Abstract
Although praziquantel (PZQ) has been used to treat schistosomiasis for over 20 years its mechanism of action remains unknown. We have developed an assay based on the transcriptional response of Schistosoma mansoni PR-1 to heat shock to confirm that while 6-week post-infection (p.i.) schistosomes are sensitive to PZQ, 4-week p.i. schistosomes are not. Further, we have used this assay to demonstrate that in mice this sensitivity develops between days 37 and 40 p.i. When PZQ is linked to the fluorophore BODIPY to aid microscopic visualization, it appears to enter the cells of intact 4 and 6-week p.i. schistosomes as well as mammalian NIH 3T3 cells with ease suggesting that the differential effects of PZQ is not based on cell exclusion. A transcriptomal analysis of gene expression between 4 and 6 weeks p.i. revealed 607 up-regulated candidate genes whose products are potential PZQ targets. A comparison of this gene list with that of genes expressed by PZQ sensitive miracidia reduced this target list to 247 genes, including a number involved in aerobic metabolism and cytosolic calcium regulation. Finally, we also report the effect of an in vitro sub-lethal exposure of PZQ on the transcriptome of S. mansoni PR-1. Annotation of genes differentially regulated by PZQ exposure suggests that schistosomes may undergo a transcriptomic response similar to that observed during oxidative stress.
Keywords: Schistosoma mansoni, Praziquantel, Oxidative phosphorylation, Calcineurin, Schistosomiasis, Oxidative stress
1. Introduction
Praziquantel (PZQ) was first selected for its anti-helminthic action in the mid-1970s and was used initially to treat veterinary cestode and trematode infections. It has been used subsequently to treat a variety of human trematode infections (reviewed by [1]) and is currently the drug of choice for all forms of schistosomiasis.
Schistosomes have complex life cycles involving invertebrate and vertebrate hosts as well as free-swimming stages. While PZQ kills all Schistosoma species known to infect humans, it has been established using animal models and in vitro testing that it is not equally effective against all the life cycle stages of these species. For example, free-swimming Schistosomamansoni miracidia are rapidly killed by concentrations of PZQ as low as 1 μg/mL (~3 μM) and the same concentration reduces the infection rate of S. mansoni cercariae by 80% [2]. Similarly, Liang et al. [3] reported that 10−3M PZQ stops 84% of S. mansoni miracidia swimming while 10−4M PZQ stops the swimming behavior of cercariae and promotes subsequent tail shedding. In contrast, 10 μg/mL (~30 μM) PZQ does not kill S. mansoni sporocysts though 20–30 μg/mL (~60–90 μM) PZQ fed in the diet of Biomphalaria glabrata did interfere temporarily with S. mansoni cercarial shedding [4]. Of more clinical importance are studies using S. mansoni infected experimental mammals [5–7], together with in vitro studies [7], which show that PZQ does not kill juvenile schistosomes 3–4 weeks after infection of the definitive host. Sexually mature worms from mixed sex infections regain susceptibility, however, at around 6–7 weeks post-infection. Male, and especially female worms of this age, obtained from single sex infections are more refractory to the drug [7].
Although PZQ has now been used routinely in humans for over 20 years, its precise mechanism of action has not been identified. A number of observations concerning the effects of the drug on schistosomes have, however, been reported. Pax et al. demonstrated that when male S. mansoni come in contact with PZQ in vitro, they immediately undergo an intense muscular paralysis that is accompanied by a rapid influx of calcium ions, a slower influx of sodium ions and a decreased influx of potassium ions [8]. On the basis of these observations they suggested that PZQ may interfere with inorganic ion transport. A second notable feature of PZQ treatment is that the worm tegument is quickly disrupted leading to the exposure of antigens on the parasite surface [9] which has also been linked to the disruption of calcium ion homeostasis (reviewed by [10]). The notion that PZQ may act primarily through disruption of ion transport was given further credence by a series of elegant experiments that suggested PZQ may alter the function of a schistosome voltage-operated calcium channel (reviewed by [11]). Specifically, it was proposed that PZQ sensitivity is conferred on schistosomes through a Cavβ ion channel subunit with structural features distinct from those of other Cavβ subunits. Of particular interest was the observation that co-expression of the variant schistosome Cavβ subunit with the mammalian α1 subunit (Cav2.3) in Xenopus oocytes confers PZQ sensitivity on the previously insensitive α1 subunit [12]. Recently, however, Pica-Mattoccia et al. have demonstrated using 7-week-old worms from single sex male infections that while PZQ does indeed stimulate calcium ion uptake into schistosomes, uptake is enhanced by preincubation of schistosomes with cytochalasin D, a chemical that has been shown previously to suppress the schistosomicidal effects of PZQ [13,14]. A large uptake of calcium ions by day 28 post-infection (p.i.) male S. mansoni in the presence of PZQ was also noted despite the fact that worms of this age are regarded as being PZQ insensitive. Although these experiments were not performed with mixed sex infections, they led Pica-Mattoccia and colleagues to conclude that “calcium accumulation by itself, at least as measured by whole parasites maintained in vitro, may not represent an exhaustive explanation for the schistosomicidal effects of PZQ”. It has also been suggested that PZQ exerts its effect by either altering schistosomal membrane fluidity [15,16], reducing schistosomal glutathione concentrations allowing the host immune system to become more effective [17], binding to schistosomal actin leading to disruption of the tegument [18], or inhibiting nucleoside uptake [19].
In this paper, we describe: (1) the development of a quantitative real time PCR (qRT-PCR) based assay to determine the minimum lethal concentration of PZQ for mature schistosomes and we use this assay to confirm that juvenile schistosomes are refractory to the effects of PZQ. Previously, these assessments have relied mainly on the measurement of parameters such as worm length, luscence and lack of motility, for example [13,19]; (2) the use of microarrays to determine changes in gene expression after exposure of mature S. mansoni to a sub-lethal dose of PZQ in vitro; (3) experiments using a BODIPY-labeled structurally modified PZQ and confocal microscopy to determine whether PZQ can access the cells of intact juvenile and mature schistosomes as well as mammalian NIH 3T3 cells and (4) the use of microarrays to determine differences in gene expression between juvenile and mature S. mansoni.
2. Materials and methods
2.1. Schistosoma mansoni
Schistosome infected mice were supplied by Dr. Fred A. Lewis, NIAID Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD), through NIAID contract NO1-A1-30026. The abdomens of female SW mice were exposed to 125 S. mansoni PR-1 cercaria of both sexes. Four or six weeks after infection, mice were anesthetized and worms harvested by portal perfusion with RPMI 1640 media containing 20% fetal calf serum, 100 IU penicillin and 100 μg/mL streptomycin (RPMI/FCS/PS). Mice were subsequently euthanized by cervical dislocation. All animal experimentation complied with the policies, regulations and guidelines mandated by the Institutional Animal Care and Use Committee, University of New Mexico. Prior to the experiments outlined below, 4 and 6-week p.i. schistosomes were allowed to recover overnight after perfusion in RPMI/FCS/PS at 37 °C, 5% CO2. This, and all subsequent procedures that required worms to be maintained at 37 or 42 °C (see below), were performed using a water jacketed incubator with 5% CO2.
2.2. Assessing the lethality of PZQ for S. mansoni
Prior to PZQ exposure, 6-week p.i. worms were combined into a single pool before 20 worms were separated into duplicate groups containing equal numbers of each sex and incubated for 30 min at 37 °C and 5% CO2 in RPMI/FCS/PS with 0, 20, 40, 60, 80 or 100 μg/mL PZQ in 1% DMSO. In a separate experiment, 4-week p.i. worms derived from multiple mouse hosts, were combined into a single pool, then divided into groups of 50 in RPMI/FCS/PS. Duplicate groups of worms were incubated for 30 min at 37 °C and 5% CO2 in RPMI/FCS/PS with 0, 50, 100, 250, 500 or 1000 μg/mL PZQ in 1% DMSO. To remove PZQ, samples were washed twice with 2mL of RPMI 1640. After washing, one of the two groups, at each PZQ concentration, was shifted to 42 °C, while the remaining samples were maintained at 37 °C. After 4 h, the worms in each group were collected into 600 μL of RLT buffer (Qiagen) containing 1% β-mercaptoethanol (RLT/β-ME), homogenized, and placed at −80 °C prior to RNA isolation and quantitative real time PCR (qRT-PCR) analysis of heat shock related gene expression (described below).
To determine when schistosomes become sensitive to PZQ treatment during their maturation process, worms were harvested by portal perfusion every 3 days from day 28 to 43 p.i. After overnight recovery, duplicate groups of worms were incubated for 30 min at 37 °C and 5% CO2 in RPMI/FCS/PS with 0 or 80 μg/mL PZQ. To remove PZQ, samples were washed twice (described above), and one group at each PZQ concentration (0 or 80 μg/mL) was shifted to 42 °C while the remaining samples were maintained at 37 °C. After 4 h, the worms in each group were collected, homogenized and placed at −80 °C prior to RNA isolation and qRT-PCR analysis (described below).
All of the above experiments were repeated on three separate occasions.
2.3. RNA extraction and quality control
RNA was isolated from thawed, homogenized worms using an RNeasy® Mini kit (Qiagen) according to the manufacturer’s instructions, eluted with 30 μL of RNase free distilled water (dH2O) and quantified using a NanoDrop® ND-1000 spectrophotometer using ND-1000 3.3 software (NanoDrop Technologies). RNA integrity was evaluated using an Agilent 2100 Bioanalyzer with a RNA 6000 Nano LabChip® Kit (Agilent Technologies).
2.4. qRT-PCR analysis of heat shock related gene expression
Quantitative RT-PCR was used to measure the transcriptional activity of 3 S. mansoni genes, HSP40, HSP70 and HSP86, to heat shock. We have previously shown these genes to be induced significantly by a temperature shift from 37 to 42 °C for 4h [20]. The response of two genes, β-tubulin and Ribo21, whose transcriptional activity are not affected by heat shock were also assessed [20]. The sequences of the forward and reverse oligonucleotide primers used to amplify each of these genes are listed in the supplementary data. All supplementary material can be found at http://biology.unm.edu/Cunningham/Papers/PZQ.htm. Primer pairs were designed using Primer Express 2.0 (Applied Biosystems). For qRT-PCR reactions, 250 ng of total RNA was reverse transcribed using TaqMan® Reverse Transcription Reagents (Applied Biosystems) using random hexamers as primers. Exogenous Arabidopsis thaliana CAB mRNA (Stratagene) was spiked into each RT reaction as a control. Quantitative RT-PCR reactions (95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 58 °C for 1 min)were performed, in triplicate (technical replicates) for each sample, using Power SYBR® Green PCR Master Mix (Applied Biosystems), forward and reverse primers (0.6 μMeach), and a cDNA template (1–2 ng). Cycle thresh old values for each reaction were determined using the ABI Prism 7000 SDS 1.1 software package (Applied Biosystems). The mean fold change was calculated using the 2−ΔΔCT method [21].
2.5. Trancriptomic response of 6-week p.i. S. mansoni to a sub-lethal dose of PZQ
To determine the transcriptomic response of schistosomes to a sub-lethal dose of PZQ, duplicate groups (n = 30, 15 male and 15 female) were exposed to a sub-lethal dose (50 μg/mL) of PZQ for 0 (prior to the addition of PZQ), 30, 60 and 240min before total RNA was isolated as described above. cDNA synthesis, amplification, labeling and generation of the reference sample were carried out as previously described [20]. Labeled cDNA was hybridized overnight at 42 °C, to 7066 Schistosoma japonicum and 12166 S. mansoni element oligonucleotide arrays purchased from Agilent Technologies and described by Gobert et al. [22]. Microarray slides were scanned and the data processed as previously described [20]. Only the S. mansoni elements were considered in these analyses.
Each experiment was performed in duplicate and microarray analysis was performed using raw expression data for each element expressed as a ratio of the treatment, including the 0min time point (Cy5), to the common reference sample (Cy3). Expression ratios obtained for genes with the untreated samples were then subtracted from all sample ratios. Genes expressed ±1.0 log2, in both biological replicates, in either the 30 and 60 min, 60 and 240min, or 30, 60 and 240min samples after this subtraction step, were considered to be differentially expressed [23].
The accession (TC) numbers associated with elements binding differentially expressed transcripts were used to obtain their associated sequences from the S. mansoni Genome Index (http://compbio.dfci.harvard.edu/tgi/tgipage.html). These sequences were then analyzed using the gene ontology program Blast2GO (http://www.blast2go.de/) with a cut off of ≤1e-5 [24]. Sequences with ascribed functional annotations were further analyzed by blastx (www.ncbi.nlm.nih.gov/blast/Blast.cgi). Elements with an E value greater than 1e-5 were considered as having no known database homolog.
2.6. Treatment and visualization of S. mansoni and NIH 3T3 cells with modified PZQ-BODIPY
In order to determine whether PZQ has access to, or is excluded from, 4 and 6-week p.i. schistosomes and mammalian cells we conjugated a BODIPY flurophore to 4-oxo-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]isoquinoline [25], a modified PZQ (mPZQ) that meets all the structural requirements defined by Andrews et al. [26] for retention of high and broad anti-helminthic activity (see supplementary material for the structures of PZQ, mPZQ and mPZQ-BODIPY). The mPZQ-BODIPY conjugate was synthesized by coupling mPZQ and 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-proprionic acid (BODIPY FL) in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and 4-dimethylaminopyridine using pyridine as the solvent over 24 h. The crude product was purified by flash chromatography using 2% methanol/dichloromethane to elute the purified mPZQ-BODIPY.
S. mansoni were harvested at 4 and 6 weeks p.i. as described above and exposed to either 10 μg/mL mPZQ-BODIPY or 5 μg/mL BODIPY in RPMI 1640 for 10 min at 37 °C in 5% CO2. After exposure, schistosomes were fixed in 4% (w/v) paraformaldehyde. Samples were placed in Tissue-Tek® O.C.T. Compound (Sakura Finetek) and frozen on dry ice for cryosectioning. Sections of 10–15 μm thickness were cut using a Minotome Plus (Triangle Biomedical Sciences) and placed on Colorfrost PLUS microscope slides (Thermo Scientific). Nuclear staining was carried out directly on the mounted sections by adding 1 μL of 0.5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) to 1mL ProLong® Antifade (Invitrogen), which was used to affix the coverslip; samples were allowed to incubate overnight in the dark. Images were obtained using an LSM510 confocal microscope (Zeiss) and processed using LSM Image Browser (Zeiss).
Mouse NIH 3T3 fibroblast cells were seeded onto 13mm glass coverslips and exposed to 0.5 μg/mL DAPI with either 10 μg/mL mPZQ-BODIPY or 5 μg/mL BODIPY in DMEM for 10 min at 37 °C in 5% CO2 and mounted on glass microscope slides using 100 μL ProLong® Antifade. Images were obtained using a Zeiss Axioscope 2mot plus fluorescent microscope with Axiovision software (Zeiss).
A further group of 4 and 6 weeks p.i. worms and NIH 3T3 cells were processed as described above but were not exposed to either mPZQ-BODIPY or BODIPY.
2.7. Comparison of the transcriptomic expression profiles of 4 and 6-week p.i. S. mansoni
To determine which genes are differentially expressed between week 4 and 6 p.i., schistosomes were harvested by portal perfusion and maintained overnight in RPMI/FCS/PS media. RNA was then collected from groups of 30, 4-week p.i. worms derived from a mixed sex infection and 24 (12 male and 12 female), 6-week p.i. worms as described above. cDNA synthesis, amplification, labeling and microarray hybridization were carried out as previously described [20] except that the reference sample consisted of 99% total RNA from 6-week p.i. worms with the remaining 1% coming from 4-week p.i. worms.
Microarray analysis was performed using samples derived from four biological replicates for both 4 and 6-week p.i. schistosomes. Raw expression data for each element was expressed as a ratio of the schistosomes, 4 or 6 weeks p.i. (Cy5), to the common reference sample (Cy3). Differentially expressed genes were evaluated using Significance Analysis of Microarrays (SAM) [27], with a significance cutoff of a 2-fold increase or decrease in the 4-week p.i. sample relative to the 6-week p.i. sample.
All microarray information contained in this paper is MIAME compliant (http://www.mged.org/Workgroups/MIAME/miame.html) and all data were submitted to Gene Express Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE12850.
3. Results and discussion
3.1. Assessing the effect of PZQ on S. mansoni using a heat shock gene based qRT-PCR assay
Schistosomes exposed to at least 0.1 μg/mL PZQ in vitro immediately exhibit a paralysis of musculature resulting in a contracted state [7]. Previous studies have used the degree of contraction as a measure of PZQ sensitivity, as well as other parameters such as immobility and opacity, as markers for worm death [7,14,19]. Pica-Mattoccia and Cioli noted, using adult male worms from unisex infections, that there was no correlation between worm length after overnight treatment with various PZQ concentrations between 0.05 and 1.0 μg/mL and subsequent worm survival after removal of the drug [7]. While these observations are at variance with those of Ismail et al. [28] who described a correlation between ‘worm muscle tension’ and PZQ concentration for a number of Egyptian field isolates, they are in agreement with our own observations where we found no correlation between the length or circularity of mature worms from mixed sex infections and the concentration of PZQ (ranging from 0 to 100 μg/mL) that the worms were exposed to in vitro (supplemental data). As one of the goals of this study was to determine the effect of PZQ on the transcriptome of mature worms we were reluctant to rely on the measurement of worm length, curvature, muscle tension or simply observing worm movement or opacity to determine a protocol that would deliver a sub-lethal dose of the drug. Thus, we developed a molecular assay to more precisely determine the minimal lethal dose/exposure time regime of PZQ for S. mansoni PR-1.
Quantitative RT-PCR was used to determine if schistosome HSP40, HSP70 and HSP86 genes were induced in response to a shift in temperature in vitro from 37 to 42 °C for 4 h after exposure of the worms to 0, 20, 40, 60, 80 or 100 μg/mL PZQ for 30min at 37 °C. These genes have been shown previously to be induced by heat-shock [20,29–31]. When exposed to 0, 20, 40 and 60 μg/mL PZQ, the mixed sex population exhibited, on average, an 5-fold, 13-fold and 4-fold increase in the abundance of HSP40, HSP70 and HSP86 mRNAs, respectively, while the abundance of two constitutively expressed genes, Ribo21 and β-tubulin, remained unchanged (Fig. 1A). At a concentration of 80 μg/mL or greater, there was no significant increase in expression of HSP40, HSP70 or HSP86 mRNAs after 4 h of heat shock. These results suggest that exposure to PZQ concentrations of 80 μg/mL or greater for 30min is sufficient to halt transcription in vitro. To determine whether the heat shock genes are actually induced in response to PZQ exposure rather than by heat shock per se, schistosomes were exposed to a sub-lethal dose of PZQ (50 μg/mL) for 30min at 37 °C then subjected to qRT-PCR analysis without heat shock. Only HSP40 was induced (approximately 2-fold) in response to PZQ exposure (supplemental data). We were also able to demonstrate that the ability to mount a heat shock response was not recovered by 6-week p.i. schistosomes treated with 100 μg/mL PZQ or higher, if they were allowed to recover overnight at 37 °C before the heat shock was delivered (supplemental data). Together, these observations suggest that exposure to 80 μg/mL PZQ for 30min at 37 °C in vitro is sufficient to kill S. mansoni PR-1. This outcome can be compared to that obtained by Xiao et al. [32] who exposed day 42 p.i. S. mansoni to PZQ in vitro before observing worm behavior 24 h after removal of the drug. Exposure to 10 μg/mL PZQ for 60min produced little effect on worm behavior while exposure to 10 μg/mL for 240min or 30 μg/mL PZQ for 15 min resulted in contracted, immobile worms containing many vesicles. It was not clear, however, whether the failure of these latter worms to recover normal activity and shape correlated with worm death. As the data of Xiao and colleagues suggests, the length of time that worms are exposed to PZQ in vitro increases the effectiveness of lower concentrations of PZQ. This is supported by the observations of Pica-Mattoccia and Cioli [7] who found that ‘overnight’ exposure of mature male and female worms from mixed sex infections to a minimum of 2 and 10 μg/mL PZQ, respectively resulted in the death of all worms when inspected 8 days later. In these experiments, worms were judged to have died when they ‘remained contracted, did not resume movements and acquired an opaque appearance’. In contrast, however, using the qRT-PCR heat shock assay, we found male and female worms from mixed sex infections to be equally susceptible (supplemental data) though each of these apparent differences in outcome may reflect the different exposure protocols employed.
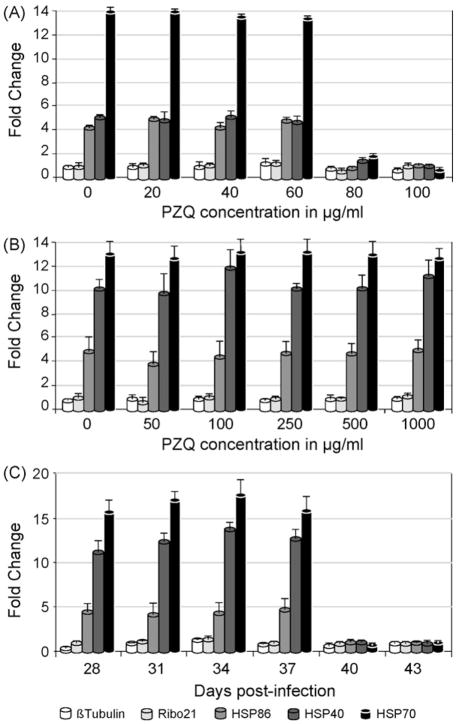
Fig. 1.
Quantitative RT-PCR assays of the transcriptomal response of S. mansoni PR-1 to heat shock after treatment with PZQ. The effect of 30 min exposure to increasing concentrations of PZQ on the transcriptomal response of (A) a 6-week and (B) a 4-week p.i. mixed sex population. (C) The effect of 80 μg/mL PZQ for 30min on the transcriptomal response of schistosomes harvested every 3 days from day 28 to 43 p.i. The fold change for each of the five transcripts indicated in the key was calculated using the 2−ΔΔCT method [21]. Error bars represent the standard deviation of three measurements and a fold change >1 indicates an increase in transcript abundance.
While the concentration of PZQ required to kill mature schistosomes in vitro is much higher than the maximal plasma concentration obtained in humans, (for example, Andrews [33] estimated this to be approximately 0.9 μg/mL after 2 h following oral administration of 46 mg/kg) it is not clear that PZQ works in vivo by killing schistosomes directly. Andrews suggests that the effective therapeutic plasma concentration is above 0.3 μg/mL which is sufficient to cause muscle contraction and paralysis of schistosomes; however, how this phenomenon is related to worm death is still an open question. Pica-Mattoccia et al. observed that exposure of schistosomes to 0.5 μg/mL PZQ for an ‘overnight’ period in vitro resulted in 89% male and 100% female worm survival while 1 μg/mL PZQ left 38% of males and 98% of females alive [7]. Thus, exposure to ‘therapeutic’ doses for extended periods of time in vitro does not appear to uniformly kill schistosomes, at least as measured by the methods of Pica-Mattoccia and colleagues [7].
Though schistosomula are known to be sensitive to the effects of PZQ for the first 1–2 weeks of infection there are numerous reports in the literature of the relative insensitivity of 2–4-week-old schistosomes to the drug [5–7]. Pica-Mattoccia and Cioli demonstrated using an in vivo assay that 28-day p.i. mixed sex worm infections of mice had a 30-fold higher ED50 compared with 49-day p.i. mixed sex worm infections [7]. These results were borne out by their in vitro data showing that 4-week p.i. worms from mixed sex infections had a 250-fold higher EC50 compared with those from 7-week infections. We have confirmed these observations using the heat shock assay and observed that even at PZQ concentrations as high as 1000 μg/mL there was no effect on the ability of 28-day p.i. schistosomes to respond to heat stress (Fig. 1B).
To determine the time at which schistosomes regain susceptibility to PZQ, schistosomes from a mixed sex infection were harvested every 3 days, starting at 28 days p.i. continuing through day 43 p.i. Each group of worms was subsequently tested for its ability to respond to heat shock following treatment with 80 μg/mL PZQ for 30 min. From 28 to 37 days p.i. schistosomes exhibited an increase in heat shock related transcripts following exposure to PZQ (Fig. 1C) with the level of response similar across all time points. At day 40 p.i., however, schistosomes could no longer respond transcriptionally to heat shock following PZQ exposure, indicating the onset of PZQ susceptibility. Thus, it would appear that the schistosomes do not slowly regain their susceptibility to the drug but rather there is a relatively sudden onset (at least between days 37 and 40) of full susceptibility. Interestingly, this timing coincides with the start of significant egg production by PR S. mansoni. Fallon et al. noted that although there were a very small number of eggs present in the liver (though none in the gut) of mice exposed to 200 PR cercariae at day 30 p.i., by day 38 p.i., there were approximately 500 and 300 eggs per worm pair in the liver and gut, respectively [34]. These numbers then rose to 1062 (liver) and 2183 (gut) eggs per worm pair at day 49. Eggs were first found in feces of mice infected with PR isolates between days 36 and 38 p.i.
To develop a drug protocol that would deliver an extended sub-lethal dose of PZQ, 6-week p.i. mixed sex schistosomes were exposed to 50 μg/mL PZQ for 4 h prior to heat shock in the absence of the drug. Under these conditions, schistosomes were able to respond to heat shock (supplemental data). Thus, mRNA was prepared from S. mansoni PR-1 exposed to 50 μg/mL PZQ for up to 4 h and used in the microarray experiments reported below.
3.2. The effect of a sub-lethal dose of PZQ on the transcriptome of S. mansoni PR-1
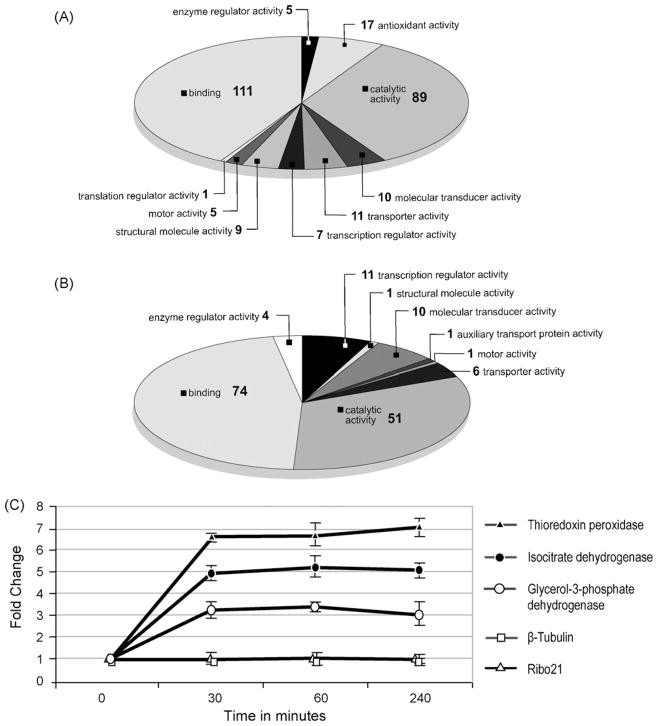
To understand how the mature S. mansoni PR-1 transcriptome changes on exposure to PZQ, schistosomes were incubated with 50 μg/mL PZQ for 0 (unexposed), 30, 60 or 240min and the mRNA used to generate cDNA that was labeled and hybridized to microarrays containing 12166 S. mansoni elements. Seven hundred and twenty six elements were identified that increased in abundance after treatment with PZQ, while 347 decreased. Both groups of genes were analyzed using the blast2GO gene ontology program for annotation of molecular function. Of the 726 genes that were induced, 251 were successfully annotated (supplemental data). Here, the two most prominent categories were ‘binding’ (29%) and ‘catalytic activity’ (23%) (Fig. 2A). Of the 347 genes that decreased in abundance, 131 were successfully annotated (supplemental data) and the two most prominent categories of molecules identified were those involved in ‘binding’ (40%) and ‘cellular processes’ (27%) (Fig. 2B). Interestingly, among the groups of annotated genes that were induced in response to PZQ treatment, 4% fell into the ‘antioxidant activity’ category. These included, an extracellular superoxide dismutase precursor (e-value = 6−23), glycerol-3-phosphate dehydrogenase (e-value = 6−166), isocitrate dehydrogenase (e-value = 2−62), cytochrome c oxidase subunit 2 (e-value = 2−95), and thioredoxin peroxidase (e-value = 8−108). Induction of glycerol-3-phosphate dehydrogenase, isocitrate dehydrogenase, and thioredoxin peroxidase was verified by qRT-PCR (Fig. 2C). These results suggest that treatment with PZQ may result in a molecular response similar to that observed when schistosomes undergo oxidative stress [20]. It should be noted, however, that this conclusion is in contrast to that of El-Bassiouni et al. [35] who observed that treatment of 3 or 6-week p.i. S. mansoni with up to 100 ng/mL PZQ in vitro for 1 or 3 h had no effect on the activities of superoxide dismutase, glutathione peroxidase or glutathione transferase though this may be due to the relatively low levels of PZQ employed.
Fig. 2.
Analysis of the transcriptome of 6-week p.i. S. mansoni exposed to a sub-lethal dose (50 μg/mL) of PZQ for up to 4 h. Gene ontology annotation of (A) 251 array elements up-regulated in response to PZQ and (B) 131 array elements down-regulated in response to PZQ. Transcripts were evaluated that had a 2-fold increase or decrease in abundance after PZQ exposure and were present in at least 80% of the data points. S. japonicum array elements were excluded from gene ontology analysis. Substances with multiple annotated functions were included and could be in more than one category. (C) qRT-PCR results plotted as fold change calculated using the 2−ΔΔCT method [21] as a function of time after PZQ exposure. Error bars represent the standard deviation of three measurements. Fold change >1 indicates an increase in transcript abundance.
3.3. mPZQ-BODIPY labeling of schistosomes and mammalian cells
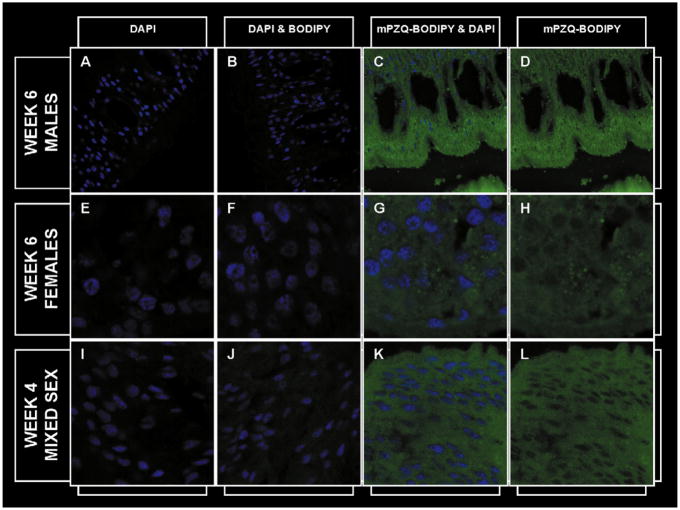
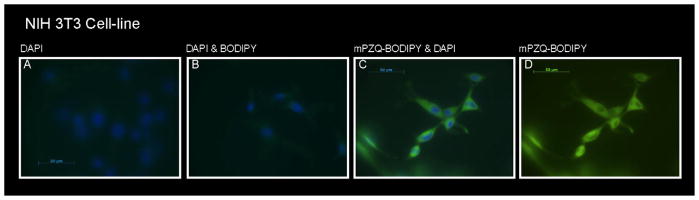
To determine whether the relative resistance of 4-week p.i. worms to PZQ was related to the ability of the schistosome cells to exclude or expel PZQ we incubated worms of this age, as well as 6-week p.i. male and female worms with 10 μg/mL of an active, structurally modified PZQ molecule linked to BODIPY (mPZQBODIPY) and examined cryosections using confocal microscopy. mPZQ-BODIPY was distributed equally throughout the 6-week p.i. male (Fig. 3A–D) and female (Fig. 3E–H) schistosomes, but appeared to be absent from the nucleus. A similar distribution of the compound was evident in 4-week p.i. schistosomes (Fig. 3I–L) suggesting that the resistance of 4-week p.i. worms is not the based on their ability to either prevent drug entry into, or actively expel the drug from, cells. Additionally, exposure of mouse NIH 3T3 cells to 10 μg/mL mPZQ-BODIPY for 10min demonstrated that PZQ easily penetrated these cells also (Fig. 4A–D). As PZQ is a relatively small (MW= 312.4), hydrophobic molecule, its easy penetration into both schistosomal and mammalian cells comes as no surprise.
Fig. 3.
A–D: Six-week p.i. male schistosomes at 88× magnification stained with (A) DAPI (B) DAPI and BODIPY, (C) mPZQ-BODIPY and DAPI and (D) mPZQ-BODIPY with DAPI staining optically removed. E–H: Six-week p.i. female schistosomes at 353× magnification stained with (E) DAPI, (F) DAPI and BODIPY, (G) mPZQ-BODIPY and DAPI and (H) mPZQ-BODIPY with DAPI staining optically removed. I–L: Four-week p.i. mixed sex schistosomes at 353× magnification stained with (I) DAPI, (J) DAPI and BODIPY, (K) mPZQ-BODIPY and DAPI and (L) mPZQ-BODIPY with DAPI staining optically removed. DAPI stain is blue; BODIPY and mPZQ-BODIPY are green.
Fig. 4.
Mammalian NIH 3T3 cells at 40× magnification stained with (A) DAPI, (B) DAPI and BODIPY, (C) mPZQ-BODIPY and DAPI and (D) mPZQ-BODIPY with DAPI staining optically removed. DAPI stain is blue; BODIPY and mPZQ-BODIPY are green.
3.4. Comparing the transcription profiles of day 28 and 42 p.i. S. mansoni
As observed above, the difference in susceptibility between day 28 and 42 p.i. schistosomes to PZQ does not appear to be due to the inability of the drug to access juvenile schistosome cells. It is possible, however, that the drug is successfully detoxified in these worms before binding to its molecular target. Alternatively, it can be hypothesized that the target molecule of PZQ is either unique to PZQ sensitive life cycle stages and/or has restricted sequence or structural similarity with the same molecule in mammals, allowing differential binding. In order to determine whether PZQ may be detoxified by 28-day p.i. worms and which proteins and/or pathways PZQ might be targeted in day 42 p.i. worms we used microarrays [22] to investigate the difference in gene expression between these two groups.
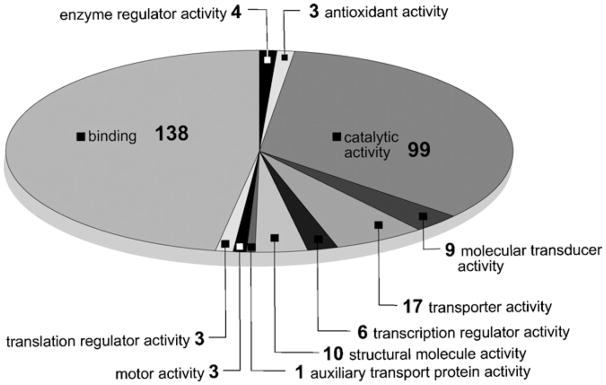
Six hundred and seven array elements were identified that were up-regulated at day 42 compared to day 28 p.i. schistosomes with a false discovery rate of 5.6%. Gene ontology was determined for 272 of these elements, which fell into 11 categories (Fig. 5 and supplemental data). The categories with by far the largest number of genes were ‘binding’ (36%) and ‘catalytic activity’ (26%). In comparison, 425 elements were up-regulated in day 28 compared to day 42 worms (supplemental data). When the above analysis was performed with a significance cut off of 1.8, 721 and 1805 elements were identified as being up-regulated at days 28 and 42 p.i., respectively. As a result of a false discovery rate of 10.2%, however, this analysis was discarded.
Fig. 5.
Geneontology annotation of 272microarray elements that were up-regulated at day 42 compared to day 28 p.i. S. japonicum elements were excluded from this analysis. Elements with multiple annotated functions were included and could be in more than one category.
On analysis of the 425 genes up-regulated at day 28 compared to day 42 p.i. we found no obvious constitutively expressed candidate genes, such as those encoding cytochrome P450’s, that might detoxify PZQ. This, of course, does not rule out the possibility that such a system might be induced by PZQ or that schistosomes have an effective novel detoxification mechanism available at day 28 but not day 42 p.i. It is worth noting, however, that day 42 p.i. worms exposed to a sub-lethal dose of PZQ (see Section 3.2) had no increased expression of genes encoding cytochrome P450s.
A number of hypotheses have been forwarded as to how PZQ might act on schistosomes including the suggestion that the drug might insert itself into membranes in such a way as to alter membrane fluidity [15,16]. Thus, while it is possible that the mechanism of action of PZQ does not directly involve proteins or that the drug may be detoxified at day 28 p.i., we have proceeded on the assumption that either the target of PZQ is itself a protein or the pathways directly influenced by PZQ contain protein components and that in either case, these proteins are differentially expressed between days 28 and 42. To further refine the group of 607 candidate genes up-regulated at day 42 p.i. and whose products could be considered as potential binding targets of PZQ, we compared these genes to a list of 4302 genes expressed by miracidia [36], which are PZQ sensitive, and identified 247 genes common to both data sets. Sixteen of these potential candidate genes were products of retrotransposon(s) while 102 had no ascribed function based on homology. The remaining 129 genes were analyzed for any insights they might provided regarding the potential mode of action of PZQ.
3.4.1. Proteins involved in aerobic metabolism
Among this cohort were a number of transcripts whose products are likely to be active in aerobic metabolism including homologues of NADH dehydrogenase subunits 2 (TC7217, 2e-28 and TC15703, 7e-24) and 6 (TC16807, 3e-25), succinate dehydrogenase iron-sulfur subunit (TC13682, e-114), succinate dehydrogenase flavoprotein subunit A (TC13974, e0.0), ATP synthase F0 subunit 6 (TC17477, 3e-93), cytochrome c oxidase subunit III (TC16772, 2e-46), glycogen phosphorylase (TC13593, 5e-19), pyruvate dehydrogenase phosphatase catalytic subunit 2 (TC8323, 4e-28) and glycerol-3-phosphate dehydrogenase 2 (TC17241, 8e-74). The presence of these transcripts at day 42 p.i. may be indicative of an increased reliance on aerobic metabolism as egg production gets underway. Coles [37] and van Oordt et al. [38] estimated that adult schistosomes in vitro derive one quarter or one-third of their energy demands, respectively, from aerobic respiration. In addition, Coles was able to show that egg production was reliant on oxygen consumption and speculated that oxidative phosphorylation is especially important in reproductive metabolism, particularly in females [37,39]. It is unknown whether 4-week p.i. worms generate energy by aerobic metabolism, however, when cercariae lose their tails and transform into schistosomula they move from aerobic to a much more anaerobic metabolism [40].
These, together with our own observations, suggest the possibility that PZQ may act on some component of aerobic respiratory pathways that are essential not only for reproductive success in males and females but also, during copulation and egg production, for survival. This is supported by the observation that each of the inferred schistosomal proteins highlighted above showed no more than 67% sequence identity with their human homologues, suggesting differential binding of PZQ would be feasible. Counter to this idea, Ross and Bueding found that schistosomes could survive for 5–6 days under nitrogen or in the presence of 10−3M cyanide. Whether these worms were actively producing significant numbers of eggs is, however, unknown and probably unlikely [41].
Should an aerobic metabolism necessitated by active egg production be central to the action of PZQ, then it can be predicted that worms from single sex infections should be insensitive to the effects of PZQ at an age when those from mixed sex infections succumb to the drug. Pica-Mattoccia and Cioli have demonstrated that 7-week-old schistosomes from such infections, especially females, have a reduced sensitivity to PZQ [7]. Thus, though there is evidence that worms from single sex infections do express some genes encoding homologues of aerobic respiratory proteins [42,43], they may not be as dependent on this source of energy as worms that are actively reproducing.
PZQ inhibits the movement of, and probably kills, swimming S. mansoni miracidia and cercariae [2,3]. These life cycle stages also have an aerobic metabolism in which endogenous glycogen is catabolized mainly to CO2 via the Krebs cycle [44,45]. In contrast, sporocysts are not sensitive to PZQ. Furthermore, while they generate energy aerobically, their survival is not dependent on it. Tielens et al. demonstrated that sporocysts have a large anaerobic capacity and survive comfortably in the presence of cyanide [46]. Together, these data suggest that PZQ may exert its lethal effect by inhibiting some aspect of aerobic energy production in miracidia, cercariae and reproductively active schistosomes in vivo. What is not clear under this scenario, however, is why anaerobic metabolism would not keep adult worms from mixed sex infections alive in the presence of PZQ, even if not reproductively active.
3.4.2. Proteins involved in calcium signaling
Calcineurin (CN) is a calcium/calmodulin dependent protein phosphatase that is present throughout eukaryotes. The protein consists of a catalytic subunit (CNA) and a regulatory subunit (CNB) and homologues of both CNA (TC10034, 2e-56) and CNB (TC11261, 3e-79) were identified in this analysis. Mecozzi et al. demonstrated using immunoblots that CNA and CNB are present in both the soluble and membrane fraction of adult worms as well as in miracidia, cercariae, schistosomula and eggs, though juvenile worms were not tested [47]. Relatively high concentrations of CNA have been localized to the excretory systems including flame cells in schistosomula and adult worms, perhaps suggesting a role for CN in the regulation of ion fluxes in the schistosome excretory system [47,48].
CN is a participant in many signal transduction pathways including the regulation of ion homeostasis (reviewed by [49]). In muscle cells, calcium ions are stored within the sarco(endo)plasmic reticulum to prevent their lethal accumulation in the cytosol. These ions are moved into this compartment by sarco(endo)plasmic reticulum Ca2+-ATPase pumps (SERCs). One such pump, the SMA2 Ca2+-ATPase, is expressed by S. mansoni and, using SERC mutants of Saccaromyces cerevisiae, has been shown to be negatively regulated by S. cerevisiae CN [50]. There is no evidence, however, that S. mansoni CNA (SmCNA) inhibits SMA2 when they are co-expressed in mutant yeast though this may have been due to the poor expression of SmCNA transcripts [51]. If PZQ is acting directly on SmCN then its likely effect would be to deplete the cytosol of Ca2+, perhaps leading to the observed influx of extracellular Ca2+ into the worm cells.
Finally, Greenberg and colleagues have put forward a large body of evidence (reviewed by [11]) suggesting that PZQ sensitivity is conferred on schistosomes through a Cavβ ion channel subunit with structural features distinct from those of other Cavβ subunits. Using qRT-PCR we found no evidence that the gene encoding this subunit is differentially regulated between 28 and 42-day p.i. schistosomes (supplemental data). This confirms the findings of Valle et al. [52] who used Northern blots of Cavβ1 and Cavβ2 gene expression in day 28 and 49 p.i. S. mansoni to reach the same conclusion.
3.4.3. Other proteins of interest
It has been suggested that PZQ may act by reducing schistosomal glutathione concentrations allowing the host immune system to become more effective [17]. We observed that glutathione-S-transferase (TC10486, 2e-67), as well as thioredoxin (TC11430, 4e-23), thioredoxin peroxidase (TC14049, 8e-67) and extracellular superoxide dismutase (TC16777, 2e-94; TC16780, 2e-94; TC16781, 3e-18) transcripts were significantly more abundant in mature schistosomes and miracidia compared to juveniles suggesting that potential anti-oxidant targets for PZQ do exist at these stages. As discussed above, when mature schistosomes are exposed to sub-lethal doses of PZQ, one outcome is the increased expression of several genes that are partitioned into the ‘anti-oxidant’ ontology category suggesting that this response may actually be induced by PZQ.
Tallima and El Ridi have suggested that PZQ may bind to schistosomal actin leading to disruption of the tegument[18]. Ahomologue of supervillin (TC17535, 3e-07) was up-regulated in mature worms and miracidia. Supervillin binds to the sides of actin filaments, has actin bundling activity and may also mediate binding to non-muscle myosin II [53,54]. Two transcripts encoding muscle proteins were also up-regulated in mature worms and miracidia. These encoded titin (TC12891, 1e-22) and muscle LIM protein (MLP) (TC17700, 3e-07). In Drosophila, MLP mutants arrest during pupation with impaired muscle function. The site of action of MLP is at the Z disc where it co-localizes with D-titin, which is itself crucial for sarcomere organization and stretch mechanics. These two proteins have been suggested to co-operate to stabilize Drosophila muscle sarcomeres [55]. Clearly, if PZQ were to bind to any of these proteins there would be significant potential for the disruption of muscle function.
These data provide new insights into the possible mechanism of action of PZQ. We are currently conducting experiments to further elucidate the potential role of a number of the products of these genes, either as the direct target of PZQ or as members of pathways that are immediately affected by the binding of the drug to its target.
Supplementary Material
Acknowledgments
We would like to thank Dr. Geoffrey N. Gobert of the Queensland Institute of Medical Research and Australian Centre for International Health and Nutrition, Brisbane, Qld, Australia for his permission to use the microarrays employed in this study and Dr. Karl F. Hoffmann, University of Cambridge, UK for generously providing the microarray protocols used in this paper. We would also like to thank Dr. Stephen Stricker, Dept. of Biology, University of New Mexico for his help with the confocal microscopy reported in this paper and Dr. Bruce Hofkin, Dept. of Biology, University of New Mexico for reading the manuscript. Images in this paper were generated in the University of New Mexico Cancer Center Fluorescence Microscopy Facility, supported as detailed on the webpage: http://hsc.unm.edu/crtc/microscopy/Facility.html. Technical support was supplied by the Molecular Biology Core Facility of the Dept. of Biology, University of New Mexico. This work was funded by NIH grant number 1P20RR18754 from the Institute Development Award (IDeA) Program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Cioli D, Pica-Mattoccia L. Praziquantel. Parasitol Res. 2003;90:S3–9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- 2.Andrews P. Effect of praziquantel on the free living stages of Schistosoma mansoni. Z Parasitenkd. 1978;56:99–106. doi: 10.1007/BF00925943. [DOI] [PubMed] [Google Scholar]
- 3.Liang YS, Coles GC, Doenhoff MJ, Southgate VR. In vitro responses of praziquantel-resistant and-susceptible Schistosoma mansoni to praziquantel. Int J Parasitol. 2001;31:1227–35. doi: 10.1016/s0020-7519(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 4.Mattos ACA, Kusel JR, Pimenta PFP, Coelho PMZ. Activity of praziquantel on in vitro transformed Schistosoma mansoni sporocysts. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):283–7. doi: 10.1590/s0074-02762006000900044. [DOI] [PubMed] [Google Scholar]
- 5.Gönnert R, Andrews P. Praziquantel a new board-spectrum antischistosomal agent. Z Parasitenkd. 1977;52:129–50. doi: 10.1007/BF00389899. [DOI] [PubMed] [Google Scholar]
- 6.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 7.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol. 2004;34:527–33. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Pax R, Bennett JL, Fetterer R. A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn-Schmiedebergs Arch Pharmacol. 1978;304:309–15. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- 9.Harnett W, Kusel JR. Increased exposure of parasite antigens at the surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology. 1986;93:401–5. doi: 10.1017/s0031182000051568. [DOI] [PubMed] [Google Scholar]
- 10.Redman CA, Robertson A, Fallon PG, et al. Praziquantel: an urgent and exciting challenge. Parasitol Today. 1996;12:14–20. doi: 10.1016/0169-4758(96)80640-5. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg RM. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Kohn AB, Anderson PAV, Roberts-Misterly JM, Greenberg RM. Schistosome calcium channel β subunits—unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J Biol Chem. 2001;276:36873–6. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- 13.Pica-Mattoccia L, Orsini T, Basso A, et al. Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp Parasitol. 2008;119:332–5. doi: 10.1016/j.exppara.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Pica-Mattoccia L, Valle C, Basso A, et al. Cytochalasin D abolishes the schistosomicidal activity of praziquantel. Exp Parasitol. 2007;115:344–51. doi: 10.1016/j.exppara.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Lima SF, Vieira LQ, Harder A, Kusel JR. Effects of culture and praziquantel on membrane fluidity parameters of adult Schistosoma mansoni. Parasitology. 1994;109:57–64. doi: 10.1017/s0031182000077763. [DOI] [PubMed] [Google Scholar]
- 16.Harder A, Goossens J, Andrews P. Influence of praziquantel and Ca2+ on the bilayer-isotropic-hexagonal transition of model membranes. Mol Biochem Parasitol. 1988;29:55–9. doi: 10.1016/0166-6851(88)90119-3. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro F, Coelho PM, Vieira LQ, Watson DG, Kusel JR. The effect of praziquantel treatment on glutathione concentration in Schistosoma mansoni. Parasitology. 1998;116:229–36. doi: 10.1017/s0031182097002291. [DOI] [PubMed] [Google Scholar]
- 18.Tallima H, El Ridi R. Praziquantel binds Schistosoma mansoni adult worm actin. Int J Antimicrob Agents. 2007;29:570–5. doi: 10.1016/j.ijantimicag.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Angelucci F, Basso A, Bellelli A, Brunori M, Pica-Mattoccia L, Valle C. The anti-schistosomal drug praziquantel is an adenosine antagonist. Parasitology. 2007;134:1215–21. doi: 10.1017/S0031182007002600. [DOI] [PubMed] [Google Scholar]
- 20.Aragon AD, Imani RA, Blackburn VR, Cunningham C. Microarray based analysis of temperature and oxidative stress induced messenger RNA in Schistosoma mansoni. Mol Biochem Parasitol. 2008;162:134–41. doi: 10.1016/j.molbiopara.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Gobert GN, McInnes R, Moertel L, et al. Transcriptomics tool for the human Schistosoma blood flukes using microarray gene expression profiling. Exp Parasitol. 2006;114:160–72. doi: 10.1016/j.exppara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Vencio RZN, Koide T. HTself: self-self based statistical test for low replication microarray studies. DNA Res. 2005;12:211–4. doi: 10.1093/dnares/dsi007. [DOI] [PubMed] [Google Scholar]
- 24.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–6. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Lee YS, Park H, Kim CS. Formation of pyrazinoisoquinoline ring system by the tandem amidoalkylation and N-acyliminium ion cyclization: an efficient synthesis of Praziquantel. Tetrahedron. 1998;54:7395–400. [Google Scholar]
- 26.Andrews P, Thomas H, Pohlke R, Seubert J. Praziquantel. Med Res Rev. 1983;3:147–200. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- 27.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail M, Botros S, Metwally A, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–5. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 29.Nene V, Dunne DW, Johnson KS, Taylor DW, Cordingley JS. Sequence and expression of a major egg antigen from Schistosoma mansoni—homologies to heat-shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986;21:179–88. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KS, Wells K, Bock JV, Nene V, Taylor DW, Cordingley JS. The 86-Kilodalton antigen from Schistosoma mansoni is a heat-shock protein homologous to yeast HSP90. Mol Biochem Parasitol. 1989;36:19–28. doi: 10.1016/0166-6851(89)90196-5. [DOI] [PubMed] [Google Scholar]
- 31.Neumann S, Ziv E, Lantner F, Schechter I. Regulation of HSP70 gene-expression during the life-cycle of the parasitic helminth Schistosoma mansoni. Eur J Biochem. 1993;212:589–96. doi: 10.1111/j.1432-1033.1993.tb17697.x. [DOI] [PubMed] [Google Scholar]
- 32.Xiao SH, Catto BA, Webster LT. Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J Infect Dis. 1985;151:1130–7. doi: 10.1093/infdis/151.6.1130. [DOI] [PubMed] [Google Scholar]
- 33.Andrews P. A summary of the efficacy of praziquantel against schistosomes in animal experiments and notes on its mode of action. Arzneimittel-Forschung. 1981;31:538–41. [PubMed] [Google Scholar]
- 34.Fallon PG, Mubarak JS, Fookes RE, et al. Schistosoma mansoni: maturation rate and drug susceptibility of different geographic isolates. Exp Parasitol. 1997;86:29–36. doi: 10.1006/expr.1997.4149. [DOI] [PubMed] [Google Scholar]
- 35.El-Bassiouni EA, Helmy MH, Saad EI, El-Nabi Kamel MA, Abdel-Meguid E, Hussein HS. Modulation of the antioxidant defence in different developmental stages of Schistosoma mansoni by praziquantel and artemether. Br J Biomed Sci. 2007;64:168–74. doi: 10.1080/09674845.2007.11732782. [DOI] [PubMed] [Google Scholar]
- 36.Vermeire JJ, Taft AS, Hoffmann KF, Fitzpatrick JM, Yoshino TP. Schistosoma mansoni: DNA microarray gene expression profiling during the miracidiumto-mother sporocyst transformation. Mol Biochem Parasitol. 2006;147:39–47. doi: 10.1016/j.molbiopara.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Coles GC. Oxidative phosphorylation in Schistosoma mansoni. Nature. 1972;240:488–9. doi: 10.1038/240488a0. [DOI] [PubMed] [Google Scholar]
- 38.van Oordt BE, van den Heuvel JM, Tielens AGM, van den Bergh SG. The energy production of the adult Schistosoma mansoni is for a large part aerobic. Mol Biochem Parasitol. 1985;16:117–26. doi: 10.1016/0166-6851(85)90080-5. [DOI] [PubMed] [Google Scholar]
- 39.Coles GC. The metabolism of schistosomes: a review. Int J Biochem. 1973;4:319–37. [Google Scholar]
- 40.Horemans AMC, Tielens AGM, van den Bergh SG. The reversible effect of glucose on the energy metabolism of Schistosomamansoni cercariae and schistosomula. Mol Biochem Parasitol. 1992;51:73–9. doi: 10.1016/0166-6851(92)90202-u. [DOI] [PubMed] [Google Scholar]
- 41.Ross OA, Bueding E. Survival of Schistosoma mansoni in vitro. Proc Soc Exp Biol Med. 1950;73:179–82. doi: 10.3181/00379727-73-17755. [DOI] [PubMed] [Google Scholar]
- 42.Waisberg M, Lobo FP, Cerqueira GC, et al. Microarray analysis of gene expression induced by sexual contact in Schistosoma mansoni. BMC Genomics. 2007;8:181. doi: 10.1186/1471-2164-8-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitzpatrick JM, Hoffmann KF. Dioecious Schistosoma mansoni express divergent gene repertoires regulated by pairing. Int J Parasitol. 2006;36:1081–9. doi: 10.1016/j.ijpara.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Van Oordt BE, Tielens AGM, Van den Bergh SG. Aerobic to anaerobic transition in the carbohydrate metabolism of Schistosoma mansoni cercariae during transformation in vitro. Parasitology. 1989;98:409–15. doi: 10.1017/s0031182000061497. [DOI] [PubMed] [Google Scholar]
- 45.Tielens AGM, van de Pas FAM, van den Heuvel JM, van den Bergh SG. The aerobic energy metabolism of Schistosoma mansoni miracidia. Mol Biochem Parasitol. 1991;46:181–4. doi: 10.1016/0166-6851(91)90211-n. [DOI] [PubMed] [Google Scholar]
- 46.Tielens AGM, Horemans AMC, Dunnewijk R, van der Meer P, van den Bergh SG. The facultative anaerobic energy metabolism of Schistosoma mansoni sporocysts. Mol Biochem Parasitol. 1992;56:49–57. doi: 10.1016/0166-6851(92)90153-b. [DOI] [PubMed] [Google Scholar]
- 47.Mecozzi B, Rossi A, Lazzaretti P, et al. Molecular cloning of Schistosoma mansoni calcineurin subunits and immunolocalization to the excretory system. Mol Biochem Parasitol. 2000;110:333–43. doi: 10.1016/s0166-6851(00)00287-5. [DOI] [PubMed] [Google Scholar]
- 48.Rossi A, Wippersteg V, Klinkert MQ, Grevelding CG. Cloning of 5′ and 3′ flanking regions of the Schistosoma mansoni calcineurin A gene and their characterization in transiently transformed parasites. Mol Biochem Parasitol. 2003;130:133–8. doi: 10.1016/s0166-6851(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 49.Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–70. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 50.Talla E, de Mendonça RL, Degand I, Goffeau A, Ghislain M. Schistosoma mansoni Ca2+-ATPase SMA2 restores viability to yeast Ca2+-ATPase-deficient strains and functions in calcineurin-mediated Ca2+ tolerance. J Biol Chem. 1998;273:27831–40. doi: 10.1074/jbc.273.43.27831. [DOI] [PubMed] [Google Scholar]
- 51.Rossi A, Ghislain M, Klinkert MQ. Regulatory pathways in ion homeostasis involving calcineurin and a calcium transporting ATPase are different between yeast and schistosomes. Mol Biochem Parasitol. 2004;135:165–9. doi: 10.1016/j.molbiopara.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Valle C, Troiani AR, Festucci A, et al. Sequence and level of endogenous expression of calcium channel beta subunits in Schistosoma mansoni displaying different susceptibilities to praziquantel. Mol Biochem Parasitol. 2003;130:111–5. doi: 10.1016/s0166-6851(03)00171-3. [DOI] [PubMed] [Google Scholar]
- 53.Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61:2614–23. doi: 10.1007/s00018-004-4225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Takizawa N, Crowley JL, et al. F-actin and myosin II binding domains in supervillin. J Biol Chem. 2003;278:46094–106. doi: 10.1074/jbc.M305311200. [DOI] [PubMed] [Google Scholar]
- 55.Clark KA, Bland JM, Beckerle MC. The Drosophila muscle LIM protein, Mlp84B, cooperates with D-titin to maintain muscle structural integrity. J Cell Sci. 2007;120:2066–77. doi: 10.1242/jcs.000695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.