Abstract
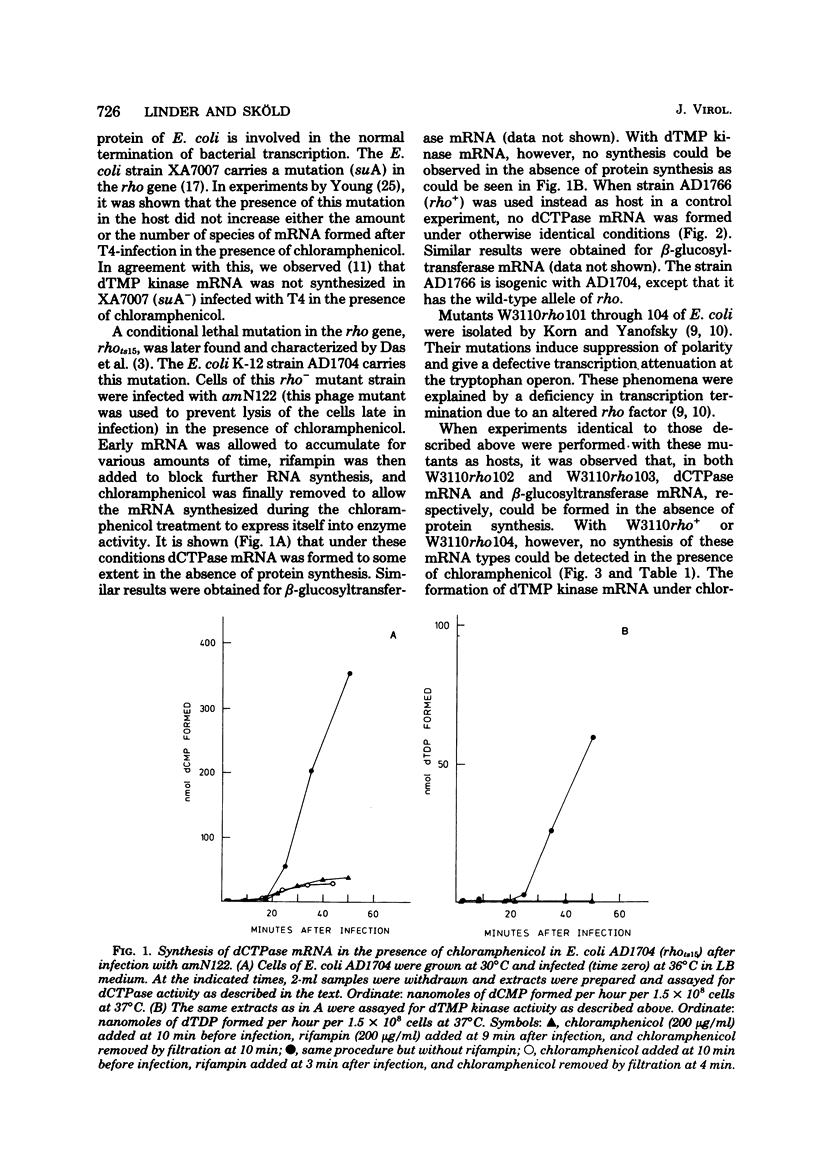
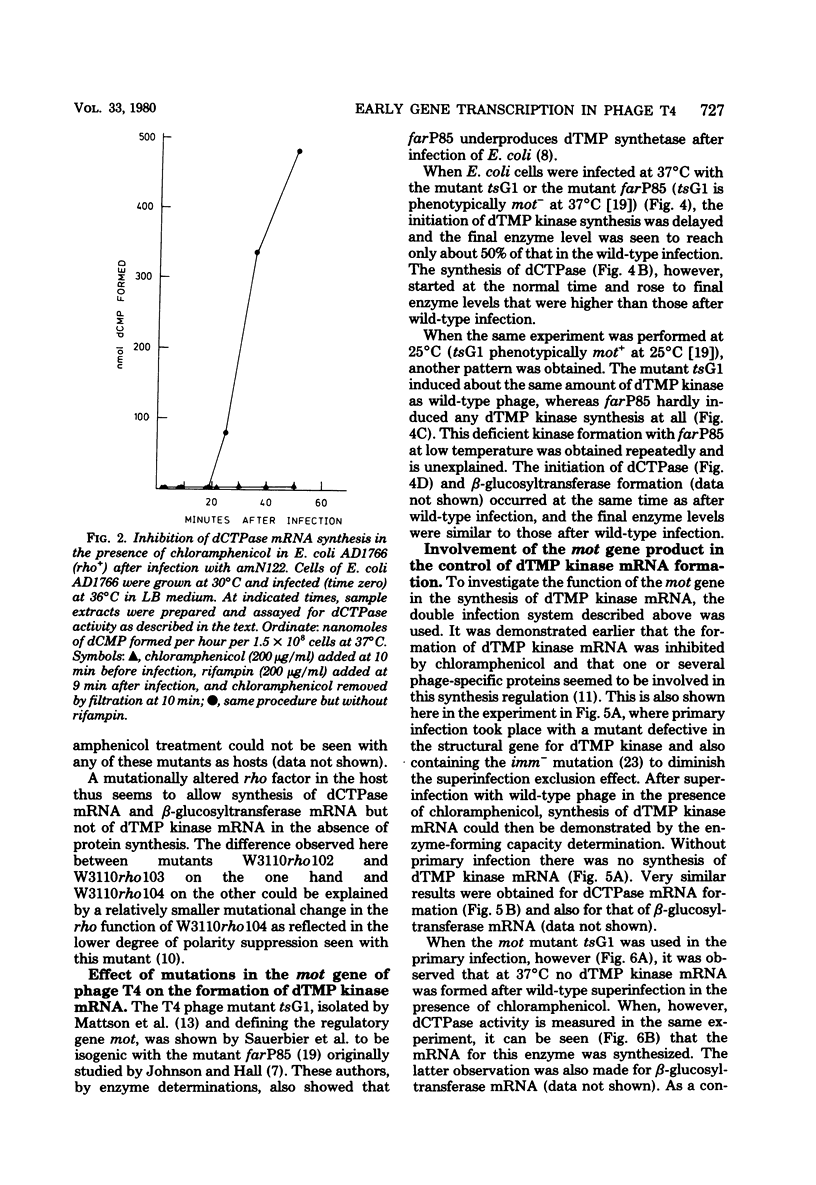
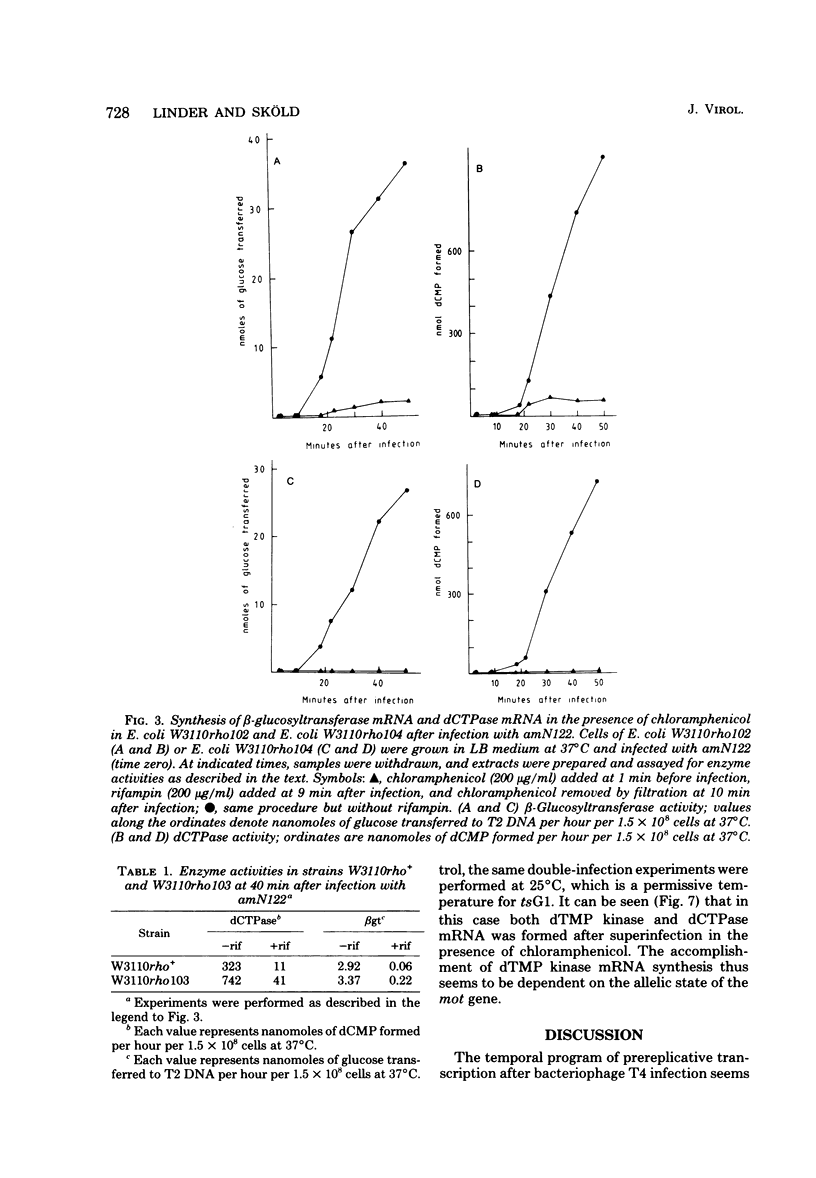
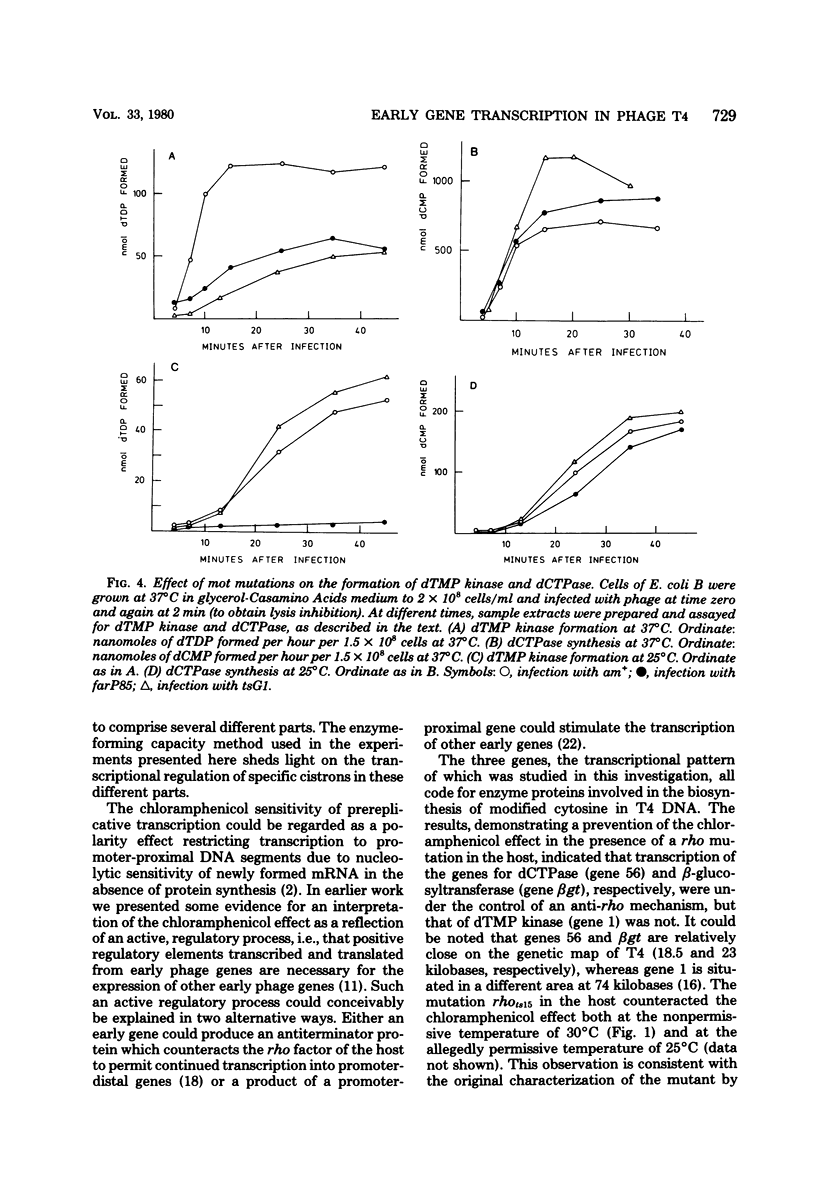
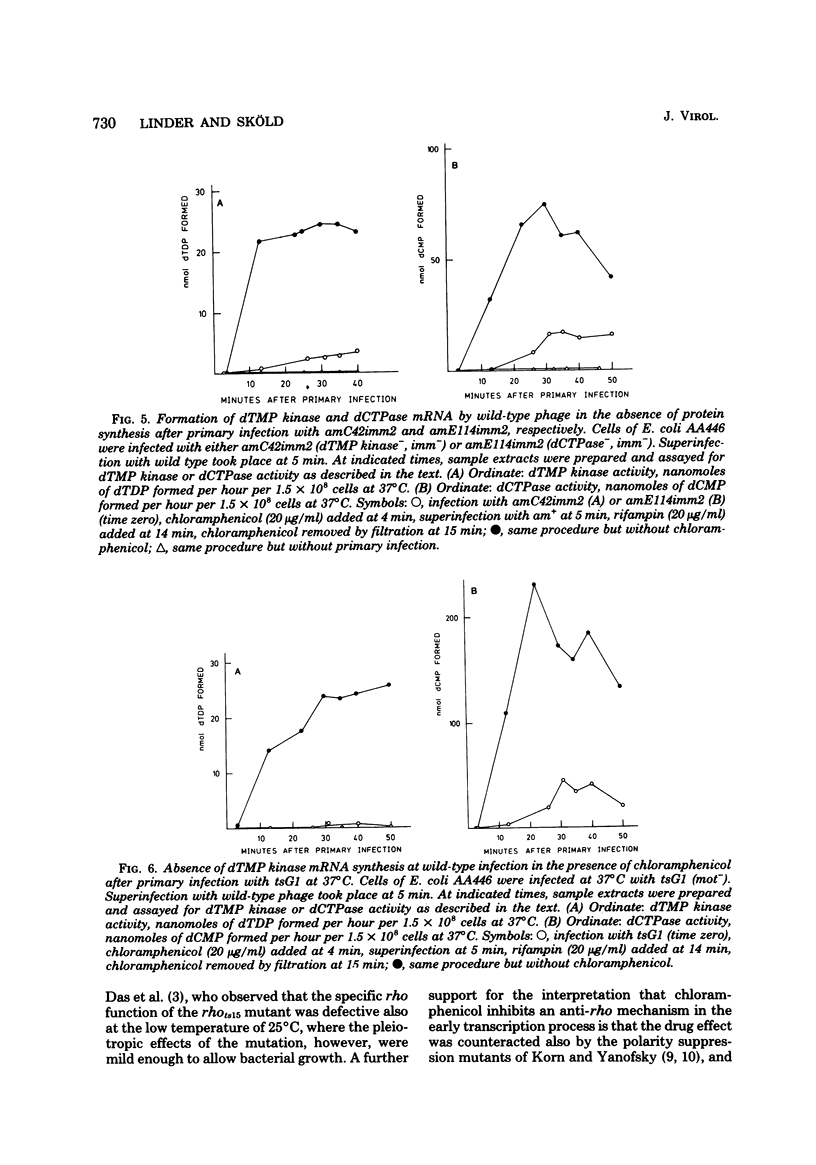
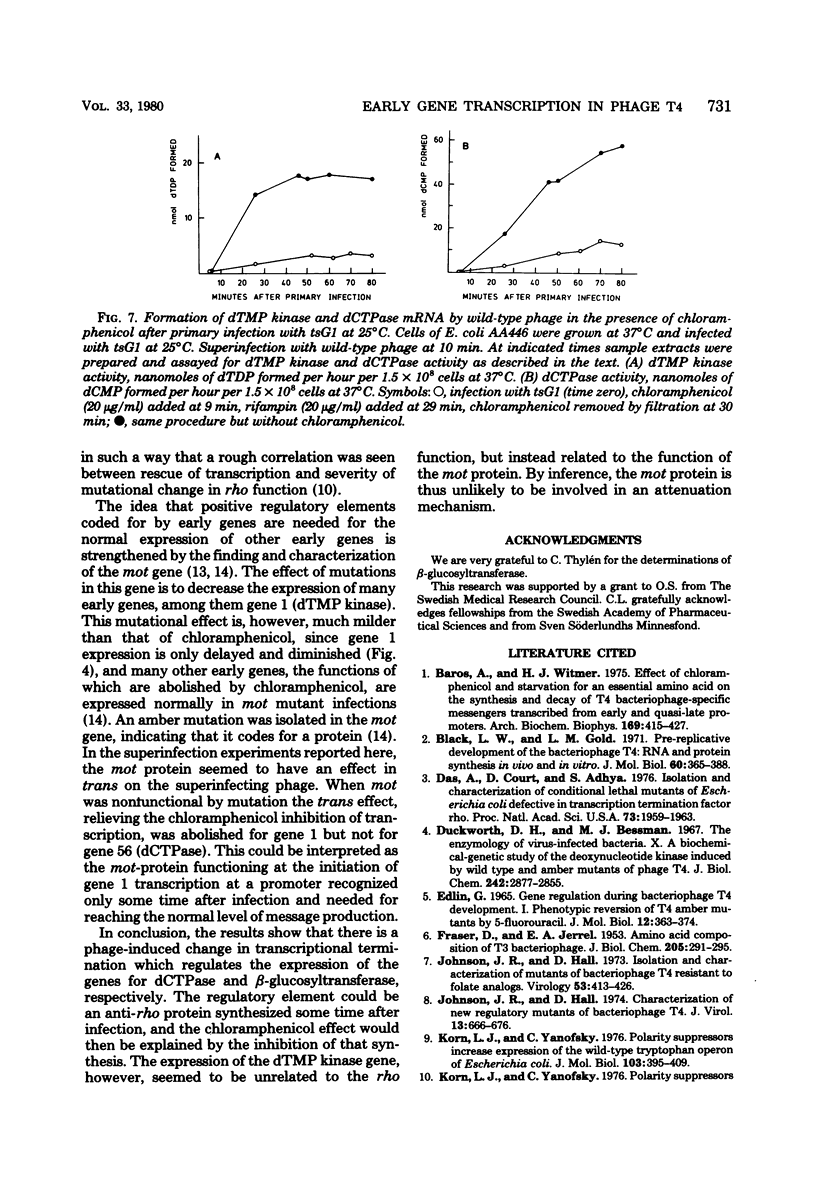
Many early mRNA species of bacteriophage T4 are not synthesized after infection of Escherichia coli in the presence of chloramphenicol. This has been interpreted as a need for T4 protein(s) to be synthesized to allow expression of some early genes, e.g., those for deoxycytidinetriphosphatase, deoxynucleosidemonophosphate kinase and UDP-glucose-DNA β-glucosyltransferase. In the experiments described here, early mRNA of bacteriophage T4 was allowed to accumulate during chloramphenicol treatment. After the addition of rifampin to inhibit further RNA synthesis, and subsequent removal of chloramphenicol, the accumulated mRNA was permitted to express itself into measured enzyme activities. It was shown that the early mRNA species coding for deoxycytidinetriphosphatase and UDP-glucose-DNA β-glucosyltransferase could be formed in the presence of chloramphenicol if the E. coli host cell carried a mutation in the structural gene for the RNA chain termination factor rho. This was interpreted to mean that T4 protein(s) with anti-rho activity is normally required for the expression of these two early genes. An altered rho-factor could not, however, relieve the need of phage protein synthesis for the formation of another early mRNA, that coding for deoxynucleosidemonophosphate kinase. In this case the mot gene of T4 seemed to be involved, since the primary infection of E. coli cells with the mot gene mutant tsG1 did not allow subsequent deoxynucleoside monophosphate kinase mRNA synthesis after wild-type phage infection in the presence of chloramphenicol. In control experiments, deoxynucleoside monophosphate kinase mRNA synthesis induced by wild-type phage superinfecting in the presence of chloramphenicol was facilitated by the primary infection with T4 phage containing an unmutated mot gene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baros A., Witmer H. J. Effect of chloramphenicol and starvation for an essential amino acid on the synthesis and decay of T4 bacteriophage-specific messengers transcribed from early and quasi-late promoters. Arch Biochem Biophys. 1975 Aug;169(2):415–427. doi: 10.1016/0003-9861(75)90183-6. [DOI] [PubMed] [Google Scholar]
- Black L. W., Gold L. M. Pre-replicative development of the bacteriophage T4: RNA and protein synthesis in vivo and in vitro. J Mol Biol. 1971 Sep 14;60(2):365–388. doi: 10.1016/0022-2836(71)90300-7. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. The enzymology of virus-infected bacteria. X. A biochemical-genetic study of the deoxynucleotide kinase induced by wild type and amber mutants of phage T4. J Biol Chem. 1967 Jun 25;242(12):2877–2885. [PubMed] [Google Scholar]
- EDLIN G. GENE REGULATION DURING BACTERIOPHAGE T4 DEVLOPMENT. I. PHENOTYPIC REVERSION OF T4 AMBER MUTANTS BY 5-FLUOROURACIL. J Mol Biol. 1965 Jun;12:363–374. doi: 10.1016/s0022-2836(65)80260-1. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Johnson J. R., Hall D. H. Characterization of new regulatory mutants of bacteriophage T4. J Virol. 1974 Mar;13(3):666–676. doi: 10.1128/jvi.13.3.666-676.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Hall D. H. Isolation and characterization of mutants of bacteriophage T4 resistant to folate analogs. Virology. 1973 Jun;53(2):413–426. doi: 10.1016/0042-6822(73)90221-3. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors increase expression of the wild-type tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):395–409. doi: 10.1016/0022-2836(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Linder C. H., Sköld O. Evidence for a diffusible T4 bacteriophage protein governing the initiation of delayed early RNA synthesis. J Virol. 1977 Jan;21(1):7–15. doi: 10.1128/jvi.21.1.7-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Mattson T., Richardson J., Goodin D. Mutant of bacteriophage T4D affecting expression of many early genes. Nature. 1974 Jul 5;250(461):48–50. doi: 10.1038/250048a0. [DOI] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Epstein R. H. Isolation and characterization of conditional lethal mutations in the mot gene of bacteriophage T4. J Mol Biol. 1978 Dec 15;126(3):551–570. doi: 10.1016/0022-2836(78)90058-x. [DOI] [PubMed] [Google Scholar]
- Peterson R. F., Cohen P. S., Ennis H. L. Properties of phage T4 messenger RNA synthesized in the absence of protein synthesis. Virology. 1972 Apr;48(1):201–206. doi: 10.1016/0042-6822(72)90127-4. [DOI] [PubMed] [Google Scholar]
- Ratner D. Evidence that mutations in the suA polarity suppressing gene directly affect termination factor rho. Nature. 1976 Jan 15;259(5539):151–153. doi: 10.1038/259151a0. [DOI] [PubMed] [Google Scholar]
- STRETTON A. O., BRENNER S. MOLECULAR CONSEQUENCES OF THE AMBER MUTATION AND ITS SUPPRESSION. J Mol Biol. 1965 Jun;12:456–465. doi: 10.1016/s0022-2836(65)80268-6. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K., Hall D. H. Utilization of early promotors in mutant far P85 of bacteriophage T4. J Virol. 1976 Aug;19(2):668–674. doi: 10.1128/jvi.19.2.668-674.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]
- Vallée M., Cornett J. B. A new gene of bacteriophage T4 determining immunity against superinfecting ghosts and phage in T4-infected Escherichia coli. Virology. 1972 Jun;48(3):777–784. doi: 10.1016/0042-6822(72)90161-4. [DOI] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. T. Analysis of bacteriophage T4 chloramphenicol RNA by DNA-RNA hybridization and by cell-free protein synthesis, and the effect of Escherichia coli polarity-suppressing alleles on its synthesis. J Mol Biol. 1975 Aug 15;96(3):393–424. doi: 10.1016/0022-2836(75)90168-0. [DOI] [PubMed] [Google Scholar]


