitative differences. Notably, the cone single-photon response is typically 102-fold smaller than that of rods and individually undetectable, as well as several-fold faster in kinetics (Baylor, 1987). Their faster responses make them better motion detectors than rods. Cones also adapt to light much more effectively than rods. The adaptive properties of cones, and the underlying mechanisms, still require much exploration. On the whole, cone phototransduction is less well understood than rod phototransduction. Nonetheless, knowledge is steadily emerging.
Besides the pigment, the other primary phototransduction proteins, including Gtα, Gtβγ, PDE, and cGMP-gated channel, also have different isoforms in rods and cones (for review, Fu and Yau, 2007; Kawamura and Tachibanaki, 2008). The cone PDE, like the rod PDE, is a tetramer but has only two different subunits: one catalytic (PDEα’) and the other inhibitory (PDEγ’), in a stoichiometry of 2PDEα’:2PDEγ’. The cone cGMP-gated channel is composed of CNGA3 and CNGB3, in a reported symmetrical 2CNGA3:2CNGB3 stoichiometry (Peng et al., 2004), different from the rod channel and still surprising. The situation with GC is complex. There are two homologous GCs: retGC-1 and retGC-2 (both belonging to the multimember “particulate” family of GCs, with a single transmembrane domain). In mouse, rods have both forms, whereas cones primarily express retGC-1. Similarly, rods have both GCAP1 and GCAP2, but cones primarily express GCAP1. There are two pigment kinases, GRK1 and GRK7. GRK1 is present in rods, but GRK1 and GRK7 are typically both present in cones. Mouse is an exception to this, as it only has GRK1, present in both rods and cones. There are also rod and cone versions of arrestin; in at least mouse, however, cones express both versions (Nikonov et al., 2008). RGS9 appears to be common to both rods and cones, as do R9AP and Gβ5. So far, only one recoverin isoform has been described, and it is present in both rods and cones. Finally, there are rod and cone versions of NCKX (Schnetkamp, 2004). Much work remains to sort out the functional significance of all of this complexity.
The mechanisms underlying the lower sensitivity and faster response kinetics of cones compared to rods are gradually becoming understood (Fu and Yau, 2007; Kawamura and Tachibanaki, 2008; Luo et al., 2008a). The pigment content is not necessarily very different between rods and cones. Instead, Gt is less efficiently activated by Rh*, and the effective lifetime of cone Rh* is also much shorter, because GRK7 has a much higher specific activity than GRK1 and is present at a much higher concentration in cones than GRK1 in rods (Kawamura and Tachibanaki, 2008). Furthermore, the GTPase activity of Gtα*.GTP and hence its deactivation are more rapid in cones because RGS9, a key component in the GAP complex, is present at a much higher concentration than in rods (Cowan et al., 2001). In concert with the faster kinetics of the forward phototransduction cascade, the Ca2+ feedback is also faster in cones due to a faster Ca2+ decline in the light because of a larger surface-to-volume ratio of the cone outer segment for pumping down Ca2+ by NCKX (Yau, 1994). The Ca2+ feedback on the cGMP-gated channel is also greater in cones (Korenbrot and Rebrik, 2002), making this a potentially significant factor, unlike in rods. With different phototransduction steps being sped up, it remains to be determined which of them dominates the decline of the cone response. Surprisingly, except for their difference in spectral sensitivity, rod and cone pigments signal in quantitatively similar ways; that is, they interact in an identical manner with a given Gt, GRK1 and 7, and arrestin (Kefalov et al., 2003; Fu et al., 2008).
Besides the well-known pigment-regeneration pathway residing in the retinal pigment epithelial (RPE) cells, which serve both rods and cones, it now appears that a dedicated regeneration pathway exists for cones (Muniz et al., 2007; Travis et al., 2007; Wang et al., 2009). This pathway is somewhat different mechanistically from the one in RPE cells and resides in the Müller glial cells, which individually span almost the entire thickness of the retina. In this case, all-trans-retinol is reisomerized directly to 11-cis-retinol in these cells, then returned as such to the cones, which are capable of uptaking it at the cell body and converting it to 11-cis-retinal, both feats lacked by rods (Jones et al., 1989) (Figure 3A, right). Unlike rhodopsin, which has no tendency to dissociate into opsin and 11-cis-retinal in darkness, cone pigments do have some tendency to dissociate, probably because their chromophore-binding pocket is more open (e.g., Kefalov et al., 2005). Thus, in bleaching light, rod opsin outcompetes cone opsins in acquiring chromophore and acts as a huge sink for 11-cis-retinal, making it necessary for cones to have an additional chromophore source. Cone pigments also need to be regenerated rapidly and continuously because they operate in bright-light conditions. This rapid recycling likely requires a rapid dissociation of all-trans-retinal from the cone opsin in order for rapid reisomerization to follow, thus presumably requiring a looser (or relatively open) chromophore-binding pocket on cone opsin. This more open binding pocket is also likely to be partially responsible for a higher thermal isomerization rate, and thus higher noise, of cone pigments relative to rod pigments (Kefalov et al., 2003; Fu et al., 2008). In other words, the molecular design for achieving rapid regeneration of the bleached pigment may come with the price of greater noise in darkness.
Other Vertebrate Photoreceptors
Although the existence of extraocular photoreceptors has been known for some time in reptiles, birds, amphibians, and fish, the discoveries in recent years of additional photoreceptors in the retina, including that of mammals, have been greatly surprising. Most notably, a small subset of retinal ganglion cells are now known to be intrinsically photosensitive by virtue of the presence of the pigment melanopsin, an r-opsin (for review, Rollag et al., 2003; Fu et al., 2005; Hankins et al., 2008). In the retina of vertebrates such as amphibians, fish, and birds, melanopsin is also expressed in at least some retinal horizontal cells, HCs (e.g., Provencio et al., 1998). These HCs are also intrinsically photosensitive (Cheng et al., 2009; Jenkins et al., 2003). In addition, putative photoreceptors have been detected in small regions of the brains of lower vertebrates (based largely on the localization of opsins), with some referred to as “deep-brain photoreceptors” (e.g., Provencio et al., 1998; Halford et al., 2009). Besides rod/cone pigments and melanopsin, other opsins found in vertebrates include pinopsin, parapinopsin, peropsin, vertebrate-ancient (VA) opsin and its alternatively spliced variant, vertebrate-ancient-long (VAL) opsin, RPE-retinal G protein-coupled receptor (RGR), neuropsin (Opn5), parietopsin, encephalopsin, and tmt-opsin (Figure 1A) (Terakita, 2005). Among these, only pinopsin and parietopsin, first identified in the photosensitive bird pineal gland (Okano et al., 1994) and the lizard parietal eye (Su et al., 2006), respectively, have so far been shown to have clear light-signaling functions in their native cells. RGR appears to be a photoisomerase, which contributes to pigment regeneration by converting all-trans-retinal to 11-cis-retinal with the help of an appropriate photon.
Light-Sensitive Pinealocytes
Like the retina itself, the pineal gland is an outgrowth of the diencephalon of the brain. Like rods and cones, pinealocytes also have a cilium-derived outer segment with tightly stacked membrane discs, although their outer segments often lack the highly regular shape of rod and cone outer segments (for review, Eakin, 1973; Klein, 2004; Mano and Fukada, 2007). Fish and bird pinealocytes are light sensitive, and the green-sensitive pigment, pinopsin, was first identified in the chicken pineal (Okano et al., 1994), although rod and cone pigments are also present. The phototransduction mechanism in light-sensitive pinealocytes appears similar to that in rods and cones, involving a pinopsin-driven and Gt-mediated hyperpolarizing light response produced by the closure of a cGMP-gated, nonselective cation channel (Pu and Dowling, 1981; Dryer and Henderson, 1991; Mano and Fukada, 2007). In addition, pinopsin appears to interact with Gα11 leading to circadian phase shifting of the gland (Mano and Fukada, 2007). The exact mechanism underlying the latter function remains unclear. Mammalian pinealocytes are not photosensitive, but, reflecting their phylogenetic link to retinal rods and cones, they do express some retinal phototransduction genes (although little opsin and no transducin) presumably for participating in other G protein-signaling pathways (Klein, 2004). This gland supplies the hormone melatonin to the animal’s body. Despite the lack of photosensitivity in the mammalian pineal gland, the melatonin release is under circadian control from the suprachiasmatic nucleus (SCN), with a high release in darkness.
Parietal-Eye Photoreceptor
The parietal eye (sometimes called the third eye), present on the forehead of some lizards and amphibians (Eakin, 1973), is also an outgrowth of the diencephalon and often coexists with the pineal gland. Unlike the less-structured pineal, this eye resembles the lateral eyes by having a cornea, a lens, and a structured retina. The retina, however, has only photoreceptors and ganglion cells. It is also not inverted as the lateral-eye retinas are, so its photoreceptors face forward and are the first neurons to encounter incident light. There is no retinal pigment epithelium overlying the photoreceptors, so the mechanism for pigment regeneration is unclear. These ciliary photoreceptors have a well-formed outer segment, which resembles the cone outer segment in shape and by also having lamellar evaginations of the plasma membrane instead of completely internalized membrane discs as in rods. The precise function of the parietal eye is unknown, with one suggestion being that it enhances the animal’s detection of dawn and dusk based on color changes in the sky (Solessio and Engbretson, 1993). In the lateral eyes, colors are perceived based on chromatic antagonism between the outputs from different spectral cone types. Interestingly, in the parietal eye, chromatic antagonism exists within a single photoreceptor, with green light depolarizing the cell and blue light in the steady presence of green light hyperpolarizing it (Solessio and Engbretson, 1993). The underlying mechanism is now understood. There are two pigments in each cell: one green-sensitive and the other blue-sensitive. Activation of the green pigment inhibits a PDE, causing a rise in the cGMP level (presumably due to constitutive cGMP synthesis by a GC) and the opening of a cGMP-gated, nonselective cation channel to produce a depolarization (Finn et al., 1997; Xiong et al., 1998). In the steady presence of green light (and hence steady depolarization), activation of the blue-sensitive pigment hyperpolarizes the cell by triggering a rod/cone-like transduction pathway, namely, activation of the same PDE to hydrolyze cGMP and thus closure of the same cGMP-gated channel (Xiong et al., 1998). This color opponency within one cell may be one of the most primitive forms of color vision. The molecular components have been identified and are rather surprising (Su et al., 2006). The blue-sensitive pigment is pinopsin, whereas the green-sensitive pigment is a hitherto-unknown, phylogenetically ancient pigment named parietopsin. They act through different G proteins, neither being Gt. Instead, pinopsin appears coupled to gustducin (Ggust), a G protein mediating gustation in vertebrate taste receptors. Nonetheless, Ggust is the closest homolog of Gt and is capable of activating the retinal PDE (Hoon et al., 1995). Parietopsin appears coupled to Go. This involvement of Go in light detection resembles that found in the scallop hyperpolarizing photoreceptor, an invertebrate ciliary photoreceptor. From this perspective, the parietal-eye photoreceptor, with the copresence of vertebrate and invertebrate components, appears to be an evolutionary missing link between invertebrate ciliary photoreceptors and vertebrate rods and cones (Su et al., 2006). The photodetection mediated by Go is possibly more ancient than that mediated by Gt or its close relative Ggust. Because cones are thought to have evolved before rods (Okano et al., 1992), the closer morphological resemblance of the parietal-eye photoreceptor to cones than to rods is also consistent with this missing-link notion. The PDE in this photoreceptor is of the cone variety, although its cGMP-gated channel is of the rod type (Su et al., 2006). As a mechanism, the antagonistic controls of the PDE by Ggust and Go are strikingly analogous to the antagonistic controls of the adenylate cyclase in the heart by Gs and Gi.
Intrinsically Photosensitive Retinal Ganglion Cells
One of the most surprising discoveries in vision is that the retina harbors a third type of photoreceptor, intrinsically photosensitive retinal ganglion cells (ipRGCs), which use a distinct visual pigment, melanopsin, for light detection (Rollag et al., 2003; Fu et al., 2005; Hankins et al., 2008). They constitute ~1% of all retinal ganglion cells and project to the hypothalamic SCN (the central circadian pacemaker), the olivary pretectal nucleus (the brain center controlling the pupillary light reflex), and a number of other brain nuclei for accessory (non-image or subconscious) visual functions (Fu et al., 2005). Non-image vision informs the organism of the presence or absence of ambient light as well as its intensity and possibly spectral composition for the purpose of tracking the time of day or seasonal changes, among other functions. The SCN is innervated almost exclusively by ipRGCs. The other nuclei receive more mixed inputs from ipRGCs and conventional RGCs (the latter also mediating image vision). Even the dorsal lateral geniculate nucleus, which is the first central station for image vision, receives a weak input from the ipRGCs. IpRGCs also appear to signal within the retina, influencing rod and cone pathways (Hankins and Lucas, 2002; Zhang et al., 2008). Thus, melanopsin is involved in both image and non-image vision, although perhaps only very weakly in the former. Besides rods, cones, and ipRGCs, there appears to be no other photodetection system that signals to the brain. The melanopsin system is conserved across all mammals, including humans (e.g., Dacey et al., 2005).
The ipRGCs are orders of magnitude less sensitive to light than rods and cones. This low sensitivity comes primarily from a low photon-capture probability due to the extremely low density of melanopsin in the plasma membrane, which is 104 times lower than that of the pigments in rod/cone disc membrane (Do et al., 2009). Moreover, unlike rods and cones, ipRGCs have no membrane elaborations, and melanopsin is found only in the plasma membrane (Belenky et al., 2003). This low photon capture may serve to avoid intercepting the incident light to the rods and cones and degrading image vision.
The poor photon capture notwithstanding, the single-photon response in the ipRGCs is substantial, being ~1 pA in mouse, or twice that of mouse rods and 100 times that of rodent cones (Do et al., 2009). The ipRGC single-photon response is very slow, lasting many seconds, or 20-fold slower than the rod response and 100-fold slower than the cone response. Thus, ipRGCs emphasize temporal integration of light signals, a hallmark of accessory visual functions such as circadian photoentrainment.
The ipRGCs have no overt ciliary or rhabdomeric features, but melanopsin itself belongs to the r-opsin subfamily. Like many invertebrate pigments and unlike rod and cone pigments, it may also be bistable, i.e., with a stable photoproduct. Retinal ganglion cells in general also share key developmental genes with rhabdomeric photoreceptors, including the transcription factors Pax6, atonal, and BarH (Arendt, 2003). Thus, perhaps not surprisingly, ipRGCs functionally resemble rhabdomeric photoreceptors (and light-sensitive melanophores, where melanopsin was originally discovered; Provencio et al., 1998; Isoldi et al., 2005) by apparently using a PLC-type phototransduction mechanism (Berson, 2007; Hartwick et al., 2007). However, the underlying mechanistic details and molecular components are still very unclear and a subject of intense research. So far, one approach for molecularly identifying these components has been based on the tenet that the phototransduction proteins ought to be selectively, or at least predominantly, present in ipRGCs and not conventional RGCs. This assumption may not be valid, especially given the recent finding that viral transduction of conventional RGCs with the melanopsin gene alone is sufficient for making them intrinsically photosensitive (Lin et al., 2008). Ultimate verification of the phototransducing components should involve gene-knockout experiments, although the interpretation of any negative findings from such experiments may potentially be confounded by genetic compensation or redundancy.
Intrinsically Photosensitive Retinal Horizontal Cells
As mentioned earlier, in amphibians, fish, and bird, melanopsin is expressed not just in ipRGCs but also in other neurons, including some retinal HCs. Most recently, some dissociated fish HCs have indeed been found to be intrinsically photosensitive (ipHCs) (Cheng et al., 2009; Jenkins et al., 2003). In catfish, these are cone-driven HCs (Cheng et al., 2009). Interestingly, light does not appear to affect a dedicated, phototransducing ion channel, but simply modulates the common L-type Ca channel (in amplitude but not voltage dependence). In this respect, the ipHC possibly stands out from all other photoreceptors. The mechanism underlying this Ca-channel modulation remains entirely unknown. In catfish at least, the signaling pigment indeed appears to be melanopsin (Cheng et al., 2009). The light response is extremely slow, lasting tens of minutes. Possibly, this light sensitivity serves to modulate, through intracellular Ca2+, electrical coupling or synaptic transmission of HCs. There may also be nonelectrical effects of light. Because HCs have a central role in creating the receptive fields of visual neurons for detecting objects, their intrinsic photosensitivity must somehow affect image vision. VA/VAL opsins are also present in some fish HCs (Soni et al., 1998; Kojima et al., 2000), but their actions remain unclear. Like ipRGCs, ipHCs show no morphological signs of being ciliary or rhabdomeric. Developmentally, nonetheless, HCs appear to also show homology to rhabdomeric photoreceptors (Arendt, 2003). Thus, tentatively, the ipHCs may use a PLC pathway for signaling light, although at least one of its end points is the modulation of the voltage-gated Ca channel.
Amphioxus Rhabdomeric Photoreceptors
The cephalochordate amphioxus is strictly speaking an invertebrate but is included here because it is regarded as the most primitive living chordate ancestor of the vertebrate lineage. Besides a primitive eye containing ciliary photoreceptors, the animal has rhabdomeric photoreceptors, which might be forerunners of the vertebrate ipRGCs. In this sense, these cells are the most clear-cut rhabdomeric photoreceptors close to our lineage. There are two groups of these cells, called pigmented ocelli and Joseph cells, both expressing an apparently bistable melanopsin and also Gq (Koyanagi et al., 2005). Both cell types depolarize to light by activating a conductance, one with a high Na+ permeability and the other not (Gomez et al., 2009). Being inward-rectifying, neither of these conductances seems to be similar to the outward-rectifying, light-sensitive conductance of ipRGCs. More interestingly, internal mobilization of Ca2+ appears to be important for the production of the light response (Gomez et al., 2009), different from the ipRGCs (Berson, 2007; Hartwick et al., 2007).
Given that the ciliary photoreceptors in amphioxus are likely also the evolutionary forerunner of vertebrate rods and cones, it would be of great interest to know how phototransduction works in these cells. Unfortunately, their physiological properties are so far completely unknown, although the animal’s genome does contain Go-opsin-like and peropsin-like homologs (Koyanagi et al., 2002).
Invertebrates
The protostome invertebrates account for the vast majority of animal species, with diverse eyes and photoreceptors. Rhabdomeric photoreceptors dominate, particularly in arthropods and most molluscs. They all appear to use r-opsins, Gq and PLC, and depolarize to light. However, there is a dichotomy in that an inositol 1,4,5-trisphosphate (InsP3)-induced Ca2+ release appears important for photoexcitation in some species (e.g., Limulus and bee), whereas a membrane-delimited messenger linked to channel gating appears important in others (e.g., dipteran flies). Although rarer, numerous examples of ciliary photoreceptors do exist, especially in Cnidaria, polychaetes, and molluscs. Thus far, they are all based on a cyclicnucleotide signaling motif. There are no unequivocal ciliary photoreceptors described among the arthropods, but several insects, including bees and mosquitoes, express a c-opsin-like pigment (pteropsin; Figure 1) that hints at yet-undiscovered extraocular ciliary photoreceptors (Velarde et al., 2005).
Drosophila
Drosophila has the best studied rhabdomeric photoreceptor. Typical of a compound eye, the Drosophila retina is composed of repeating units called ommatidia. Each ommatidium contains, in addition to accessory glia, etc., eight photoreceptors, each of which contains ~4 × 104 tightly packed microvilli, forming a long (~80 µm), light-guiding rhabdomere. Six photoreceptors (R1–R6) are essentially identical, with a blue-green-absorbing rhodopsin (Rh1, λmax ~480 nm) in peripherally arranged rhabdomeres. The central rhabdomere is a tandem arrangement of microvilli from cells R7 and R8, each expressing two distinct opsins, giving altogether four opsins with λmax ranging from 330 to 520 nm. Like vertebrate cones, R7 and R8 mediate color vision (for review, Hardie and Postma, 2008; Katz and Minke, 2009). Unlike the vertebrate rod/cone dichotomy in photosensitivity, however, R1–R6 and R7/8 all respond to single photons with a similar gain, and they all adapt up to the brightest daylight intensities. Moreover, except for their distinct visual pigments, the other key phototransduction elements in R1–R6 and R7/8 appear to be molecularly identical (i.e., coded by the same genes).
Phototransduction Motif
Phototransduction in Drosophila is mediated by a Gq-coupled PLC signaling cascade that has become an influential genetic model for this pathway (for review, Pak, 1995; Hardie and Raghu, 2001; Wang and Montell, 2007; Hardie and Postma, 2008; Katz and Minke, 2009; Figure 4). The PLC, a β isoform, is coded by the norpA gene, a close homolog of the vertebrate PLCβ4. Intriguingly, PLCβ4 is also expressed in retinal rods and/or cones (Ferreira and Pak, 1994; Peng et al., 1997) and PLCβ4 knockout mice show subtle visual defects, although phototransduction per se appears unaffected (Jiang et al., 1996). The final step in phototransduction consists of the opening of two tetrameric transient-receptor-potential channels (TRP and TRPL). The light response is dominated by TRP, which has an unusually high Ca2+ selectivity (PCa:PNa > 50:1). TRP is the prototypical member of a large family of nonselective cation channels (Montell and Rubin, 1989; Hardie and Minke, 1992; Phillips et al., 1992), with 28 mammalian members distributed among 6 subfamilies (for review, Montell, 2005). TRP and TRPL define the TRPC (canonical TRP) subfamily, with all members being activated downstream of PLC.
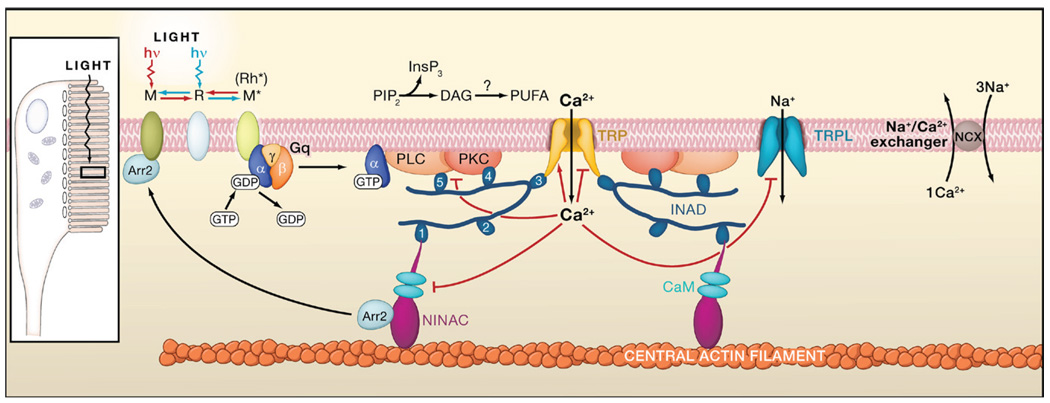
Figure 4. Phototransduction in Drosophila Rhabdomeric Photoreceptors.
Absorption of a photon by rhodopsin (R) converts it to the thermostable, active metarhodopsin state (M* or Rh*), which activates heterotrimeric Gq by GTP-GDP exchange essentially the same as in vertebrate rods. Active Gαq binds to and activates phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG), with the latter potentially producing polyunsaturated fatty acids (PUFAs) via a DAG lipase. Two classes of light-sensitive channels (TRP and TRPL, with the first being primarily Ca2+ permeable) are activated by a still-unknown membrane-delimited effect of PLC activity. Ca2+ influx feeds back positively and negatively at multiple sites (indicated by red lines ending in arrowheads and small bars, respectively), including PKC (required for inactivation of PLC), NINAC/arrestin (Arr2), and the TRP/TRPL channels. Ca2+ is extruded by a Na/Ca exchanger. TRP, PKC, and PLC are assembled into a signaling complex by the scaffolding protein INAD, possibly linked to the F-actin core via NINAC, a CaM-binding class III myosin. INAD has 5 PDZ domains, associated preferentially with different targets. The precise composition of the native complex is uncertain. Inset: schematic diagram of the rhabdomeric Drosophila photoreceptor, with microvilli forming a light-guiding rhabdomere. Submicrovillar cisternae at 10–100 nm beneath the base of the microvilli may release Ca2+ via InsP3 receptors in many rhabdomeric photoreceptors. However, InsP3 appears to play no role in photoactivation in Drosophila.
Several cascade elements are organized, with an ~1:1 stoichiometry, into multimolecular signaling complexes by a scaffolding protein, INAD. INAD contains 5 PDZ domains each with specific binding targets (for review, Tsunoda et al., 1998; Huber, 2001), with the core ones being PLC, TRP, and an eye-specific protein kinase C (PKC) required for response termination. Each microvillus, with a membrane surface area of ~0.2 µm2, contains ~1000 Rh, 50 Gq, 100 INAD, 100 PLC, 100 PKC, 25 TRP, and 2–3 TRPL (Hardie and Raghu, 2001).
Activation
Light isomerizes the chromophore 11-cis 3-hydroxy retinal to the all-trans configuration to generate metarhodopsin (named “M,” as opposed to “R,” the inactive 11-cis state; for consistency in this Review, we shall refer to the active M-state as Rh* unless for specific reasons otherwise). With Rh molecules arranged in an apparently rigid helical array (Suzuki et al., 1993), and PLC and TRP effectively immobilized in INAD complexes, only Gq is likely free to diffuse. Each Rh* probably activates 5–10 Gq molecules by diffusional encounters at 100–200 per second, not greatly different from the Gt*/Rh* signaling ratio in rods noted earlier. Each Gαq*.GTP activates one PLC molecule, which hydrolyzes the minor membrane phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2), to release InsP3 and diacylglycerol (DAG). Like the rod PDE*, this PLC* has a very high enzymatic rate (Hardie et al., 2001).
Exactly how PLC* activity opens TRP and TRPL channels remains controversial (Katz and Minke, 2009; Raghu and Hardie, 2009). In contrast to some other rhabdomeric photoreceptors, there is minimal light-induced Ca2+ release from internal stores, and InsP3-receptor mutants have no defects in phototransduction. Attention has thus turned to the lipid effects of PLC* activity. These include: (1) the generation of DAG, (2) the generation of downstream metabolites of DAG, such as polyunsaturated fatty acids possibly released from DAG by an additional enzyme (DAG lipase), and (3) a reduction in PIP2. There is evidence for each of these three substances being involved in channel gating, but none is conclusive. Given that TRP channels are often polymodally regulated (Rohacs and Nilius, 2007), it is even possible that all three take part. At any rate, the key channel-gating messenger is likely a membrane-delimited lipid effect resulting from PLC* activity. This scenario resonates with the situation for mammalian TRPC homologs, all of which are also gated downstream of PLC by unresolved mechanisms. For instance, DAG activates a subset of mammalian TRPCs, although whether the activation is direct is unclear; in addition, PIP2 has recently been reported to have both inhibitory and excitatory effects on certain TRPCs (for review, Beech et al., 2009; Raghu and Hardie, 2009). One difficulty is the failure to identify binding domains for lipids such as DAG and PIP2 (but see Kwon et al., 2007 for a reported binding site on TRPC6 for PIP3), raising the possibility that channel gating involves not a ligand in the classic sense but, instead, PLC-induced alterations in the physical properties of the bilayer (Katz and Minke, 2009).
Quantum Bump (Single-Photon Response)
In Drosophila, a single Rh* generates a quantum bump of ~10 pA, corresponding to ~15 open TRP channels (Hardie and Postma, 2008; Katz and Minke, 2009). Quantum bumps have a stereotypic waveform with a half-width of ~20 ms but a finite and characteristically variable latency of 15–100 ms. This variable latency contrasts with the single-photon response of vertebrate rods, which are 10–100 times slower but of relatively constant latency. Under voltage clamp, the response to a flash delivering even up to several hundred absorbed photons still consists of a linear summation of the underlying bumps, with a waveform given by the mathematical convolution of the latency distribution and the bump waveform. The quantum bump most probably represents an event restricted to a single microvillus, with its tiny dimensions ensuring minimal diffusion times and extremely rapid rise and fall of reactant concentrations. Within a microvillus, a single ion or molecule already represents a concentration close to 1 µM!
The amplification and rapid kinetics of the quantum bump depend critically on Ca2+ influx through the TRP channels, which induces sequential positive and negative feedbacks. The negative Ca2+ feedback is shared with vertebrate rods and cones, albeit by distinct molecular mechanisms. The positive Ca2+ feedback appears unique to rhabdomeric photoreceptors, although its molecular basis remains unclear. Although Ca2+ by itself does not activate the channels, it appears to greatly increase the channel’s open probability. According to current understanding, a membrane-delimited second messenger gradually increases in concentration with successive PLC molecules activated. At some stochastic point in time and space, the local second-messenger concentration overcomes the threshold for the first TRP channel to open. With a flux of ~106 Ca2+ per second per channel, the rapid rise in internal Ca2+ throughout the microvillus facilitates the opening of most of the remaining channels. Subsequently, however, this rise in microvillar Ca2+ to near-millimolar levels (Oberwinkler and Stavenga, 2000) triggers negative feedback acting at multiple targets, including the channels, and rapidly terminates the bump (Figure 4). Once the channels are inactivated, Ca2+ is rapidly cleared by combined diffusion into the cell body and extrusion via a powerful Na/Ca exchanger (NCX) on the microvilli (Wang et al., 2005). A quantum bump can then be generated again in the same microvillus after a refractory period of ~100 ms (i.e., at ~10 Hz). Computational models based on this framework can quantitatively account for the major features of the light response, including the quantum-bump amplification, its kinetics, and stochastic variability (Hardie and Postma, 2008; Pumir et al., 2008).
During light adaptation, the accumulated Ca2+ influx raises steady-state Ca2+ to a level as high as ~10 µM throughout the whole cell (Oberwinkler and Stavenga, 2000). Because the channels are inhibited by Ca2+, both the amplitude and the duration of the bumps are now greatly reduced, and the refractory period probably shortened. With ~4 × 104 microvilli, each capable of signaling photons at 10 Hz or faster, this strategy allows the photoreceptor to process daylight intensities, which approach 106 absorbed photons per second per cell.
Deactivation
As in vertebrate rods and cones, each phototransduction step needs to terminate in a timely fashion. Ca2+ again plays a major role. Drosophila expresses two arrestin isoforms, Arr1 and Arr2, but only Arr2 appears important for response termination (Dolph et al., 1993). Although Rh* is phosphorylated on C-terminal serine residues, this phosphorylation, unlike in rods, appears unnecessary for response termination or Arr2 binding (Hardie and Postma, 2008). Nevertheless, inactivation of Rh* by Arr2 is tightly regulated by Ca2+, taking ~200 ms without Ca2+ influx and only 20 ms with physiological Ca2+ influx. Mutation analysis shows that this Ca2+ dependence requires calmodulin and myosin III (NINAC). A working model is that Arr2 is constitutively bound to NINAC in the microvilli under low-Ca2+ conditions, but the Ca2+ influx promotes, via calmodulin, the release of Arr2, allowing Arr2 to rapidly inactivate Rh* (Figures 3B and 4) (Liu et al., 2008).
The active Gαq*.PLC is deactivated by the generic mechanism of intrinsic GTPase activity as in rods and cones. No dedicated GAP proteins (such as the RGS9 complex in rods) have yet been identified, but, analogous to PDEγ in rods and cones, PLC itself is an obligatory GAP protein. In severe PLC hypomorphic mutants, Gαq*.GTP can remain active for many minutes before encountering any PLC (Katz and Minke, 2009). Rapid termination of PLC* activity also requires Ca2+ influx. Thus, in trp mutants lacking TRP (the more Ca2+-permeable light-sensitive channel), the PLC* stays active long enough to deplete the entire PIP2 reserve in the rhabdomere within ~1 s of bright illumination (Hardie et al., 2001). In fact, this depletion of PIP2 appears to underlie the phenotype of the trp mutants. The Ca2+ dependence of PLC* inactivation also requires PKC (Gu et al., 2005). Interestingly, the INAD scaffolding protein appears to undergo a light- and PKC-dependent conformational change (involving the formation of an intramolecular disulfide cystine bridge) that disrupts one of its PDZ domains (Mishra et al., 2007). Given that PLC is one of the core members of the INAD complex, this INAD conformational switch may underlie the rapid inactivation of PLC, but this has yet to be confirmed.
Finally, analogous to the rod CNG channel, the Drosophila light-sensitive channels, TRP and TRPL, are also negatively regulated by Ca2+, with an IC50 (half-maximal inhibitory concentration) of ~1 µM, perhaps via their CaM-binding sites. This Ca2+-dependent channel inactivation appears to be the dominant mechanism for light adaptation (Gu et al., 2005).
Pigment Cycle
A key difference between rhabdomeric and ciliary pigments is that the metarhodopsin state (M-state) of rhabdomeric pigments is usually thermostable, i.e., the all-trans-retinal in M does not dissociate from the opsin moiety. Instead, the holopigment can be reisomerized to the R-state by another photon. The M-state absorption peak (λmax ~570 nm) in Drosophila is red-shifted compared to that of the R-state (λmax ~480 nm), so long-wavelength light passing through a red screening pigment in the eye always favors reconversion to R. Drosophila does have the biochemical machinery for chromophore biogenesis (Wang and Montell, 2007), but photoreisomerization mediated by ambient illumination is the typical mechanism under normal conditions (Figure 3) (for review, Stavenga, 1996).
As mentioned above, Rh* is phosphorylated (by a kinase that is presumably a homolog of vertebrate GRK1), but this is not required for arrestin (Arr2) binding and it is questionable if it plays any direct role in response termination. Arr2 is also phosphorylated at a single serine residue by CamKII (Matsumoto et al., 1994), but this is likewise not required for Arr2′s binding to the M-state of Rh. Instead, Arr2 needs to be phosphorylated in order to dissociate from the R-state of the pigment after photoreisomerization. Following Arr2 dissociation, the phosphorylated R is dephosphorylated by a Ca2+-CaM-dependent phosphatase coded by the rdgC gene (Wang and Montell, 2007; Hardie and Postma, 2008; Katz and Minke, 2009). A range of genetic defects affect this cycle directly or indirectly (e.g., due to compromised Ca2+ influx), resulting in light-dependent apoptotic retinal degeneration due to accumulated hyperphosphorylated Rh*.Arr2 complexes, which are targets for clathrin-mediated endocytosis (Wang and Montell, 2007).
Limulus Ventral Photoreceptor
In Drosophila, InsP3-induced Ca2+ release does not appear to play a role in phototransduction, but this mechanism is clearly important in many other species (bee, amphioxus, Limulus, and at least some molluscs). The most thoroughly investigated of these is the Limulus (horseshoe crab) ventral photoreceptor. For many years, this cell was actually the preferred invertebrate photoreceptor of study because its large size allows multiple-microelectrode insertions for two-electrode voltage clamp and pharmacological injections (for review, Dorlochter and Stieve, 1997; Nasi et al., 2000).
Exogenous InsP3 or Ca2+ introduced into Limulus photoreceptors clearly activates what appears to be the light-sensitive current. Light also triggers InsP3-induced Ca2+ release from intracellular stores (the submicrovillar cisternae) that can elevate free cytosolic Ca2+ to ~150 µM (Nasi et al., 2000). On the other hand, cGMP is the only substance found so far to activate ion channels in an excised membrane patch from the microvilli, prompting the proposal that cGMP may be produced by a Ca2+-dependent GC and constitutes an additional, penultimate step in phototransduction. There is no biochemical or molecular evidence for this enzyme, but inhibitors of the nonsoluble type of GC severely attenuated the light response (Garger et al., 2001).
Such a “linear” cascade has been challenged, however. Other studies have suggested as many as three different kinds of light-sensitive channels possibly operating in parallel, controlled by distinct G protein-mediated pathways: one signaling via PLC and InsP3, a second via GC, and a third via adenylate cyclase (Dorlochter and Stieve, 1997; Nasi et al., 2000). Consistent with the electrophysiological evidence mentioned above, a cGMP-gated cation channel homologous to the vertebrate CNG channels has been cloned and immunolocalized to the microvillar membrane (Chen et al., 2001). A Limulus trp homolog was also found in the mRNA from the ventral photoreceptor (Bandyopadhyay and Payne, 2004). Finally, the latter authors have found that a DAG analog injected into the cell activated an inward current with properties similar to the light-activated current.
Quantum bumps in Limulus share several features with those in Drosophila, including the variable latency, comparable first-stage (Rh*-Gα*) gain, threshold, and negative feedback via Ca2+ (Nasi et al., 2000). However, the Limulus quantum bump is up to 2 nA in amplitude and mediated by several thousand ion channels spread over dozens of microvilli. Presumably, the InsP3 generated initially in one microvillus diffuses to its base and releases Ca2+ from InsP3-sensitive stores, resulting in a rapid, large, but still relatively local Ca2+ release that can activate, or facilitate the opening of, ion channels on several microvilli up to ~2 µm from the release site. Limulus differs from Drosophila in that the light-sensitive channels have very little permeability for Ca2+; yet, in both species, Ca2+ has both excitatory and inhibitory roles in transduction. In Drosophila, this is mediated by Ca2+ influx; in Limulus, the Ca2+ comes from InsP3-induced Ca2+ release, with at least one negative-feedback target being the InsP3 receptor (Nasi et al., 2000).
Scallop Hyperpolarizing and Depolarizing Photoreceptors
When ciliary and rhabdomeric photoreceptors coexist in the same animal, one type (rhabdomeric in most invertebrates, ciliary in vertebrates) typically dominates in the eyes, whereas the other performs nonvisual functions or is present as extraocular photoreceptors. However, in some marine molluscs such as the scallop (Pecten), the retina of the image-forming eye is more or less equally divided into two layers, one with ciliary and the other with rhabdomeric photoreceptors. In scallop, the ciliary photoreceptors are hyperpolarizing and the rhabdomeric photoreceptors are depolarizing (Nasi et al., 2000).
The hyperpolarizing photoreceptor is the best studied example of an invertebrate ciliary photoreceptor and, as might be expected, uses a cGMP-gated channel for phototransduction. Most surprisingly, however, the channel in this case is K+ selective and opens in response to light (Gomez and Nasi, 1995). Equally surprising, the pigment (SCOP2) appears to be coupled to Go rather than Gt (which may not even exist in invertebrates) (Kojima et al., 1997). Finally, light changes the cGMP level by affecting the activity of a GC rather than a PDE (Gomez and Nasi, 2000). Thus, there are two distinct ways to achieve a hyperpolarizing light response: a Gt-PDE pathway (which leads to a decrease in cGMP) coupled to a cGMP-gated, nonselective cation channel as in rods and cones and a Go-GC pathway (which leads to an increase in cGMP) coupled to a cGMP-gated K channel. Because the latter pathway occurs also in other invertebrates, it appears to be a separate submotif in cyclic-nucleotide signaling for ciliary photoreceptors, and perhaps more ancient than the Gt-PDE pathway. The GC involved here is not a nitric-oxide-activated soluble GC (Gomez and Nasi, 2000) and, being likely G-protein coupled, is presumably not a “particulate” GC of the kind found in rods and cones. Instead, it may be an adenylate-cyclase-related GC (for review, Linder and Schultz, 2002). The Go-opsins, of which SCOP2 is an example, diverged phylogenetically from a common ancestral opsin prior to the protostome/deuterostome split. Consistent with the above notion that the Go-GC pathway may be more ancient than the Gt-PDE pathway, the Go-opsins also appear to be more ancient than Gt-opsins and are most closely related to vertebrate neuropsins, peropsins, and retinochromes (photoisomerases) (Terakita, 2005). Recently, the first gene coding for a cGMP-gated K channel has been identified in sea urchins, situated phylogenetically between the classic CNG nonselective cation channels and the ERG K channel family (Galindo et al., 2007). As another departure from rods and cones, light adaptation in this photoreceptor appears completely independent of Ca2+ because there is neither intracellular Ca2+ release nor Ca2+ influx. Instead, cGMP may also mediate adaptation, possibly via protein kinase G (Gomez and Nasi, 2005a). It will be interesting to know whether this feature is typical of Go-mediated phototransduction pathways.
By contrast, the scallop-depolarizing photoreceptors appear to be of the canonical rhabdomeric type. They express an r-opsin (SCOP1) and Gαq (Kojima et al., 1997) and are believed to respond to light via a PLC cascade and the opening of a nonselective cation channel. Although InsP3-induced Ca2+ release clearly has an excitatory role, this may not be essential for photoexcitation, Moreover, as in Drosophila, light-induced DAG production and/or PIP2 decrease appear important (Gomez and Nasi, 2005b; Nasi et al., 2000).
Cephalopod Photoreceptors
The lensed eyes of cephalopods such as squid and octopus are populated entirely by rhabdomeric photoreceptors. Their large sizes have proven valuable for biochemical studies and for the purification of rhabdomeric proteins. They have the canonical components of PLC signaling: Rh, Gq, PLC, and TRP channels, along with Rh kinase and arrestin (for review, Lott et al., 1999; Mayeenuddin and Mitchell, 2003). Recently, the crystal structure of squid rhodopsin has been resolved down to 2.5 Å, providing the first structure of a Gq-coupled GPCR (Murakami and Kouyama, 2008). Unfortunately, cephalopod photoreceptors have proven less amenable to physiological experiments, so there is essentially no information about the mechanistic details downstream of PLC.
Single Photosensitive Neurons in Onchidium
The central nervous system of many invertebrates contains light-sensitive interneurons with no overt ciliary or rhabdomeric features. Among the best studied are four giant interneurons in the abdominal ganglion of the marine slug, Onchidium (for review, Gotow and Nishi, 2008). Two of these cells (AP1 and Es1) depolarize to light due to the closure of a cGMP-gated K conductance, whereas the others (Ip1 and Ip2) hyperpolarize to light due to the opening of a similar conductance. Pharmacological data suggest the involvement of Go and GC (as in the scallop ciliary photoreceptor) in the hyperpolarizing cells and of Gt and PDE (as in vertebrate rods and cones) in the depolarizing cells, but no molecular information is available. Molecular confirmations are especially important in this case because Gt has not been reported in invertebrates so far; instead, the G-protein may be a more ancient homolog of Gt/Ggust. In any case, these cells clearly follow one or the other cyclic-nucleotide submotifs. The response polarity of the AP1 and Es1 cells also reveals a new feature; namely, the choice of a cGMP-gated K channel can still lead to a depolarizing light response, provided the upstream pathway involves PDE activation and a cGMP decrease. As for the Ip1 and Ip2 cells, the mechanism underlying the hyperpolarizing response pretty much follows that in the scallop hyperpolarizing response described earlier. It would be interesting to know whether the submotif of Go → GC → cGMP increase → cGMP-gated K channel opening → hyperpolarization is stereotyped in invertebrates.
Jellyfish
The most primitive extant animals with image-forming eyes are the Cnidaria, which are prebilaterians—evolving prior to the protostome/deuterostome split (Figure 1B). Until recently, very little was known about phototransduction in these organisms, which include jellyfish, box jellyfish, and hydrozoans. The photoreceptors in elaborate lensed eyes of box jellyfish have a ciliary morphology. Numerous cloned Cnidarian opsins cluster as a group most closely related to the c-opsins (Suga et al., 2008). Surprisingly, the only G protein identified so far in photoreceptors of the box jellyfish Carybdea is Gs. As expected from this, light is found to induce cAMP production in the eye (Koyanagi et al., 2008). If this effect indeed underlies the electrical response to light, this cell type would probably be the first example of an opsin-based photoreceptor signaling via neither cGMP nor PLC (albeit a CNG pathway nonetheless). For another box jellyfish, Tripedalia, gene expression for a cGMP cascade typical of ciliary-type phototransduction has been found, including PDE, phosducin, and GC (Kozmik et al., 2008).
A Gustatory Receptor Sensing Ultraviolet Light in Caenorhabditis
The nematode worm, C. elegans, is generally considered blind. It has no eyes, no morphologically distinguishable photoreceptors, and no opsins in its genome. Surprisingly, two recent studies reported its locomotory response to intense UV illumination, presumably for evading harmful sunlight. Based on high-throughput mutagenesis screening, a gene, lite-1, required for this behavioral response to light was identified (Edwards et al., 2008). Remarkably, lite-1, along with two homologs, lite-2 and lite-3, is most closely related to a family of insect gustatory receptors (Gr), coding for proteins with 7–8 predicted transmembrane domains and no sequence homology to opsins or other GPCR family members. Ectopic expression of lite-1 in muscle cells rendered them likewise photosensitive, implicating lite-1 as a novel photosensitive protein. It is still possible, however, that lite-1 is activated by a free radical or photo-oxidation product generated by the intense UV illumination, in which case it would not be a bona fide photosensitive pigment. In a separate study, a similar UV response was defective in tax mutants lacking functional CNG channels (Ward et al., 2008), but this has been challenged by the former group. This intriguing system clearly requires further investigation.
Summary and Conclusions
In this Review, we have surveyed the phototransduction mechanisms in a range of ciliary and rhabdomeric photoreceptors from both vertebrates and invertebrates. Vertebrate rods in particular have been investigated in unparalleled detail and the analysis of their transduction cascade represents a real triumph in modern biology. A beneficiary of this information is clinical ophthalmology, with many retina-afflicting diseases becoming understood and therapies currently being devised. The Drosophila photoreceptor, with an apparently more challenging PLC phototransduction pathway, is nonetheless also understood in considerable detail and represents an influential genetic model for this ubiquitous cascade. Our survey has revealed a degree of diversity. Nevertheless, a principle first suggested over 10 years ago on the basis of only a few examples (Finn et al., 1997; Xiong et al., 1998; Nasi et al., 2000) remains true: namely, ciliary photoreceptors use a cyclic-nucleotide motif, and rhabdomeric photoreceptors use a PLC motif, for signaling light. This dichotomy applies even to photoreceptors with no ciliary or rhabdomeric morphological features, consistent with their evolutionary link to ciliary or rhabdomeric photoreceptors based on their expression of certain developmental genes (Arendt, 2003).
The motifs of phototransduction and their variations are summarized in Figure 5. For ciliary photoreceptors, there are two submotifs, one mediated by Gt (or its close homolog Ggust) and the other by Go. The Go motif may be more ancient than the Gt motif. Current knowledge indicates that Gt invariably activates a PDE and hence cGMP hydrolysis. Go, on the other hand, can activate a GC or inhibit a PDE, although the result in either case is a rise in cGMP. It is possible that the coupling of Go to PDE inhibition is unique to the vertebrate parietal-eye photoreceptor in connection with its unusual chromatic antagonism, whereas the coupling of Go to GC is more mainstream, say, in invertebrates. Downstream from cGMP, the light-transducing, cGMP-gated channel can be nonselective among cations (as in vertebrate ciliary photoreceptors) or selective for K+ (as in protostome invertebrate ciliary photoreceptors), with the open channel leading to a depolarization or a hyperpolarization, respectively. Thus, the response polarity depends on the choice between Gt and Go pathways and also the choice between a nonselective cation channel and a K channel. More fundamentally, the question is often asked whether ethological or signaling factors might dictate the choice between a hyperpolarizing and a depolarizing response to light. There is probably no simple answer to this question. Certainly, in both cases, photosensitivity can be high and signaling can be effective because vertebrate rods as well as Drosophila photoreceptors can signal single-photon absorption. One might have thought that a hyperpolarizing photoreceptor such as the rod would be metabolically disadvantageous in a mostly dark habitat because its dark current requires a lot of energy to sustain. However, it turns out that the Drosophila photoreceptor, which depolarizes to light, uses as much energy in darkness as the rod, and it further increases its energy consumption as the light level increases (Okawa et al., 2008). In contrast, the rod actually becomes more energy efficient when the dark current decreases or disappears in the light (Braun et al., 1995).
Figure 5. Phototransduction Motifs in Vertebrates and Invertebrates.
There are two primary motifs, one mediated by cyclic nucleotides and the other by phospholipase C (PLC), segregated in ciliary and rhabdomeric photoreceptors, respectively. Within each primary motif, the canonical pathways are shown in black, and the noncanonical ones in brown to indicate their rare occurrence as currently known. The cyclic-nucleotide motif has two submotifs, mediated by Gt (or its close homolog, Ggust) and Go. In the Gt (?) pathway, found in the abdominal ganglion of the marine slug, Onchidium, the involvement of Gt is by inference only, without molecular identity, hence the question mark. The Gs pathway, reported in a box jellyfish, is tentative at present. The second, noncanonical Go pathway, found in the vertebrate parietal-eye photoreceptor, differs from the canonical Go pathway by involving a decrease in phosphodiesterase (PDE) activity rather than an increase in guanylate cyclase (GC) activity, with the same end result, namely, an increase in cyclic GMP (cGMP). The Go-PDE pathway may be unique to the parietal-eye photoreceptor, called for by the chromatic antagonism in this cell. In the PLC canonical pathway, the dashed lines indicate that the channel-activating messenger is still unclear. PLC depletes phosphatidylinositol 4,5-bisphosphate (PIP2) by hydrolyzing it into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (InsP3) with the latter releasing Ca2+ from intracellular stores; there is evidence implicating PIP2, DAG, InsP3 (via intracellular Ca2+ release), and a metabolite of DAG in the gating of the TRP-family nonselective cation channels. Ca2+ may also act synergistically with lipid messengers rather than as an activator in its own right. The canonical Gq pathway is typified by Drosophila and Limulus photoreceptors. The noncanonical Gq pathway is tentative, found in vertebrate intrinsically photosensitive horizontal cells (ipHCs), and suggested by current evidence to use melanopsin (an r-opsin) as pigment; however, there is no information on the intermediate steps or their molecular components. There are other differences between motifs or variations within a motif. For example, prominent negative feedbacks mediated by Ca2+ exist in the canonical Gt-mediated pathway but are apparently absent in the canonical Go-mediated pathway.
There are also variations within the PLC motif for rhabdomeric photoreceptors. Perhaps the most significant one is the divergence with respect to whether or not InsP3-induced Ca2+ release is a key step in producing the light response. In addition, there may be differences in the details of channel gating, but, remarkably, no channel-gating mechanism has yet been unequivocally established for any rhabdomeric photoreceptor. With this caveat, the phototransduction cascade in rhabdomeric photoreceptors seems otherwise well conserved, always employing Gq and PLC, and almost always resulting in a depolarization mediated by the activation of a nonselective cation channel. We are aware of only one possible hyperpolarizing light response, from a supposedly rhabdomeric photoreceptor of the tunicate Salpia (McReynolds and Gorman, 1975). There is, however, no information on the mechanism, and even its identification as a true rhabdomeric photoreceptor has been questioned (Salvini-Plawen, 2008).
Not only can ciliary and rhabdomeric photoreceptors coexist in the same animal, but both phototransduction motifs can coexist and signal light in the same cell, although not exactly for the same purpose. Thus, in the chicken pinealocyte, pinopsin (a c-opsin) and Gt are involved in producing the hyperpolarizing light response for directly curtailing melatonin release; additionally, pinopsin appears to couple to Gα11 (a close homolog of Gq) for phase-shifting the circadian rhythm of the cell, presumably via a PLC pathway. Even in rods and/or cones, Gα11 and PLCβ4 are present (Ferreira and Pak, 1994; Peng et al., 1997) but are apparently not involved in the phototransduction pathway (Jiang et al., 1996). Conversely, a cGMP-gated channel has been found in the rhabdomeric ventral photoreceptor of Limulus (Chen et al., 2001), which may be responsible for at least one component of the light response. CNG channels and a soluble GC are also expressed in Drosophila photoreceptors, although in this case they are implicated in axonal path-finding during development rather than phototransduction (Baumann et al., 1994; Gibbs and Truman, 1998). In this perspective, one interesting research direction would be to continue exploring the potential divergence or intersections of the two motifs triggered by a pigment in a given ciliary or rhabdomeric photoreceptor, with one serving a canonical phototransduction role and the other playing a modulatory role or carrying out an unrelated function.
Another area currently attracting much interest concerns the light-dependent translocations of phototransduction proteins in photoreceptors. In vertebrate rods, massive translocation of Gt takes place from the outer segment to elsewhere in the cell under light-adapted conditions, returning in darkness; arrestin moves in the opposite direction (for review, Calvert et al., 2006). Similar light-induced movements of Gq and arrestin, as well as TRPL, occur in and out of the rhabdomeric compartment in flies (Katz and Minke, 2009). One suggested function of these translocations is to provide long-term light adaptation. It is conceivable that the less-central light-triggered pathway in a photoreceptor is involved in such a function.
Finally, this Review has focused on animal photoreceptors that typically signal light to the brain via opsin and a vitamin A-based chromophore. These photoreceptors belong to the overwhelming majority in the animal kingdom. There is, however, at least one other known photoprotein, namely, the blue-absorbing flavoprotein cryptochrome. In Drosophila, there is good evidence that cryptochrome absorbs light and signals it to the molecular clock mechanism (for review, Ashmore and Sehgal, 2003). In vertebrates, the two cryptochromes (CRY1 and CRY2) are components of the molecular clock, but here their role appears to be unrelated to light signaling. In a potentially fascinating development, cryptochromes have been proposed to underlie magnetic-compass orientation in birds (Mouritsen and Ritz, 2005) and insects (Yoshii et al., 2009). The recent discovery in C. elegans of UV light avoidance via a gustatory receptor also raises the possibility of yet another photoprotein. However, we must wait to see whether the receptor, lite-1, indeed absorbs light directly.
ACKNOWLEDGMENTS
We thank Drs. M. Tri Do, D.-G. Luo, and G. Travis for helpful discussions and comments.
REFERENCES
- Arendt D. Evolution of eyes and photoreceptor cell types. Int. J. Dev. Biol. 2003;47:563–571. [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD, Pugh EN., Jr G Proteins and phototransduction. Annu. Rev. Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- Ashmore LJ, Sehgal A. A fly’s eye view of circadian entrainment. J. Biol. Rhythms. 2003;18:206–216. doi: 10.1177/0748730403018003003. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay BC, Payne R. Variants of TRP ion channel mRNA present in horseshoe crab ventral eye and brain. J. Neurochem. 2004;91:825–835. doi: 10.1111/j.1471-4159.2004.02773.x. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Increment thresholds at low intensities considered as signal/noise discriminations. J. Physiol. 1957;136:469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A, Frings S, Godde M, Seifert R, Kaupp UB. Primary structure and functional expression of a Drosophila cyclic nucleotide-gated channel present in eyes and antennae. EMBO J. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA. Photoreceptor signals and vision. Proctor lecture. Invest. Ophthalmol. Vis. Sci. 1987;28:34–49. [PubMed] [Google Scholar]
- Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E. TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium. 2009;45:583–588. doi: 10.1016/j.ceca.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J. Comp. Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- Bhandawat V, Reisert J, Yau K-W. Elementary response of olfactory receptor neurons to odorants. Science. 2005;308:1931–1934. doi: 10.1126/science.1109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RD, Linsenmeier RA, Goldstick TK. Oxygen consumption in the inner and outer retina of the cat. Invest. Ophthalmol. Vis. Sci. 1995;36:542–554. [PubMed] [Google Scholar]
- Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu. Rev. Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Burns ME, Mendez A, Chen J, Baylor D. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. doi: 10.1016/s0896-6273(02)00911-x. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Chen FH, Baumann A, Payne R, Lisman JE. A cGMP-gated channel subunit in Limulus photoreceptors. Vis. Neurosci. 2001;18:517–526. doi: 10.1017/s0952523801184026. [DOI] [PubMed] [Google Scholar]
- Cheng N, Tsunenari T, Yau K-W. Intrinsic light response of retinal horizontal cells of teleosts. Nature. 2009;460:899–903. doi: 10.1038/nature08175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote RH, Bownds MD, Arshavsky VY. cGMP binding sites on photoreceptor phosphodiesterase: Role in feedback regulation of visual transduction. Proc. Natl. Acad. Sci. USA. 1994;91:4845–4849. doi: 10.1073/pnas.91.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CW, He W, Wensel TG. RGS proteins: lessons from the RGS9 subfamily. Prog. Nucleic Acid Res. Mol. Biol. 2001;65:341–359. doi: 10.1016/s0079-6603(00)65009-2. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau K-W, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Do MTH, Shin SH, Xue T, Zhong H, Liao H-W, Bergles DE, Yau K-W. Photon capture and signaling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan T, Mendez A, Detwiler PB, Chen J, Rieke F. Multiple phosphorylation sites confer reproducibility of the rod’s single-photon responses. Science. 2006;313:530–533. doi: 10.1126/science.1126612. [DOI] [PubMed] [Google Scholar]
- Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993;260:1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- Dorlochter M, Stieve H. The Limulus ventral photoreceptor: Light response and the role of calcium in a classic preparation. Prog. Neurobiol. 1997;53:451–515. doi: 10.1016/s0301-0082(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Dryer SE, Henderson D. A cyclic GMP-activated channel in dissociated cells of the chick pineal gland. Nature. 1991;353:756–758. doi: 10.1038/353756a0. [DOI] [PubMed] [Google Scholar]
- Eakin RM. The Third Eye. Berkeley, CA: University of California Press; 1973. [Google Scholar]
- Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6:e198. doi: 10.1371/journal.pbio.0060198. 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol. Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Ferreira PA, Pak WL. Bovine phospholipase C highly homologous to the NorpA protein of Drosophila is expressed specifically in cones. J. Biol. Chem. 1994;269:3129–3131. [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu. Rev. Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Finn JT, Grunwald ME, Yau K-W. Cyclic nucleotide-gated ion channels: An extended family with diverse functions. Annu. Rev. Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- Finn JT, Solessio EC, Yau K-W. A cGMP-gated cation channel in depolarizing photoreceptors of the lizard parietal eye. Nature. 1997;385:815–819. doi: 10.1038/385815a0. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr. Opin. Struct. Biol. 2006;16:252–259. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Fu Y, Liao H-W, Do M, Yau K-W. Non-image-forming ocular photoreception in vertebrates. Curr. Opin. Neurobiol. 2005;15:415–422. doi: 10.1016/j.conb.2005.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Kefalov V, Luo DG, Xue T, Yau K-W. Quantal noise from human red cone pigment. Nat. Neurosci. 2008;11:565–571. doi: 10.1038/nn.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo BE, de la Vega-Beltran JL, Labarca P, Vacquier VD, Darszon A. Sp-tetraKCNG: A novel cyclic nucleotide gated K+ channel. Biochem. Biophys. Res. Commun. 2007;354:668–675. doi: 10.1016/j.bbrc.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Garger A, Richard EA, Lisman JE. Inhibitors of guanylate cyclase inhibit phototransduction in Limulus ventral photoreceptors. Vis. Neurosci. 2001;18:625–632. doi: 10.1017/s0952523801184129. [DOI] [PubMed] [Google Scholar]
- Gibbs SM, Truman JW. Nitric oxide and cyclic GMP regulate retinal patterning in the optic lobe of Drosophila. Neuron. 1998;20:83–93. doi: 10.1016/s0896-6273(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Gomez M, Nasi E. Activation of light-dependent K+ channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca2+ cascade. Neuron. 1995;15:607–618. doi: 10.1016/0896-6273(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Gomez M, Nasi E. Light transduction in invertebrate hyperpolarizing photoreceptors: Possible involvement of a Go-regulated guanylate cyclase. J. Neurosci. 2000;20:5254–5263. doi: 10.1523/JNEUROSCI.20-14-05254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M, Nasi E. Calcium-independent, cGMP-mediated light adaptation in invertebrate ciliary photoreceptors. J. Neurosci. 2005a;25:2042–2049. doi: 10.1523/JNEUROSCI.5129-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M, Nasi E. A direct signaling role for phosphatidylinositol 4,5-bisphosphate (PIP2) in the visual excitation process of microvillar receptors. J. Biol. Chem. 2005b;280:16784–16789. doi: 10.1074/jbc.M414538200. [DOI] [PubMed] [Google Scholar]
- Gomez M, Angueyra JM, Nasi E. Light-transduction in melanopsin-expressing photoreceptors of Amphioxus. Proc. Natl. Acad. Sci. USA. 2009;106:9081–9086. doi: 10.1073/pnas.0900708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow T, Nishi T. Simple photoreceptors in some invertebrates: Physiological properties of a new photosensory modality. Brain Res. 2008;1225:3–16. doi: 10.1016/j.brainres.2008.04.059. [DOI] [PubMed] [Google Scholar]
- Gu Y, Oberwinkler J, Postma M, Hardie RC. Mechanisms of light adaptation in Drosophila photoreceptors. Curr. Biol. 2005;15:1228–1234. doi: 10.1016/j.cub.2005.05.058. [DOI] [PubMed] [Google Scholar]
- Halford S, Pires SS, Turton M, Zheng L, Gonzalez-Menendez I, Davies WL, Peirson SN, Garcia-Fernandez JM, Hankins MW, Foster RG. VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 2009;19:1396–1402. doi: 10.1016/j.cub.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr. Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Peirson SN, Foster RG. Melanopsin: An exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Postma M. Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In: Masland RH, Albright TD, editors. The Senses - A Comprehensive Reference, Volume 1. New York: Elsevier; 2008. pp. 77–130. [Google Scholar]
- Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 2001;30:149–159. doi: 10.1016/s0896-6273(01)00269-0. [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J. Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S, Shlaer S, Pirenne M. Energy, quanta and vision. J. Gen. Physiol. 1942;25:819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F, Biel M, Kaupp UB. International Union of Pharmacology. LI. Nomenclature and structure-function relationships of cyclic nucleotide-regulated channels. Pharmacol. Rev. 2005;57:455–462. doi: 10.1124/pr.57.4.8. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Northup JK, Margolskee RF, Ryba NJ. Functional expression of the taste specific G-protein, alpha-gustducin. Biochem. J. 1995;309:629–636. doi: 10.1042/bj3090629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A. Scaffolding proteins organize multimolecular protein complexes for sensory signal transduction. Eur. J. Neurosci. 2001;14:769–776. doi: 10.1046/j.0953-816x.2001.01704.x. [DOI] [PubMed] [Google Scholar]
- Isoldi MC, Rollag MD, Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc. Natl. Acad. Sci. USA. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A, Munoz M, Tarttelin EE, Bellingham J, Foster RG, Hankins MW. VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr. Biol. 2003;13:1269–1278. doi: 10.1016/s0960-9822(03)00509-8. [DOI] [PubMed] [Google Scholar]
- Jiang HP, Lyubarsky A, Dodd R, Vardi N, Pugh E, Baylor D, Simon MI, Wu DQ. Phospholipase-C beta-4 is involved in modulating the visual response in mice. Proc. Natl. Acad. Sci. USA. 1996;93:14598–14601. doi: 10.1073/pnas.93.25.14598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc. Natl. Acad. Sci. USA. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front. Cell. Neurosci. 2009;3:2. doi: 10.3389/neuro.03.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Tachibanaki S. S-modulin. Adv. Exp. Med. Biol. 2002;514:61–68. doi: 10.1007/978-1-4615-0121-3_4. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Tachibanaki S. Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008;150:369–377. doi: 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- Kefalov VJ, Fu Y, Marsh-Armstrong N, Yau K-W. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425:526–531. doi: 10.1038/nature01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau K-W. Breaking the covalent bond—a pigment property that contributes to desensitization in cones. Neuron. 2005;46:879–890. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC. The 2004 Aschoff/Pittendrigh lecture: Theory of the origin of the pineal gland–a tale of confict and resolution. J. Biol. Rhythms. 2004;19:264–279. doi: 10.1177/0748730404267340. [DOI] [PubMed] [Google Scholar]
- Kojima D, Terakita A, Ishikawa T, Tsukahara Y, Maeda A, Shichida Y. A novel Go-mediated phototransduction cascade in scallop visual cells. J. Biol. Chem. 1997;272:22979–22982. doi: 10.1074/jbc.272.37.22979. [DOI] [PubMed] [Google Scholar]
- Kojima D, Mano H, Fukada Y. Vertebrate ancient-long opsin: a green-sensitive photoreceptive molecule present in zebrafish deep brain and retinal horizontal cells. J. Neurosci. 2000;20:2845–2851. doi: 10.1523/JNEUROSCI.20-08-02845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI, Rebrik TI. Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones. Adv. Exp. Med. Biol. 2002;514:179–203. doi: 10.1007/978-1-4615-0121-3_11. [DOI] [PubMed] [Google Scholar]
- Koutalos Y, Yau K-W. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Terakita A, Kubokawa K, Shichida Y. Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis-and all-trans-retinals as their chromophores. FEBS Lett. 2002;531:525–528. doi: 10.1016/s0014-5793(02)03616-5. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A. Jellyfish vision starts with cAMP signaling mediated by opsin-Gs cascade. Proc. Natl. Acad. Sci. USA. 2008;105:15576–15580. doi: 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z, Ruzickova J, Jonasova K, Matsumoto Y, Vopalensky P, Kozmikova I, Strnad H, Kawamura S, Piatigorsky J, Paces V, Vlcek C. Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc. Natl. Acad. Sci. USA. 2008;105:8989–8993. doi: 10.1073/pnas.0800388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol. Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN., Jr Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl. Acad. Sci. USA. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JU, Schultz JE. Guanylyl cyclases in unicellular organisms. Mol. Cell. Biochem. 2002;230:149–158. [PubMed] [Google Scholar]
- Liu CH, Satoh AK, Postma M, Huang J, Ready DF, Hardie RC. Ca2+-dependent metarhodopsin inactivation mediated by calmodulin and NINAC myosin III. Neuron. 2008;59:778–789. doi: 10.1016/j.neuron.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott JS, Wilde JI, Carne A, Evans N, Findlay JB. The ordered visual transduction complex of the squid photoreceptor membrane. Mol. Neurobiol. 1999;20:61–80. doi: 10.1007/BF02741365. [DOI] [PubMed] [Google Scholar]
- Luo D-G, Kefalov V, Yau K-W. Phototransduction in retinal rods and cones. In: Masland RH, Albright TD, editors. The Senses - A Comprehensive Reference, Volume 1. New York: Elsevier; 2008a. pp. 269–301. [Google Scholar]
- Luo DG, Xue T, Yau K-W. How vision begins: An odyssey. Proc. Natl. Acad. Sci. USA. 2008b;105:9855–9862. doi: 10.1073/pnas.0708405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H, Fukada Y. A median third eye: pineal gland retraces evolution of vertebrate photoreceptive organs. Photochem. Photobiol. 2007;83:11–18. doi: 10.1562/2006-02-24-IR-813. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kurien BT, Takagi Y, Kahn ES, Kinumi T, Komori N, Yamada T, Hayashi F, Isono K, Pak WL, et al. Phosrestin I undergoes the earliest light-induced phosphorylation by a calcium/calmodulin-dependent protein kinase in Drosophila photoreceptors. Neuron. 1994;12:997–1010. doi: 10.1016/0896-6273(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Mayeenuddin LH, Mitchell J. Squid visual arrestin: cDNA cloning and calcium-dependent phosphorylation by rhodopsin kinase (SQRK) J. Neurochem. 2003;85:592–600. doi: 10.1046/j.1471-4159.2003.01726.x. [DOI] [PubMed] [Google Scholar]
- McReynolds JS, Gorman AL. Hyperpolarizing photoreceptors in the eye of a primitive chordate, Salpa democratica. Vision Res. 1975;15:1181–1186. doi: 10.1016/0042-6989(75)90160-1. [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Roca A, Lem J, Wu LW, Simon MI, Baylor DA, Chen J. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 2000;28:153–164. doi: 10.1016/s0896-6273(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Mishra P, Socolich M, Wall MA, Graves J, Wang Z, Ranganathan R. Dynamic scaffolding in a G protein-coupled signaling system. Cell. 2007;131:80–92. doi: 10.1016/j.cell.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci. STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of Drosophila trp locus, a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Curr. Opin. Neurobiol. 2005;15:406–414. doi: 10.1016/j.conb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Müller DJ, Wu N, Palczewski K. Vertebrate membrane proteins: structure, function, and insights from biophysical approaches. Pharmacol. Rev. 2008;60:43–78. doi: 10.1124/pr.107.07111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, Tsin AT. A novel cone visual cycle in the cone-dominated retina. Exp. Eye Res. 2007;85:175–184. doi: 10.1016/j.exer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kouyama T. Crystal structure of squid rhodopsin. Nature. 2008;453:363–367. doi: 10.1038/nature06925. [DOI] [PubMed] [Google Scholar]
- Nasi E, Gomez M, Payne R. Phototransduction mechanisms in microvillar and ciliary photoreceptors of invertebrates. In: Stavenga DG, de Grip WJ, Pugh EN, editors. Handbook of Biological Physics. New York: Elsevier; 2000. pp. 389–448. [Google Scholar]
- Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Jr, Craft CM. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler J, Stavenga DG. Calcium transients in the rhabdomeres of dark- and light-adapted fly photoreceptor cells. J. Neurosci. 2000;20:1701–1709. doi: 10.1523/JNEUROSCI.20-05-01701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc. Natl. Acad. Sci. USA. 1992;89:5932–5936. doi: 10.1073/pnas.89.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372:94–97. doi: 10.1038/372094a0. [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak WL. Drosophila in vision research: The Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 1995;36:2340–2357. [PubMed] [Google Scholar]
- Palczewski K, Hargrave PA, McDowell JH, Ingebritsen TS. The catalytic subunit of phosphatase 2A dephosphorylates phosphoopsin. Biochemistry. 1989;28:415–419. doi: 10.1021/bi00428a001. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Sokal I, Baehr W. Guanylate cyclase-activating proteins: structure, function, and diversity. Biochem. Biophys. Res. Commun. 2004;322:1123–1130. doi: 10.1016/j.bbrc.2004.07.122. [DOI] [PubMed] [Google Scholar]
- Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron. 2004;42:401–410. doi: 10.1016/s0896-6273(04)00225-9. [DOI] [PubMed] [Google Scholar]
- Peng Y-W, Rhee SG, Yu WP, Ho YK, Schoen T, Chader GJ, Yau K-W. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proc. Natl. Acad. Sci. USA. 1997;94:1995–2000. doi: 10.1073/pnas.94.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu GA, Dowling JE. Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus. J. Neurophysiol. 1981;46:1018–1038. doi: 10.1152/jn.1981.46.5.1018. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr. Opin. Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- Pumir A, Graves J, Ranganathan R, Shraiman BI. Systems analysis of the single photon response in invertebrate photoreceptors. Proc. Natl. Acad. Sci. USA. 2008;105:10354–10359. doi: 10.1073/pnas.0711884105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P, Hardie RC. Regulation of Drosophila TRPC channels by lipid messengers. Cell Calcium. 2009;45:566–573. doi: 10.1016/j.ceca.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Rando RR. The biochemistry of the visual cycle. Chem. Rev. 2001;101:1881–1896. doi: 10.1021/cr960141c. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys. J. 1996;71:2553–2572. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch. 2007;455:157–168. doi: 10.1007/s00424-007-0275-6. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Berson DM, Provencio I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J. Biol. Rhythms. 2003;18:227–234. doi: 10.1177/0748730403018003005. [DOI] [PubMed] [Google Scholar]
- Salvini-Plawen L. Photoreception and the polyphyletic evolution of photoreceptors (with special reference to mollusca)*. Am. Malacol. Bull. 2008;26:83–100. [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- Schnetkamp PP. The SLC24 Na+/Ca2+-K+ exchanger family: vision and beyond. Pflugers Arch. 2004;447:683–688. doi: 10.1007/s00424-003-1069-0. [DOI] [PubMed] [Google Scholar]
- Solessio E, Engbretson GA. Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature. 1993;364:442–445. doi: 10.1038/364442a0. [DOI] [PubMed] [Google Scholar]
- Soni BG, Philp AR, Foster RG, Knox BE. Novel retinal photoreceptors. Nature. 1998;394:27–28. doi: 10.1038/27794. [DOI] [PubMed] [Google Scholar]
- Stavenga DG. Insect retinal pigments: Spectral characteristics and physiological functions. Prog. Retin. Eye Res. 1996;15:231–259. [Google Scholar]
- Su CY, Luo DG, Terakita A, Shichida Y, Liao HW, Kazmi MA, Sakmar TP, Yau K-W. Parietal-eye phototransduction components and their potential evolutionary implications. Science. 2006;311:1617–1621. doi: 10.1126/science.1123802. [DOI] [PubMed] [Google Scholar]
- Suga H, Schmid V, Gehring WJ. Evolution and functional diversity of jellyfish opsins. Curr. Biol. 2008;18:51–55. doi: 10.1016/j.cub.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Katayama E, Hirosawa K. Structure of photoreceptive membranes of Drosophila compound eyes as studied by quick-freezing electron microscopy. J. Electron Microsc. (Tokyo) 1993;42:178–184. [PubMed] [Google Scholar]
- Terakita A. The opsins. Genome Biol. 2005;6:213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Zuker CS. Specificity in signaling pathways: assembly into multimolecular signaling complexes. Curr. Opin. Genet. Dev. 1998;8:419–422. doi: 10.1016/s0959-437x(98)80112-3. [DOI] [PubMed] [Google Scholar]
- Velarde RA, Sauer CD, Walden KK, Fahrbach SE, Robertson HM. Pteropsin: a vertebrate-like non-visual opsin expressed in the honey bee brain. Insect Biochem. Mol. Biol. 2005;35:1367–1377. doi: 10.1016/j.ibmb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat. Neurosci. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, Montell C. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron. 2005;45:367–378. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Ward A, Liu J, Feng Z, Xu XZ. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 2008;11:916–922. doi: 10.1038/nn.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R, Molday RS. Regulation of the rod photoreceptor cyclic nucleotide-gated channel. Adv. Exp. Med. Biol. 2002;514:205–223. doi: 10.1007/978-1-4615-0121-3_12. [DOI] [PubMed] [Google Scholar]
- Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 2008;48:2052–2061. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J. Biol. Chem. 2008;283:4387–4394. doi: 10.1074/jbc.M703346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WH, Solessio EC, Yau K-W. An unusual cGMP pathway underlying depolarizing light response of the vertebrate parietal-eye photoreceptor. Nat. Neurosci. 1998;1:359–365. doi: 10.1038/nn0998_359. [DOI] [PubMed] [Google Scholar]
- Yau K-W. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest. Ophthalmol. Vis. Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu. Rev. Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Ahmad M, Helfrich-Forster C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 2009;7:e1000086. doi: 10.1371/journal.pbio.1000086. 10.1371/journal.pbio.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc. Natl. Acad. Sci. USA. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS, Yau K-W. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420:193–198. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]