Abstract
The resistance of a plant community against herbivore attack may depend on plant species richness, with monocultures often much more severely affected than mixtures of plant species. Here, we used a plant–herbivore system to study the effects of selective herbivory on consumption resistance and recovery after herbivory in 81 experimental grassland plots. Communities were established from seed in 2002 and contained 1, 2, 4, 8, 16 or 60 plant species of 1, 2, 3 or 4 functional groups. In 2004, pairs of enclosure cages (1 m tall, 0.5 m diameter) were set up on all 81 plots. One randomly selected cage of each pair was stocked with 10 male and 10 female nymphs of the meadow grasshopper, Chorthippus parallelus. The grasshoppers fed for 2 months, and the vegetation was monitored over 1 year. Consumption resistance and recovery of vegetation were calculated as proportional changes in vegetation biomass. Overall, grasshopper herbivory averaged 6.8%. Herbivory resistance and recovery were influenced by plant functional group identity, but independent of plant species richness and number of functional groups. However, herbivory induced shifts in vegetation composition that depended on plant species richness. Grasshopper herbivory led to increases in herb cover at the expense of grasses. Herb cover increased more strongly in species-rich mixtures. We conclude that selective herbivory changes the functional composition of plant communities and that compositional changes due to selective herbivory depend on plant species richness.
Keywords: Biodiversity, The Jena Experiment, Herbivory, Grasshopper, Plant functional groups
Introduction
In terrestrial ecosystems, herbivores consume well over 30% of autotrophic biomass (Cebrian 2004). Insect herbivores remove on average around 10% (Crawley 1983), but there may be considerable differences in herbivory due to factors such as habitat heterogeneity (Lawton and Strong 1981), predation (Root 1973), plant nutrient content (Cebrian and Lartigue 2004), productivity (McNaughton et al. 1989), plant species and functional identity (Scherber et al. 2006), and plant species richness (Mulder et al. 1999). Plant species richness, in particular, has long been hypothesized to be an important determinant of the amount of herbivore damage a plant community experiences (e.g., Root 1973; Mulder et al. 1999; Pfisterer et al. 2003). However, the consequences of herbivore attack for species-poor versus species-rich plant communities have so far been less frequently explored. It is poorly known how resistant species-poor versus species-rich plant communities are against herbivore attack, and how quickly they recover from herbivory. While herbivore damage is a measure of the resistance of a given plant community towards herbivory (i.e., its capability to withstand herbivore attack: Painter 1936; Wise and Carr 2008), the regrowth after herbivory measures a plant community’s recovery (also called compensation; McNaughton 1985; Trumble et al. 1993).
As shown by both experimental studies (e.g., McNaughton 1985; Unsicker et al. 2006; Lanta 2007) and recent meta-analyses (e.g., Balvanera et al. 2006), the resistance of plant communities to herbivore attack and their recovery from it may be reduced in species-poor systems when a threshold amount of damage has been exceeded. Associated shifts in plant community composition, induced by frequency-dependent selective herbivory, may potentially amplify or dampen resistance and recovery of plant communities that differ in plant species richness. Traditionally, species richness has been treated as a response rather than an explanatory variable in plant–herbivore studies (e.g., Olff and Ritchie 1998), mainly because it has been difficult to set up systems in which species richness varies independently of local abiotic conditions. Even in those cases where plant species richness has been manipulated as independently as possible (removal experiments, Díaz et al. 2003; sown diversity gradients, Schmid and Hector 2004), it has been difficult to separate the effects of species richness from functional group or even species identity effects. Further, we still know little about how plant species richness modulates herbivore-induced plant community dynamics (Crawley 1983). While a lot of work has been done on the effects of species richness on herbivory at the level of individual plant species, whole community responses to defoliation are much less explored. Paradoxically, we know that selective herbivory may change plant biodiversity (if preference is frequency-dependent; Cottam 1985; Pacala and Crawley 1992), but we do not know if the magnitude of this change will vary between species-poor and species-rich plant communities.
In the present study, we test if species-rich plant communities are more resistant to insect herbivory and recover more quickly after herbivore attack than species-poor plant communities. We deliberately use rather high herbivore densities to investigate how plant communities cope with intense herbivore attack. We use 81 experimental grassland communities grown from seed on former arable land, into which we introduce populations of a locally important insect herbivore. To test if plant functional identity or plant species richness is a better predictor of resistance and recovery, we systematically vary both plant species richness and number and identity of plant functional groups. The experimental factors are (1) ± herbivore addition, (2) number of plant species, (3) number of plant functional groups (FG), and (4) identity of plant FG. We measure above ground biomass and vegetation cover before, during and after herbivore attack of ≤55 days duration, in order to estimate consumption resistance and effects of herbivory on plant community composition (e.g., Bach 2001). Our two main a priori hypotheses are:
Both consumption resistance and recovery from selective herbivory are higher in species-rich than in species-poor plant communities;
Vegetation composition (species and functional richness, functional group composition) will change because of herbivore selectivity.
Materials and methods
Study organism
In terms of biomass turnover, Orthopterans are the most important group of phytophagous insects in temperate grasslands (van Hook 1971; Köhler et al. 1987). The meadow grasshopper Chorthippus parallelus (Zetterstedt) (Orthoptera: Acrididae, Gomphocerinae) is the locally most abundant grasshopper both in managed grasslands around the study site (Pratsch 2004) and in Central European grasslands in general (Ingrisch and Köhler 1998). Chorthippus parallelus has a feeding preference for grasses (Bernays and Chapman 1970a, b), but forbs and legumes may also be consumed in low amounts (Bernays and Chapman 1970a, b; Unsicker et al. 2008). Specht et al. (2008) reported survival and reproduction even in completely grass-free plant communities. The biology of C. parallelus is described in Richards and Waloff (1954), Bernays and Chapman (1970a, b), and Reinhardt and Köhler (1999). Chorthippus parallelus is univoltine and usually has four nymphal stages. Adults occur at the field site between July and August in densities of about 0.9–10.9 individuals per m2 (Ingrisch and Köhler 1998).
General design of the Jena Experiment
The research was conducted at a field site called The Jena Experiment (Fig. 1a; Roscher et al. 2004). The site consists of 82 experimental grassland plots that were installed in spring 2001 on former arable land (Fig. 1a). Plots measured 20 × 20 m in size and were allocated to four blocks in a randomized complete blocks design. Each plot was seeded in May 2002 with 1, 2, 4, 8, 16 or 60 plant species in combinations of 1, 2, 3 or 4 plant functional groups (FG). Species for each plot were drawn from a pool of 60 plant species of Central European Arrhenatherum meadows using randomization constrained on block and FG identity (16 grass, 12 legume, 12 small herb and 16 tall herb species). FGs were defined a priori using cluster analysis of a trait matrix. Thus, each mixture either contained either grasses, legumes, small herbs, tall herbs, or possible combinations of these (1–4 FG). Note that 1-FG plots only occurred up to the 16-species mixtures, i.e. the 60-species mixtures always contained all 4 FG; for details, see Roscher et al. (2004). Plots were mown (June, September) and weeded (April, July) every year to maintain species compositions. The resulting communities (especially the full 60-species mixture) closely resembled locally occurring Arrhenatherum communities that are also mown twice a year according to good agricultural practice.
Fig. 3.
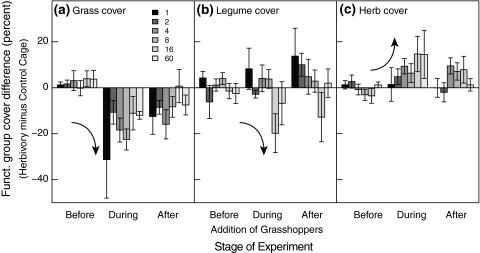
Differences in a grass, b legume and c herb cover between herbivory and control cages. Each panel shows the combined effects of experimental stage (before, during and after addition of grasshoppers) and plant species richness. Low (high) plant species richness is indicated by dark (light) shading of bars. Negative differences indicate lower functional group cover in herbivory than in control cages. Curved arrows indicate the main direction of changes after grasshopper addition. Error bars show ±1 SE of the mean. Grass cover: n = 44; legume cover: n = 43; herb cover: n = 64
Cage experiment
We used enclosure cages (Schmitz 2004) to study consumption resistance and recovery of plant communities (Fig. 1b). Between 14 and 25 June 2004, we installed two cylindrical cages (1 m high, 0.5 m diameter) per plot 1.4 m apart (n = 162 cages on 81 plots; Bellis perennis monoculture excluded because of poor establishment). Each cage consisted of a drum-shaped galvanized aluminum frame welded from 8-mm-diameter rods, covered with 2-mm aluminum mesh. Cages had a 12-cm aluminum sheet metal base of 3 mm thickness that was sunk in soil, to which the aluminum mesh was strapped. Herbivory versus control treatments were randomly assigned to each of the two cages per plot. Before adding the grasshoppers, we removed other aboveground invertebrates and predators from all cages using a D-Vac sampler. Between 6 and 15 July 2004, third and fourth instar nymphs of C. parallelus were caught from three adjacent Arrhenatherum meadows using sweep nets, and nymphs were sexed, weighed, and transferred at random to the herbivory cages one block at a time. Every ‘herbivory’ cage received 10 male and 10 female nymphs, while none were added to the ‘control’ cages. This density was chosen based on expected nymphal mortality and observed densities in unfertilized grassland (around 6–40 individuals per m2, Gyllenberg 1974; outbreak densities of up to 300/m2, Ingrisch and Köhler 1998).
Vegetation measurements
To estimate changes in vegetation biomass and composition, we measured vegetation cover and biomass before, during and after grasshopper feeding (referred to as stage 1, 2 and 3 of the experiment). Vegetation cover was estimated visually using a 1% scale. Vegetation biomass was harvested at ~3 cm above ground using garden scissors. Samples were oven-dried at 70°C for 48 h and weighed to four significant digits (e.g., 12.31 g dry weight per plant). Between 27 May and 10 June 2004, total plant biomass was harvested inside two frames of 20 × 50 cm in the center of the designated cage positions. Between 14 and 26 June 2004, all plots were mown and cages were installed. Subsequently, we visually estimated initial vegetation cover for every FG inside each cage (28 June to 1 July 2004). Grasshoppers were added between 6 and 15 July 2004 (see above). Two weeks later, at the peak of grasshopper feeding (3–9 August 2004), we estimated vegetation cover separately for each plant FG. Grasshoppers were allowed to feed for another 4 weeks. Directly after removal of grasshoppers from the cages (31 August to 7 September), we harvested vegetation biomass separately for every plant species in both herbivory and control cages at ~1 cm height above ground. About 8 months after grasshopper removal from the cages, we again measured vegetation cover (26 May to 4 June 2005) per FG and biomass per plant species (20 June to 1 July 2005) as described. Overall, the resulting dataset contained data on plant FG cover from stages 1–3, total biomass for stages 1–3, visually estimated herbivory for stages 2–3, and species-specific biomass for stages 2–3.
Calculation of consumption resistance and recovery after herbivory
Both resistance and recovery of a plant community after herbivore attack can be expressed either as a biomass difference between different damage levels (Painter 1936, 1958; Beck 1965; Stowe et al. 2000), or using a proportional scale (McNaughton 1985). Here, we use a proportional scale and define consumption resistance as H/C × 100 (%) ∀ H ≤ C, where H is biomass of the herbivory cage, and C is the biomass of the control cage at stage 2 (August 2004). Consumption resistance ranged from 0% (no resistance) to 100% (maximum resistance, H = C). Recovery was calculated using the same formula, but allowing for overcompensation (H > C) and using data from stage 3 (May 2005), i.e. after vegetation had about 8 months time to recover from herbivory. Figure 1c–f shows an example of low resistance and remarkably high recovery in a grass monoculture.
Calculation of changes in vegetation composition
Changes in plant species and plant FG richness were calculated based on species-specific biomass. Biomass per FG was calculated by summing the biomasses of the corresponding plant species of each FG. Changes in vegetation composition were analysed by calculating the differences in species richness, functional richness, and functional group cover between each H and C cage. FG cover analyses were based on those replicates that contained the corresponding FG. For example, grass cover analyses were based on n = 44 plots that contained grasses.
Statistical analysis
Data analysis was carried out using R 2.10.0 (R Development Core Team 2009). Resistance and recovery were analysed separately for each stage (n = 81 datapoints). To assess plant community changes over time, cover differences were analysed as repeated measures (n = 3 stages, each 81 plots). For example, decreasing grass cover due to herbivory might be indicated by 0% difference at stage 1, −10% difference at stage 2, and −5% difference at stage 3. For all analyses, we used linear mixed-effects models fit by maximum likelihood (nlme package, version 3.1-96; Pinheiro and Bates 2000). We started by fitting a maximal model with block included as a random effect and the following sequence of fixed effects: Stage + Logdiv + FG + Grass + Leg + Smallherb, plus two-way interactions between all terms. Stage was only included when analysing cover differences. Logdiv is log-transformed plant species richness, and Grass, Leg and Smallherb indicate grass, legume or small herb presence. We included random intercepts for each plot (1–81) and random slopes for each stage (1–3) of the experiment. Block effects were incorporated using variance functions (see below). When FG-specific variables were analysed (e.g. grass biomass), analyses were restricted to plots containing that group. Each maximal model was simplified using a modified version of stepAIC (MASS library, version 7.3-3; Venables and Ripley 2002) that computes AICc instead of AIC. Models were considered minimal adequate, when AICc reached a global minimum (Burnham and Anderson 2002). P values for parameter estimates were derived from comparisons with Student’s t distribution (by dividing parameter estimates by their standard errors). In addition, F tests were used to assess the overall significance of terms in each model (adding terms sequentially to a null model). For all models mentioned, we inspected the residuals for normality, constant mean and variance. Variance functions g(Logdiv, δ) = exp (δ × Logdiv) were used to model heteroscedasticity (where δ is estimated from the data; Pinheiro and Bates 2000). Block was included as a grouping factor in the specification of the variance function if this led to smaller AICc. Temporal autocorrelation in the residuals was accounted for by updating each model with a first-order autoregressive correlation structure (Pinheiro and Bates 2000). Parameter estimates were compared using successive difference contrasts, where the jth contrast c between successive means μ is c j = μ j+1 − μ j (Venables and Ripley 2002). Averages are given as arithmetic mean ± 1 SE.
Results
Grasshopper herbivory induced considerable differences in the response variables over time (Table 1). In particular, there were strong effects of herbivory on grass and herb cover. In addition, there were marked temporal differences in all response variables, depending on experimental stages 1–3.
Table 1.
Summary statistics of the response variables analysed in this study
| Variable (unit) | Statistic | June 2004 | August 2004 | May 2005 | |||
|---|---|---|---|---|---|---|---|
| Controla | Herbivoryb | Control | Herbivory | Control | Herbivory | ||
| Species richnessc | Mean | –f | – | 4.64 | 4.59 | 4.86 | 5.02 |
| SE | – | – | 0.54 | 0.58 | 0.58 | 0.66 | |
| FG richnessd | Mean | – | – | 2.00 | 1.93 | 2.01 | 1.99 |
| SE | – | – | 0.12 | 0.12 | 0.12 | 0.12 | |
| Total cover (%) | Mean | 60.04 | 60.69 | 84.59 | 80.52 | 85.62 | 86.04 |
| SE | 2.40 | 2.26 | 2.39 | 2.60 | 2.88 | 2.92 | |
| Grass cover (%) | Mean | 19.69 | 20.90 | 24.36 | 15.01 | 24.53 | 20.01 |
| SE | 2.95 | 2.96 | 3.26 | 2.47 | 3.26 | 3.01 | |
| Legume cover (%) | Mean | 11.32 | 11.43 | 22.94 | 21.58 | 24.57 | 25.62 |
| SE | 2.19 | 2.09 | 3.58 | 3.55 | 3.92 | 4.19 | |
| Herb cover (%) | Mean | 29.02 | 28.28 | 37.30 | 43.93 | 36.52 | 40.41 |
| SE | 2.87 | 2.71 | 3.62 | 3.83 | 3.62 | 3.65 | |
| Legume height (cm) | Mean | – | – | 19.42 | 17.37 | 19.78 | 19.05 |
| SE | – | – | 2.92 | 2.84 | 3.03 | 2.96 | |
| Grass height (cm) | Mean | – | – | 14.33 | 8.68 | 25.65 | 21.60 |
| SE | – | – | 1.91 | 1.31 | 3.06 | 2.79 | |
| Vegetation height (cm) | Mean | 13.23 | 13.00 | 30.64 | 26.60 | 40.40 | 38.83 |
| SE | 0.62 | 0.61 | 2.14 | 2.29 | 2.39 | 2.29 | |
| Cage biomass (gm−2) | Mean | – | – | 216.57 | 213.16 | 345.81 | 348.67 |
| SE | – | – | 15.26 | 19.91 | 29.91 | 32.07 | |
| Grass biomass (gm−2) | Mean | – | – | 55.92 | 25.58 | 103.38 | 76.26 |
| SE | – | – | 8.13 | 4.44 | 15.42 | 11.39 | |
| Legume biomass (gm−2) | Mean | – | – | 88.86 | 99.85 | 156.86 | 173.20 |
| SE | – | – | 16.65 | 20.44 | 29.21 | 34.32 | |
| Small herb biome (gm−2) | Mean | – | – | 28.48 | 32.35 | 29.62 | 31.29 |
| SE | – | – | 5.18 | 5.03 | 5.70 | 5.33 | |
| Tall herb biomass (gm−2) | Mean | – | – | 43.30 | 55.38 | 55.95 | 67.91 |
| SE | – | – | 7.66 | 9.32 | 10.24 | 11.10 | |
| Herb biomass (gm−2) | Mean | – | – | 14.10 | 17.22 | 16.80 | 19.48 |
| SE | – | – | 1.53 | 1.85 | 2.08 | 2.09 | |
Arithmetic means and standard error of each mean (each n = 81)
aControl cage
bHerbivory cage
cRealized species richness per 0.2 m2
dRealized number of functional groups per 0.2 m2
eSmall herb biomass
fNot measured or not applicable
Initial plant community composition inside the cages
Realized plant species richness inside control cages was highly significantly correlated with sown species richness (August 2004, r = 0.93, t = 23.19, df = 79, P < 0.0001) and ranged from 1 to 28 plant species per cage (0.2 m2); to keep the design balanced, we therefore used sown numbers of species and functional groups as explanatory variables for all subsequent analyses.
Amount of grasshopper herbivory
Overall, grasshopper herbivory averaged 6.8 ± 5.7% (n = 64; plots containing Onobrychis viciifolia L. excluded), reaching values of 16.4 ± 7.8% in plots containing grasses (n = 44, Fig. 1). Visual estimates of background herbivory in control cages (i.e. without grasshoppers added) amounted to 0.8 ± 0.1%, caused by curculionid and chrysomelid (Longitarsus sp.) beetles that had escaped initial removal or emerged later from pupae in the soil.
Consumption resistance
Consumption resistance (H/C at stage 2) averaged 64% and was significantly lower if plots contained grasses (F 1,37 = 17.12, P = 0.0002). In plots containing grasses, consumption resistance increased significantly with the number of plant functional groups present (F 1,21 = 7.94, P = 0.0103). The least consumption-resistant plots were a two-species mixture of Festuca rubra L. and Trisetum flavescens L. (resistance = 3.1%), followed by monocultures of F. rubra (3.2%, Fig. 1) and Poa pratensis L. (25.5%). The most consumption–resistant plot was an eight-species mixture containing all four plant functional groups (96.5%).
Recovery of vegetation after herbivory
Recovery after herbivory was not significantly affected by the explanatory variables and averaged 72.6%. However, individual plant communities differed greatly in recovery: while the two-species mixture between F. rubra and T. flavescens showed only 58.7% recovery, the F. rubra monoculture overcompensated and had a recovery of 123.3%. The P. pratensis monoculture recovered by 81.4%. On the other hand, there were plots that showed adverse effects of herbivory and did even worse than directly after herbivore attack. For example, plots with lowest recovery included a 16-species mixture containing all four functional groups (recovery 20.8%), followed by a F. pratensis monoculture (recovery 26.2%) and a F. pratensis mixture with Carum carvi L. (51.6%).
Effects of herbivory on total vegetation cover
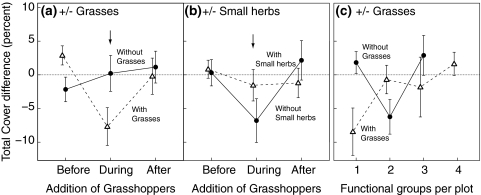
Total vegetation cover in general did not decrease significantly under herbivory (intercept term in Table 2). Total cover was about 13% lower if plots contained grasses and differed significantly between stages (Table 3). In grass-containing plots, total cover declined significantly between stages 1 and 2, while it recovered slightly and significantly between stages 2 and 3 (Fig. 2a, Stage 2-1: Grass and Stage 3-2: Grass interactions in Table 2; Stage: Grass interaction in Table 3). The converse was true for plots that contained small herbs: Small herbs had a “rescuing” effect on total cover, i.e., the decline in total cover was less severe if plots contained small herbs (Fig. 2b; Stage 2-1: Sherb and Stage 3-2: Sherb interactions in Table 2; Stage: Sherb interaction in Table 3). Plant functional group richness also had a “rescuing” effect on total cover: grass-containing plots suffered less from herbivory if more plant functional groups were present (Fig. 2c; Funcgr: Grass interaction in Table 2). In addition, there was a significant interaction between legume presence and plant species richness on total cover differences. This interaction was mainly driven by the 16-species mixtures.
Table 2.
Parameter estimates, standard errors, t and P values of the fixed-effects terms in mixed-effects models with grass, herb, legume and total cover as response variables
| Grass cover | Herb cover | Legume cover | Total cover | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est.a | SEb | DFc | t | P | Est. | SE | DF | t | P | Est. | SE | DF | t | P | Est. | SE | DF | t | P | |
| Intercept | −6.97 | 6.80 | 86 | −1.03 | 0.308 | 0.63 | 2.50 | 124 | 0.25 | 0.802 | 1.94 | 5.78 | 80 | 0.34 | 0.738 | −0.62 | 4.34 | 156 | −0.14 | 0.887 |
| Stage 2-1d | −19.43 | 2.64 | 86 | −7.35 | <0.001 | 1.02 | 3.81 | 124 | 0.27 | 0.790 | −1.97 | 6.46 | 80 | −0.31 | 0.761 | −0.76 | 3.33 | 156 | −0.23 | 0.820 |
| Stage 3-2e | 8.89 | 2.64 | 86 | 3.36 | 0.001 | −0.76 | 3.81 | 124 | −0.20 | 0.842 | 11.31 | 6.46 | 80 | 1.75 | 0.084 | 5.15 | 3.33 | 156 | 1.55 | 0.124 |
| Log Plant species richness (PSR) | –f | – | – | – | – | −0.01 | 0.94 | 61 | −0.01 | 0.993 | −3.98 | 1.82 | 40 | −2.19 | 0.034 | 0.64 | 1.21 | 72 | 0.53 | 0.595 |
| Functional group richness (FG) | −1.56 | 4.56 | 38 | −0.34 | 0.734 | – | – | – | – | – | 3.12 | 2.54 | 40 | 1.23 | 0.226 | −1.44 | 3.59 | 72 | −0.40 | 0.689 |
| Presence of grasses | – | – | – | – | – | 6.48 | 2.82 | 61 | 2.30 | 0.025 | – | – | – | – | – | −13.46 | 5.95 | 72 | −2.26 | 0.027 |
| Presence of legumes | −26.86 | 21.42 | 38 | −1.25 | 0.218 | – | – | – | – | – | – | – | – | – | – | 6.32 | 4.54 | 72 | 1.39 | 0.168 |
| Presence of small herbs | −0.93 | 6.51 | 38 | −0.14 | 0.888 | – | – | – | – | – | – | – | – | – | – | 3.88 | 3.85 | 72 | 1.01 | 0.317 |
| PSR: legume presence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | −3.61 | 1.57 | 72 | −2.30 | 0.025 |
| FG: grass presence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 8.35 | 3.41 | 72 | 2.45 | 0.017 |
| FG: legume presence | 14.27 | 7.92 | 38 | 1.81 | 0.080 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Grass: small herb presence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | −11.96 | 5.90 | 72 | −2.03 | 0.046 |
| Legume: small herb presence | −21.13 | 10.63 | 38 | −1.99 | 0.054 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Stage 2-1: PSRg | – | – | – | – | – | 3.34 | 1.31 | 124 | 2.55 | 0.012 | −5.94 | 2.03 | 80 | −2.93 | 0.004 | – | – | – | – | – |
| Stage 3-2: PSR | – | – | – | – | – | −1.10 | 1.31 | 124 | −0.84 | 0.401 | 2.05 | 2.03 | 80 | 1.01 | 0.315 | – | – | – | – | – |
| Stage 2-1: FG | – | – | – | – | – | – | – | – | – | – | 5.49 | 2.83 | 80 | 1.94 | 0.056 | – | – | – | – | – |
| Stage 2-1: grass presence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | −14.56 | 4.03 | 156 | −3.61 | <0.001 |
| Stage 2-1: small herb presence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 7.73 | 4.02 | 156 | 1.93 | 0.056 |
| Stage 3-2: FG | – | – | – | – | – | – | – | – | – | – | −4.44 | 2.83 | 80 | −1.57 | 0.121 | – | – | – | – | – |
| Stage 3-2: grass presence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 8.71 | 4.03 | 156 | 2.16 | 0.032 |
| Stage 3-2: small herb presence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | −10.41 | 4.02 | 156 | −2.60 | 0.011 |
Each parameter is tested marginally, i.e., in presence of all other terms in the model. Differences between stages of the experiment are successive difference contrasts (the Intercept term is the overall mean). Grass cover: n = 44 plots × 3 stages = 132; herb cover: n = 64 × 3 = 192; legume cover: n = 43 × 3 = 129; total cover: n = 81 × 3 = 243
aEstimate
bStandard error
cDegrees of freedom
d,eDifferences between Stages of the experiment
fTerms excluded during model simplification indicated by “–”
gInteraction terms are indicated by “:”
Table 3.
Numerator and denominator degrees of freedom, F values and P values for Wald tests for the fixed-effects terms in mixed-effects models with grass, herb, legume and total cover as response variables
| Grass cover | P | Herb cover | P | Legume cover | P | Total cover | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nDFa | dDFb | F | nDF | dDF | F | nDF | dDF | F | nDF | dDF | F | |||||
| Intercept | 1 | 86 | 13.21 | <0.001 | 1 | 124 | 6.54 | 0.012 | 1 | 80 | 0.32 | 0.571 | 1 | 156 | 0.85 | 0.359 |
| Stage | 2 | 86 | 27.1 | <0.001 | 2 | 124 | 10.44 | <0.001 | 2 | 80 | 2.05 | 0.136 | 2 | 156 | 3.71 | 0.027 |
| Log Plant species richness (PSR) | –d | – | – | – | 1 | 61 | 0.50 | 0.484 | 1 | 40 | 0.86 | 0.360 | 1 | 72 | 0.36 | 0.548 |
| Functional group richness (FG) | 1 | 38 | 1.16 | 0.288 | – | – | – | – | 1 | 40 | 1.92 | 0.174 | 1 | 72 | 2.70 | 0.105 |
| Presence of grasses | – | – | – | – | 1 | 61 | 5.28 | 0.025 | – | – | – | – | 1 | 72 | 0.84 | 0.364 |
| Presence of legumes | 1 | 38 | 1.05 | 0.313 | – | – | – | – | – | – | – | – | 1 | 72 | 0.01 | 0.905 |
| Presence of small herbs | 1 | 38 | 2.96 | 0.094 | – | – | – | – | – | – | – | – | 1 | 72 | 0.05 | 0.827 |
| PSR: legume presencec | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 72 | 3.56 | 0.063 |
| FG: grass presence | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 72 | 2.58 | 0.113 |
| FG: legume presence | 1 | 38 | 0.40 | 0.532 | – | – | – | – | – | – | – | – | – | – | – | – |
| Grass: small herb presence | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 72 | 4.11 | 0.046 |
| Legume: small herb presence | 1 | 38 | 3.95 | 0.054 | – | – | – | – | – | – | – | – | – | – | – | – |
| Stage: PSR | – | – | – | – | 2 | 124 | 3.30 | 0.040 | 2 | 80 | 2.50 | 0.089 | – | – | – | – |
| Stage: FG | – | – | – | – | – | – | – | – | 2 | 80 | 2.65 | 0.077 | – | – | – | – |
| Stage: grass presence | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 156 | 5.41 | 0.005 |
| Stage: small herb presence | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 156 | 3.69 | 0.027 |
Each term is added sequentially to a null model containing only the intercept. Grass cover: n = 44 plots × 3 stages = 132; herb cover: n = 64 × 3 = 192; legume cover: n = 43 × 3 = 129; total cover: n = 81 × 3 = 243
a,bNumerator and denominator degrees of freedom
cInteraction terms are indicated by “:”
dTerms excluded during model simplification indicated by “–”
Fig. 1.
a Aerial view of the Jena Experiment, 14 June 2006. b Overview of three of the 81 plots with two cages each (4 June 2004). c,e Effects of grasshopper herbivory on a monoculture of Festuca rubra in August 2004. d,f Recovery of the monoculture in May 2005. c and d show control cages, e and f show herbivory cages. The white pots in c and e were used to measure deposition of oothecae in another experiment. a: © A. Weigelt, W. Voigt, C. Scherber/The Jena Experiment; b–f: © C.Scherber
Changes in plant community composition induced by herbivory
We found strong and consistent effects of herbivory on plant functional group composition (Fig. 3, Tables 2 and 3). While grasses were always negatively affected by herbivory, herb cover increased, and quite remarkably, this increase was stronger in more species-rich plant communities. Grass cover declined significantly (by around 20% on average) as an immediate result of herbivore attack (Fig. 3a, Stage 2-1 difference in Table 2; Stage effect in Table 3), with subsequent recovery (by around 9%) at stage 3 (Stage 3-2 difference in Table 2). Grass cover declines were independent of plant species richness. Legume cover declined significantly at stage 2, but only in species-rich plant communities (Fig. 3b). At the same time, herb cover increased significantly (by around 6.5%), notably only in all those plots that contained grasses (Fig. 3c, Grass effects in Tables 2 and 3). Interestingly, this increase in herb cover was significantly stronger in more species-rich plant communities: while plant monocultures showed no trend in herb cover over time, more species-rich mixtures showed a strong increase in herb cover at stage 2 (Stage: Logdiv interactions in Tables 2, 3). Herb cover in 2-, 4- and 8-species mixtures increased by around 5% compared to herb monocultures, while herb cover in 16- and 60-species mixtures increased by around 13% (Fig. 3c). Overall, herbivory lead to declines in grass cover, mirrored by increases in herb cover. These shifts in vegetation composition were modified by plant species richness (as in the case of herb cover).
Fig. 2.
Total cover differences between herbivory and control cages (mean ± SE, n = 81). Arrows in (a) and (b) indicate when grasshoppers were added to the cages. Negative differences (below the dotted horizontal line) indicate lower cover in herbivory than in control cages. a,b Effects of grass and small herb presence before, during and after addition of grasshoppers. c Interaction between grass presence and functional group richness. Open triangles (filled circles) and dashed (solid) lines indicate presence (absence) of each plant functional group in (a–c)
Discussion
Resistance and recovery
Our study shows clearly that consumption resistance of plant communities depend on plant functional identity rather than on plant species richness per se. Consumption resistance was mainly affected by grass presence and only partly by plant functional group richness. These findings are in contrast to previous studies, which have mostly reported positive “biodiversity” effects on consumption resistance. For example, in a meta-analysis of biodiversity effects on trophic interactions, Balvanera et al. (2006) concluded that consumption resistance increased with plant species richness. Their study included 10 different variables from McNaughton (1985), Mulder et al. (1999) and Pfisterer et al. (2003). Pfisterer et al. (2003) used an experimental approach comparable to ours and concluded that “proportional biomass consumption was significantly reduced with increasing species richness”, indicating higher resistance of species-rich plant communities. In our study, however, plant species richness proved to be largely irrelevant to consumption resistance. One explanation for these contrasting results may be that the plant communities in Pfisterer et al. almost always contained grasses, while our experiment also contained completely grass-free communities. Thus, Pfisterer et al. tested plant species richness conditional on grass presence, while our study allowed independent tests of plant species richness and grass presence. Our findings suggest that species richness effects on consumption resistance are only to be expected if the herbivore’s preferred resource is present. Plant species richness per se may be less important for resistance and recovery from herbivory than previously anticipated. Functional group identity was clearly the most important predictor of consumption resistance in our study. Functional group effects may also explain why experimental studies published so far have either shown positive (Mulder et al. 1999), neutral (Pfisterer et al. 2003; Scherber et al. 2006) or negative (Giller and O’Donovan 2002) relationships between plant species richness and herbivory.
Considering recovery after herbivory, plant species have been sorted traditionally into “increaser” and “decreaser” species, depending on their regrowth after herbivory (e.g. del Val and Crawley 2004). Our study shows that even species within the grass functional group may exhibit considerable differences depending on local environmental conditions, including indirect influences from neighbouring plant species (e.g. Hambäck and Beckerman 2003). This may, for example, have been the case for F. rubra, which Crawley (1990) categorized as a grazing decreaser species, but in our study it had a considerable overcompensation ability. Performance of a plant species may decrease because of selective herbivory, or it may increase either because a competitively superior plant species is eaten, or because it indirectly benefits from the presence of other plant species (e.g. legumes). Likewise, host-finding of the herbivore may be masked in more species-rich communities. While our study does not allow such mechanistic insights, it does allow strong generalizations about changes in vegetation composition induced by selective herbivory, as will be shown below.
Vegetation composition
In all plots containing at least both grasses and herbs, the relative plant functional group composition shifted internally. Herbs increased at the expense of grasses wherever grass cover had been reduced by herbivory. This may explain why changes in overall vegetation biomass (i.e. resistance) and changes in overall vegetation cover were independent of plant species richness. Similar changes in plant community composition after herbivory have frequently been reported (e.g. Bach 2001; Danell and Ericson 1990; Howe et al. 2006). Pfisterer et al. (2003) suggested that consumers can change the relative biomasses and cover proportions of different species and FGs in the plant community, but they did not provide an explicit proof. Our experiment shows that selective herbivory can induce changes in plant communities that persist for at least into the next vegetation period. As has frequently been shown, selective herbivory may change competitive hierarchies in plant communities (e.g. Hambäck and Beckerman 2003; Suding and Goldberg 2001). Using a grass-feeding vertebrate herbivore, Howe et al. (2006) have found exactly the same pattern of herbs compensating for grass losses. However, tests of such compensatory effects under different levels of plant species richness and with insect herbivores have been scarce so far.
The most unexpected finding of our study, however, was that compositional changes depended on plant species richness. Herbs, once released from competition with grasses, filled up more space, the more plant species were present in the communities. This is surprising, given that Daßler et al. (2008) found (for a selection of small herbs from the Jena Experiment) that their biomass proportions actually decreased with plant species richness. Hence, selective herbivory may potentially reverse competitive hierarchies in plant communities that differ in species richness: plants that experience strong interspecific competition in species-rich communities may gain disproportionally more, once competitors have been removed by herbivory.
Conclusions
We have shown that consumption resistance and recovery of plant communities after herbivory critically depend on plant functional identity. Plant communities may compensate depending on plant species present but also depending on competitive hierarchies inside the plant communities and how these are altered by selective herbivory. Plant communities will recover more quickly after herbivory if the removal of the preferred resource can be counteracted by compensation, which is, in our case, the increased growth of herbs at the expense of grasses. From an applied point of view, this means that species richness per se is not a sufficient “insurance” against herbivore attack. Rather, each system will exhibit system-specific dynamics of resistance and recovery that are determined largely by plant functional identity (as a proxy for palatability, competitive ability, etc.). Hence, herbivory resistance and recovery do not depend on a certain “number of species”, but on specific plant traits. Internal compositional changes, however, may be modified by plant species richness, resulting in better compensatory abilities of species-rich mixtures.
Acknowledgments
We thank Bradley J. Cardinale, Oswald J. Schmitz, Bernhard Schmid, Mick Crawley, Teja Tscharntke, Markus Wagner and David Gladbach for comments and advice, Ernst-Detlef Schulze and Bernhard Schmid for experimental set-up, Christiane Roscher and Alexandra Weigelt for coordination, Sylvia Creutzburg, Ingrid Jakobi, gardeners and student helpers for field work support. All experiments conducted complied with current German laws. The authors declare that they have no conflict of interest. This study was funded by the Deutsche Forschungsgemeinschaft (grants WE2618/4-1 and FOR 456). C.S. was supported by the Studienstiftung des Deutschen Volkes.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bach CE. Long-term effects of insect herbivory and sand accretion on plant succession on sand dunes. Ecology. 2001;82:1401–1416. doi: 10.1890/0012-9658(2001)082[1401:LTEOIH]2.0.CO;2. [DOI] [Google Scholar]
- Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- Beck SD. Resistance of plants to insects. Annu Rev Entomol. 1965;10:207–232. doi: 10.1146/annurev.en.10.010165.001231. [DOI] [Google Scholar]
- Bernays EA, Chapman RF. Experiments to determine the basis of food selection by Chorthippus parallelus (Zetterstedt) (Orthoptera–Acrididae) in the field. J Anim Ecol. 1970;39:761–775. doi: 10.2307/2866. [DOI] [Google Scholar]
- Bernays EA, Chapman RF. Food selection by Chorthippus parallelus (Zetterstedt) (Orthoptera:Acrididae) in the field. J Anim Ecol. 1970;39:383–394. doi: 10.2307/2977. [DOI] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel interference. A practical information-theoretic approach. New York: Springer; 2002. [Google Scholar]
- Cebrian J. Role of first-order consumers in ecosystem carbon flow. Ecol Lett. 2004;7:232–240. doi: 10.1111/j.1461-0248.2004.00574.x. [DOI] [Google Scholar]
- Cebrian J, Lartigue J. Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecol Monogr. 2004;74:237–259. doi: 10.1890/03-4019. [DOI] [Google Scholar]
- Cottam DA. Frequency-dependent grazing by slugs and grasshoppers. J Ecol. 1985;73:925–933. doi: 10.2307/2260158. [DOI] [Google Scholar]
- Crawley MJ. Herbivory. Oxford: Blackwell; 1983. [Google Scholar]
- Crawley MJ. Rabbit grazing, plant competition and seedling recruitment in acid grassland. J Appl Ecol. 1990;27:803–820. doi: 10.2307/2404378. [DOI] [Google Scholar]
- Danell K, Ericson L. Dynamic relations between the antler moth and meadow vegetation in Northern Sweden. Ecology. 1990;71:1068–1077. doi: 10.2307/1937375. [DOI] [Google Scholar]
- Daßler A, Roscher C, Temperton VM, Schumacher J, Schulze ED. Adaptive survival mechanisms and growth limitations of small-stature herb species across a plant diversity gradient. Plant Biol. 2008;10:573–587. doi: 10.1111/j.1438-8677.2008.00073.x. [DOI] [PubMed] [Google Scholar]
- del Val E, Crawley MJ. Importance of tolerance to herbivory for plant survival in a British grassland. J Veg Sci. 2004;15:357–364. doi: 10.1111/j.1654-1103.2004.tb02272.x. [DOI] [Google Scholar]
- Díaz S, Symstad AJ, Stuart Chapin F, Wardle DA, Huenneke LF. Functional diversity revealed by removal experiments. Trends Ecol Evol. 2003;18:140–146. doi: 10.1016/S0169-5347(03)00007-7. [DOI] [Google Scholar]
- Giller PS, O‘Donovan G. Biodiversity and ecosystem function: do species matter? Biol Environ Proc R Ir Acad. 2002;102B:129–139. doi: 10.3318/BIOE.2002.102.3.129. [DOI] [Google Scholar]
- Gyllenberg G. A simulation-model for testing the dynamics of a grasshopper population. Ecology. 1974;55:645–650. doi: 10.2307/1935155. [DOI] [Google Scholar]
- Hambäck PA, Beckerman AP. Herbivory and plant resource competition: a review of two interacting interactions. Oikos. 2003;101:26–37. doi: 10.1034/j.1600-0706.2003.12568.x. [DOI] [Google Scholar]
- Howe HF, Zorn-Arnold B, Sullivan A, Brown JS. Massive and distinctive effects of meadow voles on grassland vegetation. Ecology. 2006;87:3007–3013. doi: 10.1890/0012-9658(2006)87[3007:MADEOM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ingrisch S, Köhler G. Die Heuschrecken Mitteleuropas. Magdeburg: Westarp Wissenschaften; 1998. [Google Scholar]
- Köhler G, Brodhun HP, Schäller G. Ecological energetics of Central European grasshoppers (Orthoptera: Acrididae) Oecologia. 1987;71:112–121. doi: 10.1007/BF00377354. [DOI] [PubMed] [Google Scholar]
- Lanta V. Effect of slug grazing on biomass production of a plant community during a short-term biodiversity experiment. Acta Oecol. 2007;32:145–151. doi: 10.1016/j.actao.2007.03.013. [DOI] [Google Scholar]
- Lawton JH, Strong DR. Community patterns and competition in folivorous insects. Am Nat. 1981;118:317–338. doi: 10.1086/283826. [DOI] [Google Scholar]
- McNaughton SJ. Ecology of a grazing ecosystem—the Serengeti. Ecol Monogr. 1985;55:259–294. doi: 10.2307/1942578. [DOI] [Google Scholar]
- McNaughton SJ, Oesterheld M, Frank DA, Williams KJ. Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature. 1989;341:142–144. doi: 10.1038/341142a0. [DOI] [PubMed] [Google Scholar]
- Mulder CPH, Koricheva J, Huss-Danell K, Högberg P, Joshi J. Insects affect relationships between plant species richness and ecosystem processes. Ecol Lett. 1999;2:237–246. doi: 10.1046/j.1461-0248.1999.00070.x. [DOI] [Google Scholar]
- Olff H, Ritchie ME. Effects of herbivores on grassland plant diversity. Trends Ecol Evol. 1998;13:261–265. doi: 10.1016/S0169-5347(98)01364-0. [DOI] [PubMed] [Google Scholar]
- Pacala SW, Crawley MJ. Herbivores and plant diversity. Am Nat. 1992;140:243–260. doi: 10.1086/285411. [DOI] [PubMed] [Google Scholar]
- Painter RH. The food of insects and its relation to resistance of plants to insect attack. Am Nat. 1936;70:547–567. doi: 10.1086/280696. [DOI] [Google Scholar]
- Painter RH. The resistance of plants to insects. Annu Rev Entomol. 1958;3:267–290. doi: 10.1146/annurev.en.03.010158.001411. [DOI] [Google Scholar]
- Pfisterer A, Diemer M, Schmid B. Dietary shift and lowered biomass gain of a generalist herbivore in species-poor experimental plant communities. Oecologia. 2003;135:234–241. doi: 10.1007/s00442-002-1169-0. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- Pratsch R (2004) The consumer community (Arthropoda) of selected cultivated grasslands in the surroundings of the Jena biodiversity experiment. Magister Thesis, University of Jena (in German), Jena
- R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
- Reinhardt K, Köhler G. Costs and benefits of mating in the grasshopper Chorthippus parallelus (Caelifera: Acrididae) J Insect Behav. 1999;12:283–293. doi: 10.1023/A:1020854403805. [DOI] [Google Scholar]
- Richards OW, Waloff N. Studies on the biology and population dynamics of British grasshoppers. Anti-Locust Bull. 1954;17:1–182. [Google Scholar]
- Root RB. Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea) Ecol Monogr. 1973;43:95–124. doi: 10.2307/1942161. [DOI] [Google Scholar]
- Roscher C, Schumacher J, Baade J, Wilcke W, Gleixner G, Weisser WW, Schmid B, Schulze ED. The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl Ecol. 2004;5:107–121. doi: 10.1078/1439-1791-00216. [DOI] [Google Scholar]
- Scherber C, Mwangi PN, Temperton VM, Roscher C, Schumacher J, Schmid B, Weisser WW. Effects of plant diversity on invertebrate herbivory in experimental grassland. Oecologia. 2006;147:489–500. doi: 10.1007/s00442-005-0281-3. [DOI] [PubMed] [Google Scholar]
- Schmid B, Hector A (2004) The value of biodiversity experiments. Basic Appl Ecol 5:535–542
- Schmitz OJ. From mesocosms to the field: The role and value of cage experiments in understanding top-down effects in ecosystems. In: Weisser WW, Siemann E, editors. Insects and ecosystem function. Heidelberg: Springer; 2004. pp. 277–302. [Google Scholar]
- Specht J, Scherber C, Unsicker SB, Köhler G, Weisser WW. Diversity and beyond: plant functional identity determines herbivore performance. J Anim Ecol. 2008;77:1047–1055. doi: 10.1111/j.1365-2656.2008.01395.x. [DOI] [PubMed] [Google Scholar]
- Stowe KA, Marquis RJ, Hochwender CG, Simms EL. The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst. 2000;31:565–595. doi: 10.1146/annurev.ecolsys.31.1.565. [DOI] [Google Scholar]
- Suding KN, Goldberg D. Do disturbances alter competitive hierarchies? Mechanisms of change following gap creation. Ecology. 2001;82:2133–2149. doi: 10.2307/2680221. [DOI] [Google Scholar]
- Trumble JT, Kolodnyhirsch DM, Ting IP. Plant compensation for arthropod herbivory. Annu Rev Entomol. 1993;38:93–119. doi: 10.1146/annurev.en.38.010193.000521. [DOI] [Google Scholar]
- Unsicker SB, Baer N, Kahmen A, Wagner M, Buchmann N, Weisser WW. Invertebrate herbivory along a gradient of plant species diversity in extensively managed grasslands. Oecologia. 2006;150:233–246. doi: 10.1007/s00442-006-0511-3. [DOI] [PubMed] [Google Scholar]
- Unsicker S, Oswald A, Köhler G, Weisser W. Complementarity effects through dietary mixing enhance the performance of a generalist insect herbivore. Oecologia. 2008;156:313–324. doi: 10.1007/s00442-008-0973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hook R. Energy and nutrient dynamics of spider and orthopteran populations in a grassland ecosystem. Ecol Monogr. 1971;41:1–26. doi: 10.2307/1942433. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. New York: Springer; 2002. [Google Scholar]
- Wise MJ, Carr DE. On quantifying tolerance of herbivory for comparative analyses. Evolution. 2008;62:2429–2434. doi: 10.1111/j.1558-5646.2008.00458.x. [DOI] [PubMed] [Google Scholar]