Abstract
INTRODUCTION
These experiments were designed to determine whether systemic post ischemic administration of PJ34, a Poly ADP-ribose polymerase inhibitor, decreased tissue injury and inflammation following hind limb ischemia reperfusion (I/R).
METHODS
C57BL6 mouse limbs were subjected to 1.5 hrs ischemia followed by 24 hours reperfusion. The treatment group (PJ) received intraperitoneal PJ34 (30 mg/Kg) immediately before, 15 minutes and 2 hours into reperfusion. Control group (CG) received Lactated Ringers alone at the same time intervals as PJ34 administration. Skeletal muscle levels of ATP, Macrophage Inflammatory Protein-2 (MIP-2), Keratinocyte Derived Chemokine (KC) and Myeloperoxidase (MPO) were measured. Quantitative measurement of skeletal muscle tissue injury was assessed by microscopic analysis of fiber injury.
RESULTS
ATP levels were higher in limbs of PJ vs. CG (Absolute ATP: 4.7 ± 0.35 vs. 2.3 ± 0.15 ng/mg tissue, p=0.002). Levels of MIP-2, KC and MPO were lower in PJ vs. CG (MIP-2: 1.4±0.34 vs. 3.67±0.67 pg/mg protein, p=0.014; KC: 4.97±0.97 vs. 12.65±3.05 pg/mg protein, p=0.037, MPO: 46.27±10.53 vs. 107.34±13.58 ng/mg protein, p=0.008). Muscle fiber injury was markedly reduced in PJ vs. CG (4.25±1.9% vs 22.68±3.0% total fibers, p=0.0004).
CONCLUSION
Systemic post ischemic administration of PJ34 preserved skeletal muscle energy levels, decreased inflammatory markers and preserved tissue viability post I/R. These results support PARP inhibition as a viable treatment for skeletal muscle I/R in a clinically relevant “post-hoc” scenario.
Keywords: Basic science, skeletal muscle, cytokines, inflammation, vascular disease
INTRODUCTION
Acute lower extremity ischemia remains a significant problem with a mortality rate ranging from 9 to 42% in elderly patients 1, 2, and a 20% amputation rate in survivors 3. Despite such significant morbidity and mortality, therapeutic strategies have not varied much over the past two decades, with the administration of fluid resuscitation, thrombolysis and anticoagulant therapy remaining as the mainstays of treatment. Experimental therapies have been designed to ameliorate the multiple components of I/R and include therapies directed against complement 4, neutrophils 5, reactive oxygen species 6, tissue thrombosis 7, metabolic failure 8 and other mediators such as the inhibition poly-ADP ribose Polymerase (PARP) 9–11. Most of the therapeutic interventions in acute limb ischemia have been successful using a pre-ischemic (pre-hoc) treatment scenario, only to fail when tested in a clinically relevant post-ischemic (post-hoc) protocol. Only a handful of therapies have shown significant protection in a post-hoc treatment scenario 12, 13, with only a few (catheter based thrombolysis and controlled reperfusion) having been translated into the clinical arena 14, 15. Thrombolysis decreases mortality associated with limb reperfusion as compared to surgery (58% thrombolysis vs. 84% surgery), but the mortality rate remains high and thrombolysis is associated with increased incidence of bleeding complications. Controlled reperfusion involves flushing the arterial circulation with degassed electrolyte solution, and venting the venous effluent prior to reinstituting arterial inflow. The equipment to complete this protocol is not overly expensive, but this methodology has not gained widespread popularity in the clinical arena.
PARP is one of a family of enzymes involved in the regulation of a variety of cellular functions, which include cell death and the regulation of cell proliferation and gene expression. In the nucleus, PARP functions as a sensor of DNA strand nick and breaks. Several mechanisms including radiation, sepsis, shock, inflammation and ischemia/reperfusion can result in the activation of PARP. Upon activation, PARP utilizes its catalytic domain to cleave NAD+ into nicotinamide and ADP-Ribose. ADP-Ribose is then synthesized into long (up to 200) polymers and attached to nuclear proteins, such as histones, and on to PARP itself. By virtue of its negative charge, and through electrostatic interactions, ADP-Ribosylation can affect the function of the acceptor proteins and carry out some of its functions, such as identifying areas for DNA repair. This process is a double edge sword. Excessive poly-ADP ribosylation during times of stress’ consumes the cellular NAD+ pool and ATP stores at a time when NAD+ is needed for key reactions of the glycolytic pathway 16. Furthermore, PARP activation has been shown to regulate the enhancement in the expression of inducible nitric oxide synthase (iNOS) and NF-κβ, both of which play prominent roles in the pathogenesis of I/R17. Previously, our laboratory reported the beneficial effects PJ34, an ultra-potent water soluble inhibitor of PARP 18, in a tourniquet model of skeletal muscle I/R 10. PJ34 binds to the NAD+ binding site on PARP, thereby blocking activation of PARP and preventing intracellular consumption of the NAD+ pool 19. PJ34, when given systemically before the induction of ischemia, was shown to preserve mitochondrial function and decrease markers of inflammation in a model of ischemia and reperfusion 10. Follow-up work showed that systemic (intraperitoneal) administration of PJ34 after the onset of ischemia did not show preservation of mitochondrial activity when compared with controls 9. Since completion of those studies, we have refined our experimental protocol to evaluate cellular energy levels and tissue morphology, rather than indirect measure of mitochondrial activity as an index of tissue injury/preservation upon reperfusion. In addition, experimental evaluation of skeletal muscle responses to acute hind limb ischemia reperfusion now includes extensive quantitative histologic evaluation20. Using these well established quantitative tools, the following experiments were designed to determine whether systemic administration of PJ34 can provide protection against ischemia reperfusion injury when administered using a clinically relevant post-ischemic protocol. We chose systemic administration in mice via an intraperitoneal route because PJ34 is water soluble and could be administered to humans in an intravenous manner, similar to other commonly used medications (heparin, antibiotics). Since PARP modulates both inflammatory and metabolic components of the response to ischemia, treatment with a PARP inhibitor has the potential to modulate more than one component of the response to ischemia reperfusion.
METHODS
Animal protocol
All experimental procedures were approved by the Massachusetts General Hospital IACUC in accordance with the “Principles of Laboratory Animal Care” (Guide for the Care and Use of Laboratory Animals, National Institutes of Health Publication No. 86-23, Revised 1996). C57BL6 mice (male, 20–25 gm, Jackson Laboratory, Bar Harbor, Me) were anesthetized using intraperitoneal administration of 60 mg/kg of pentobarbital. C57BL6 mice were subjected to 1.5 hours of unilateral ischemia, followed by 24 hours of reperfusion. Mice remained anesthetized throughout the duration of ischemia and the initial early reperfusion (1-hour) interval. Mice studied for 24-hour reperfusion were allowed to recover from anesthesia after the initial 1 hour of reperfusion and were returned to their cages between drug dosing.
INDUCTION OF ISCHEMIA
Reproducible levels of ischemia were obtained with the use of a 4.5 oz. Orthodontic Rubber Band (ORB, American Orthodontics, Sheboygan, WI) as previously described 21. Briefly, after anesthesia, the mice were placed on a preheated table to maintain the mouse’s monitored temperature at 37°C. The ORB was applied to the proximal limb using a McGivney Hemorrhoidal Ligator Applicator (Militex Corporation, Bethpage New York). Ischemia was confirmed (i.e. no detectable flow) using Laser Doppler imaging (Moor Instruments Inc, Wilmington, Del) as previously described22. Reperfusion was initiated by cutting the rubber band from the mouse limb.
PJ34 treatment protocol
The treatment group (PJ34, n=6) received intraperitoneal PJ34 (30 mg/Kg), in 0.4 ml of Lactated Ringers (LR), immediately before reperfusion, 15 minutes and 2 hours into the reperfusion period. These time intervals were utilized to provide PARP inhibition during the early phase of reperfusion based on literature which indicates the half life of PJ34 in serum is approximately 30 minutes23, 24. Additional doses of vehicle, LR (0.4 ml), were given 15 minutes before ischemia, at 6, 12 and 18 hours of reperfusion to provide hydration for the mice25, 26. The control group (LR, n=6) received LR only (0.4 ml) using the same treatment schedule. After 24 hours of reperfusion, the animals were euthanized (isoflurane). Ischemia-reperfusion and contralateral limbs were then harvested and the posterior hind-limb muscle was immediately frozen in liquid nitrogen for biochemical analysis.
Histological assessment
PJ34 treated (n=6) and LR treated (n=6) mice were prepared in the same manner as above, and then euthanized at the end of reperfusion. Hind limbs were harvested and fixed in 4% Paraformaldehyde overnight. The gastrocnemius muscle was isolated and rinsed in PBS for 1 hour followed by serial dehydration in graded Acetone. Muscles were embedded under vacuum condition using the acrylic compound, JB-420. Hardened molds were mounted and cut on a motorized microtome (Leica, Nuβloch Germany). Two μm thin sections were obtained using glass knifes, stained with Masson’s trichrome stain and examined under 200× power light microscope. Thirty images were taken of each muscle with a SNAP color camera (Roper Scientific, Tucson, AZ) and assigned a unique image number using SPOT Insight imaging software (Diagnostic Instruments, Sterling Heights, MI). A random number generator was used to create a random series of image numbers, which a blinded observer used to retrieve the respective images and count muscle fibers. A total of 1200 muscle fibers per gastrocnemius sample (i.e. limb) were counted and scored as uninjured or injured based on the morphology of the individual fibers20. Uninjured fibers were characterized as having well-defined borders, consistent texture, and morphologic uniformity without holes or breaks; with easily identifiable satellite cells and pericellular nuclei. In contrast, injured fibers exhibited broken, fragmented fiber morphology with inconsistent texture, color, holes throughout the cytoplasm, and frequent pericellular nuclear detachment. Summary data was expressed as percent of injured fibers per limb. Quantitative analysis of the percent of injured fibers was used for statistical analysis.
Skeletal Muscle Adenosine Tri-phosphate (ATP) content
200 mg of the gastrocnemius muscle group was homogenized on ice in 10% trichloroacetic acid using polytron tissue disruptor. Samples were cleared by centrifugation for 10 minutes 10,000 ×g at 4°C. ATP levels were measured after dilution in Dulbecco’s modified Phosphate buffered saline (DPBS with Ca++ and Mg++, pH=7.4) using ATPlite luminescence assay according to manufacturer protocol (Perkin Elmer Life, Boston, MA), and counts were read in 1450 Micro beta plate reader (PerkinElmer Life, Boston, MA). Concentrations of the unknowns were extrapolated from purified ATP standards and expressed as μM per mg tissue weight. ATP from six replicate assays per group were used for statistical analysis.
Markers of Inflammation
The entire gastrocnemius posterior calf skeletal muscle samples were snap frozen in liquid nitrogen immediately after harvest and stored at −80°C until analysis. 200 mg of the gastrocnemius muscle group was homogenized on ice for protein extraction as previously described 27. The levels of Keratinocyte Derived Chemokine (KC), Macrophage Inflammatory Protein-2(MIP-2) (R&D Systems, Minneapolis, MN) and Myelopereoxidase(MPO, marker of neutrophil activation/accumulation in tissue)(Cell Sciences, Canton, MA) were measured with ELISA kits according to manufacturers’ protocols. KC is the murine equivalent of human IL-8, a known potent neutrophil chemoattractant protein upregulated during hind limb ischemia reperfusion28. MIP-2 is a potent macrophage chemoattractant, also known to be increased in serum following hind limb ischemia reperfusion29. Values were extrapolated off the standard curve and normalized to the total protein concentration (BCA Protein Assay Reagent Kit, Pierce Biotechnology, Rockford, IL).
Western Blotting for PAR
Skeletal muscle samples were obtained from LR treated and PJ34 treated mice at 3 and 24 hours reperfusion. Muscle samples from sham mice were obtained from mice anesthetized with pentobarbital for 90 minutes without limb ischemia, allowed to recover for 24 hours and then euthanized. 10 μg total protein was solubilized with equal volume of laemmli sample buffer (0.25M Tris-HCl pH 6.8, 8%SDS, 40% glycerol, 0.4M DTT and 0.04% Bromophenol Blue, BioRad, Hercules CA), boiled for 5 min, loaded onto lanes in a 12% density Tris-HCl polyacrylamide/sodium dodecyl sulfate (SDS) gel. Samples were subjected to electrophoresis followed by electro blotting transfer to a 0.22μm nitrocellulose membrane (BioRad, Hercules, CA). The membranes were blocked in blocking buffer solution (Sigma, St. Louis, MO) for 1 h at room temperature. The blots were incubated with mouse monoclonal anti-poly-(ADP)-ribose antibody (Tulip Biolab, West Point, PA) in blocking buffer at 1:2000 dilution for 1 hour at room temperature. After washes in PBS-0.15% Tween 20, the membranes were incubated with Peroxidase-conjugated Goat polyclonal anti-mouse antibody (1:4000) (Cell Signaling Technology, Danvers, MA) in blocking buffer for 1 hour at room temperature. Finally the membranes were washed with PBS-TW and developed with the ECL chemiluminescence detection system (GE, Healthcare, Piscataway, NJ)30. Chemiluminescence light is visualized by exposing the blots into a BioMAX X-ray film. The blots were striped and reprobed using mouse monoclonal anti-mouse beta-Actin IgG (1:5000, Abcam, Cambridge, MA), followed by incubation with polyclonal goat anti-mouse IgG (1:4000). The generated specific proteins are quantified by bands densities (between 25 and 100kDa) using gel-imaging system (Alpha Innoteck Corporation, San Leandro, CA). Relative expression was quantitated as Arbitrary Units (AU).
Statistical Analysis
Statistical analysis was performed with Instat (GraphPad, San Diego, CA). Skeletal muscle ATP, tissue cytokine, MPO levels, and histological assessment in treated and untreated mice after 24 hours of reperfusion were compared with each other using unpaired t test (Mann Whitney when indicated). Expression of PAR in sham, treated and untreated mice at 3 and 24 hour reperfusion were compared with each group using Two way ANOVA(Prism, San Diego, CA), with post hoc testing (Bonferroni).
RESULTS
Histological Analysis
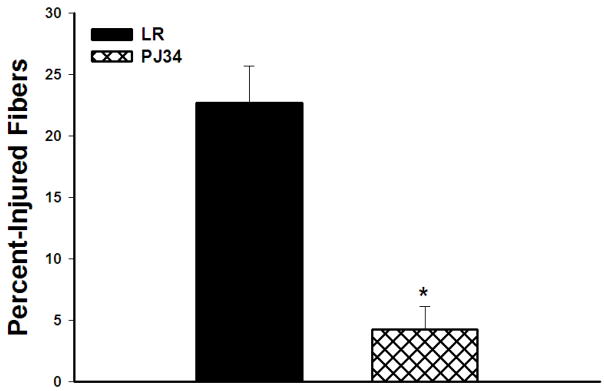
The percentage of injured fibers in PJ34 vs. LR treated hind limbs was assessed using defined histological criteria for injury. Percent-injured fibers were significantly lower in mice treated with PJ34 vs. LR treated animals (4.25±1.9% vs. 22.68±3.0%, p=0.0004, Figure 1). Representative photomicrographs of skeletal muscle for treated and untreated mice are shown in Figure 2. Treated mice had a few scattered clusters of injured cells among predominantly normal polygonal skeletal muscle cells. In contrast, untreated mice had large clusters of injured fibers, shown outlined in the photomicrograph.
Figure 1. Percent Injured Skeletal Muscle Fibers.
PJ34 treated mice had substantially fewer injured fibers than LR treated mice after 24 hours reperfusion (*p=0.0004).
Figure 2. Photomicrographs of Reperfused Skeletal Muscle (200X).
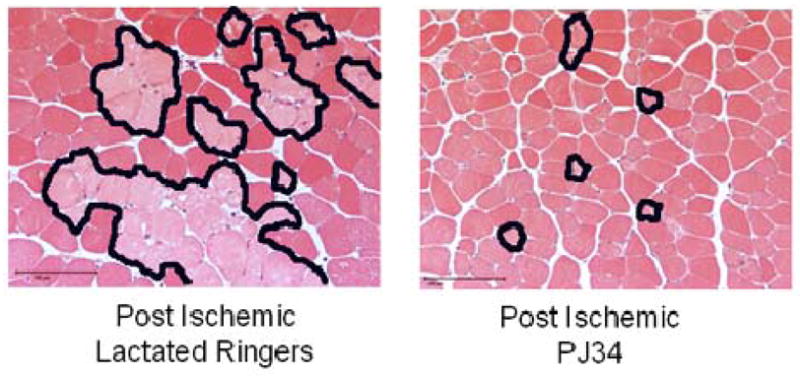
PJ34 and LR treated skeletal muscle. There are normal polygonal muscle fibers evident in the sham skeletal muscle. In contrast, there are edematous skeletal muscle fibers (arrow) and clusters of injured fibers (outlined) evident in the photomicrograph. The bar indicates 100 microns.
Skeletal Muscle Adenosine Tri-phosphate (ATP) content
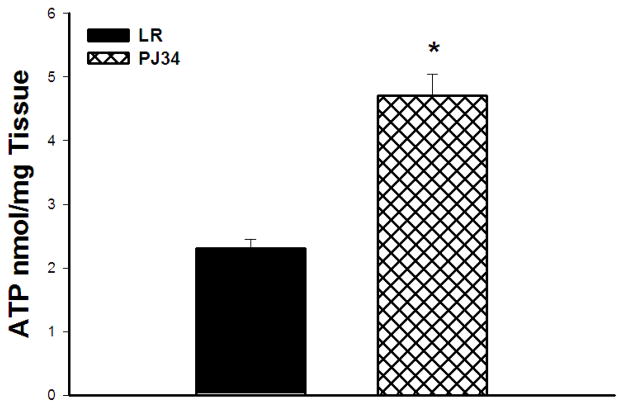
Absolute ATP levels after 24 hours of reperfusion were higher in limbs of PJ34 vs. LR treated mice (Absolute ATP: 4.7 ± 0.35 vs. 2.3 ± 0.15 nmol.mg tissue, p=0.002, Figure 3).
Figure 3. ATP levels in Reperfused Skeletal Muscle.
Absolute levels of ATP were markedly preserved in the PJ34 treated vs LR treated mice by 24 hours reperfusion (*p=0.002).
Markers of Tissue Inflammation
KC
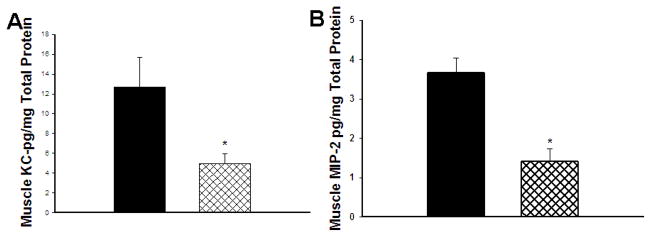
The skeletal muscle levels of KC following 90 minutes of ischemia and 24 hours of reperfusion using the post-hoc treatment protocol were decreased in limbs of post-hoc treated PJ34 LR treated mice (4.97±0.97 vs. 12.65±3.05ng/mg protein, p=0.04, Figure 4A).
Figure 4. Cytokine/Chemokine Levels in Skeletal Muscle.
(A) Keratinocyte Chemoattractant Protein (KC) levels were markedly reduced at 24 hours reperfusion in PJ34 treated mice (*p=0.04). (B) Macrophage Inflammatory Protein-2 levels in reperfused skeletal muscle were also significantly reduced in the PJ34 treated mice (p=0.014).
MIP-2
The skeletal muscle levels of MIP-2 following 90 minutes of ischemia and 24 hours of reperfusion were decreased in limbs of post-hoc treated PJ34 vs. LR treated animals (1.4±0.34 vs. 3.67±0.67 ng/mg protein, p= 0.014, Figure 4B).
MPO
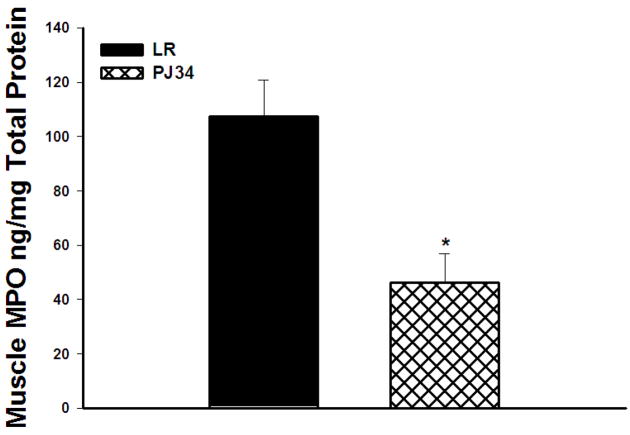
Myeloperoxidase levels were decreased in post-hoc treated PJ34 vs. LR treated animals (46.26 ± 9.4 vs. 107.34 ± 12.14 ng/mg protein, p=0.008, Figure 5).
Figure 5. Myeloperoxidase Levels in Skeletal Muscle.
Myeloperoxidase levels were markedly reduced in PJ34 treated mice as compared to LR treated mice by 24 hours reperfusion.
PAR expression in Skeletal Muscle
Sham mice treated with saline or PJ34 had nearly undetectable levels of PAR in skeletal muscle (Figure 6). At 3 hours of reperfusion, LR treated mice had significantly greater levels of PAR than PJ34 treated mice (5.0± 1.1 vs 2.13 ± 0.63 AU, p<0.01) in skeletal muscle. By 24 hours reperfusion, there was no difference in the expression of PAR in LR vs. PJ34 treated mice(3.11± 0.55 vs 4.56±0.79 AU) in skeletal muscle. Representative PAR Immunoblots from 3 hours (A) and 24 hours (B) reperfusion of skeletal muscle treated with LR and PJ34 are presented in Figure 7.
Figure 6. PAR expression following Hind Limb IR injury.
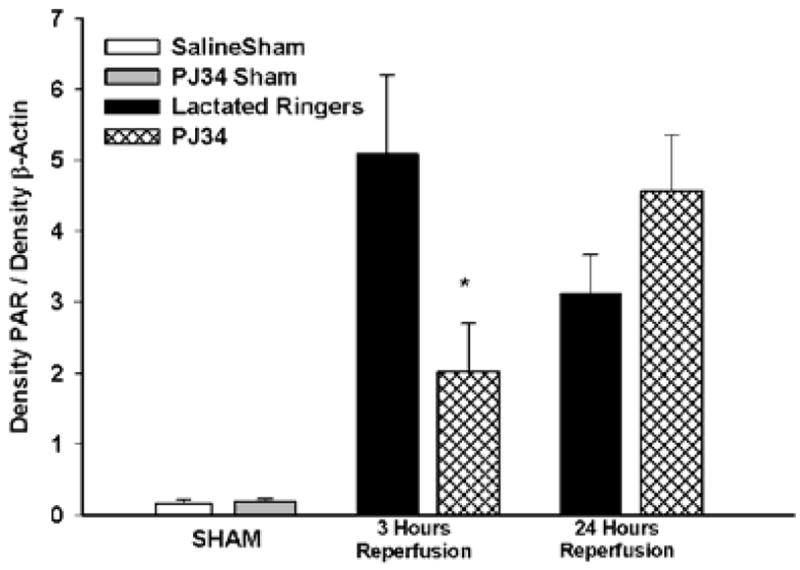
A Treatment with PJ34 resulted in decreased levels of PAR accumulation in skeletal muscle as compared to LR treated mice at 3 but not 24 hours reperfusion(*p<0.01).
Figure 7. Representative PAR Immunoblots.
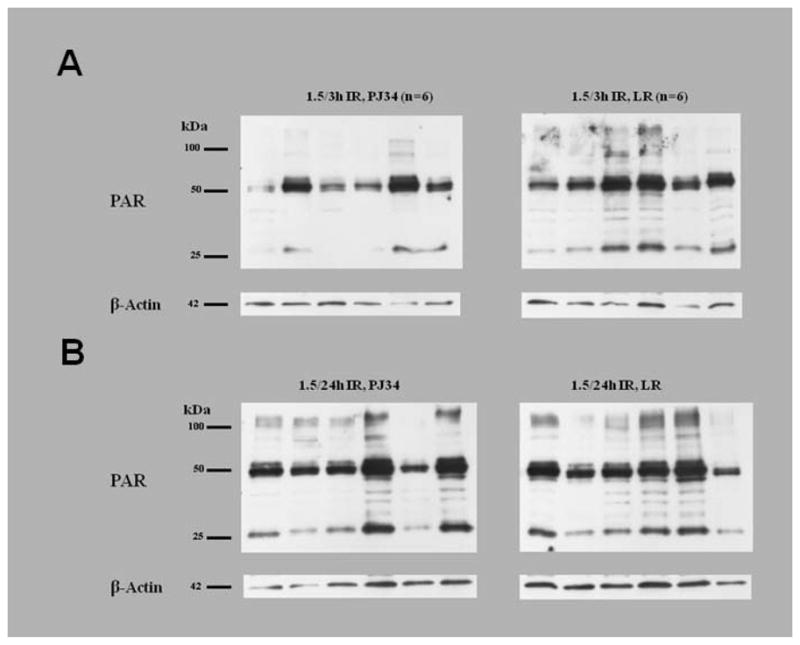
Murine skeletal muscle from LR and PJ34 treated mice exposed to 3 hours reperfusion(A) and 24 hours reperfusion(B). Each lane represents a sample from an individual mouse.
DISCUSSION
These experiments demonstrate that post ischemic treatment with PJ34 results in decreased skeletal muscle fiber injury, preservation of tissue ATP (i.e. metabolic rescue), decreased inflammation (levels of tissue cytokines, leukocyte activation) in a model of skeletal muscle ischemia and reperfusion (I/R). These results were obtained in a post-ischemic treatment model, where PJ34 was administered systemically (via intraperitoneal injections) starting immediately before reperfusion.
Previously, our laboratory reported on the beneficial effects of PJ34 in a pre-ischemic treatment model of hind-limb I/R10. In a follow-up report using a treatment protocol where PJ34 was administered intraperitoneally only during the reperfusion period failed to show preserved mitochondrial activity or anti-inflammatory action 9. Local injection of PJ34 into skeletal muscle during the ischemic period resulted in preserved mitochondrial activity after I/R without affecting local cytokine production. Since intramuscular injection of PJ34 did not alter the local levels of pro-inflammatory skeletal muscle cytokines, intraperitoneal injection prior to reperfusion, early reperfusion and at an additional interval of reperfusion was chosen with the hope of establishing a steady state level of PJ34 in the serum similar to one might see with intravenous bolus or infusion. The desire to reassess intraperitoneal injection was also driven by concerns for potential bleeding complications associated with direct intramuscular administration of PJ34 into fragile, reperfused tissue in a clinical scenario where anticoagulants and/or anti-platelet therapies are routinely utilized.
In this protocol, PJ34 was administered 15 minutes prior to reperfusion, 15 minutes and two hours after reperfusion at a dose (30 mg/kg) known to provide protection against inflammation in rodent models31. In preliminary experiments, 15mg/kg dose administered at the same time intervals did not salvage skeletal muscle ATP after 1.5 hours of ischemia followed by 24 hours reperfusion(data not shown). A dose of PJ34 was administered immediately prior to reperfusion so that PJ34 would be delivered to the limb during the earliest phase of reperfusion. Since the half life of PJ34 in plasma has been reported to be as short as 30 minutes 16, additional doses of PJ34 were administered 15 minutes and 2 hours into reperfusion because previous work from our laboratory indicated that the intense expression of mRNA for MIP-2 and KC occurred during the first 4 hours of reperfusion28. Additional rationale for the additional doses of PJ34 during reperfusion is provided by previous work from our laboratory which demonstrate stabilization of tissue flow (documented using quantitative laser Doppler imaging) after two hours reperfusion22.
The current experimental protocol involves several changes from our previous work. First, the model used to induce complete ischemia from the controlled tension tourniquet (CTT) model 22 to an orthodontic rubber band (ORB) model 21. The CTT model limited our assessment to 5 mice at a time because each there was only 5 tourniquets on the device. Thus the CTT prevented evaluation of treated and untreated mice simultaneously. Using ORB, the control and experimental mice could be analyzed concurrently since the number of mice evaluated at any time interval was not limited by the number of tourniquets. Prior assessment of skeletal muscle tissue injury with the indirect, semi-quantitative mitochondrial activity assay (MTT) was replaced with a direct quantitative histological assessment of fiber morphology 20. In addition, the current treatment protocol involved administration of PJ34 immediately prior to reperfusion and two additional doses during reperfusion (15 minutes and 2 hours). By administering this drug during three intervals, the protocol provided a more clinically relevant treatment scenario that reflects ongoing treatment during early reperfusion.
At 24 hours reperfusion, the percent-injured fibers were significantly lower in mice treated with PJ34 vs. LR treated animals (Figure 1). These experiments were terminated at 24 hours because the level of muscle fiber injury and the cytokine response associated with hind limb ischemia reperfusion is known to stabilize at 24 hours reperfusion 20, 27, 28. It remains to be determined whether the substantial degree of muscle fiber salvage correlates with a substantial preservation of skeletal muscle function.
Treatment with PJ34 also rescued the absolute amount of skeletal muscle levels of ATP compared to mice treated with LR alone during hind limb ischemia reperfusion (Figure 2). ATP is essential to maintain homeostatic functions essential to maintenance of membrane stabilization. Despite the ability of skeletal muscle to withstand periods of ischemia and utilize alternate energy substrates (derived from glycogen and free fatty acid metabolism) during times of stress, ATP becomes depleted during ischemia and skeletal muscle necrosis is a direct consequence of ATP depletion 32. The mechanism by which PARP inhibition preserves ATP during I/R involves the glycolytic pathway and mitochondrial function 16, 33. Inhibition of PARP activity decreases cleavage of NAD+ into nicotinamide and ADP-ribose during periods of ischemia and early reperfusion. PJ34’s inhibition of PARP, keeps the pool of NAD+ available for energy generation via glycolysis. While we have not directly evaluated mitochondrial function in skeletal muscle using this post ischemic treatment protocol, previous work in our lab had documented that PJ34 treatment preserved mitochondrial activity in reperfused skeletal muscle10. Evidence to support an effect of PJ34 on mitochondria is provided by experiments where PJ34 treatment decreased bax translocation in HELA cell mitochondria34 and mitochondrial superoxide production in the aorta of diabetic mice35.
Skeletal muscle ischemia reperfusion is a form of acute inflammation in which reactive oxygen species promote the activation of pro-inflammatory cytokines and leukocytes that lead to microvascular injury, membrane dysfunction and edema formation36. In these experiments, post ischemic treatment with PJ34 markedly decreased tissue levels of pro-inflammatory cytokines which promote influx of inflammatory cells during reperfusion. KC (Keratinocyte-Derived Cytokine) and MIP-2 (Macrophage Inflammatory Protein) are both members of the CXC Chemokine family of proteins implicated as neutrophil chemoattractants that regulate the migration of leukocytes to injured tissue. KC and MIP-2 are both murine equivalents of human IL-8, which is implicated in the pathogenesis of ischemia reperfusion injury37. The decreased levels of pro-inflammatory cytokines detected in skeletal muscle where treatment was administered in a post hoc scenario is consistent with findings detected when PJ34 was administered prior to the onset of ischemia/reperfusion10 (Figure 4A and 4B).
Myeloperoxidase (MPO) is an enzyme secreted by activated neutrophils and is involved in the production of tissue oxidants that contribute to reperfusion injury. MPO catalyzes the reaction of hydrogen peroxide with chloride ions that yields the toxic metabolite, hypochlorous acid (HOCl). It is not clear whether PARP inhibition with PJ34 directly or indirectly inhibited the activation of neutrophils and hence the release of MPO, or alternatively that the inhibition of KC by PJ34 administration resulted in decreased numbers of neutrophils entering the injured area. The absolute decrease in myeloperoxidase protein concentration in the muscle of PJ34 treated mice (Figure 5) is consistent with less tissue injury detected during histologic evaluation of skeletal muscle during reperfusion (Figure 1).
Quantitative analysis of PAR expression using immunoblotting following treatment with PJ34 has been used to document the effect of PJ34 treatment on diabetic mice 30, 38. PAR expression in the hind limbs of ischemia reperfused mice was markedly increased in LR treated mice at 3 and 24 hours of reperfusion. In PJ34 treated mice, PAR expression was less than LR at 3hours but not 24 hours of reperfusion (Figure 6). These findings indicate that the benefits of PARP inhibition are reflected in decrease poly-ADP ribosylation of proteins during the early, i.e. three hours of reperfusion, and remain durable at 24 hours of reperfusion even though the level of protein ADP-ribosylation eventually increases to untreated levels by 24 hours reperfusion. These findings suggest that early post ischemic therapy is effective in decreasing PARP expression, and the rebound in PAR expression at 24 hours does not appear to have a deleterious effect on skeletal muscle fiber histology, nor does it promote an ongoing inflammatory response.
CONCLUSION
These data implicate activation of Poly ADP-Ribose Polymerase in the pathogenesis of clinically relevant, i.e. “post-ischemic” muscle injury, following reperfusion. Systemic administration of PJ34 in a post-hoc treatment protocol successfully preserved tissue ATP levels, decreased the local markers of neutrophil recruitment and muscle fiber injury after reperfusion. Since PJ34 has a short half-life, clinical administration in humans will likely require intravenous administration during ischemia and throughout the early period of reperfusion. While there is concern about the chronic use of PARP inhibitors because genetic deletion of PARP-1 has been shown to result in an increased incidence of UV-light induced skin cancer in mice16, there have been no reports of spontaneous malignancy associated with use of PARP inhibitors. Furthermore, recent clinical trials using PARP inhibitors for cancer therapy have demonstrated limited side effects such as nausea and fatigue39. Thus the findings that early administration of PJ34 to mice subjected to hind limb IR is effective should ameliorate concern about malignant potential in humans. These results support PARP inhibition, using PJ34, as a viable treatment for skeletal muscle ischemia reperfusion in a clinically relevant “post-ischemic” treatment scenario.
Acknowledgments
The authors acknowledge support from the Pacific Vascular Research Foundation, the American Diabetes Association, the Geneen Fund at the Massachusetts General Hospital and the National Institutes of Health (1R01AR055843).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Wagner HJ, Starck EE, Reuter P. Long-term results of percutaneous aspiration embolectomy. Cardiovasc Intervent Radiol. 1994;17(5):241–246. doi: 10.1007/BF00192445. [DOI] [PubMed] [Google Scholar]
- 2.Braithwaite BD, Davies B, Birch PA, Heather BP, Earnshaw JJ. Management of acute leg ischaemia in the elderly. Br J Surg. 1998;85(2):217–220. doi: 10.1046/j.1365-2168.1998.00577.x. [DOI] [PubMed] [Google Scholar]
- 3.Jivegard L, Holm J, Schersten T. Acute limb ischemia due to arterial embolism or thrombosis: influence of limb ischemia versus pre-existing cardiac disease on postoperative mortality rate. J Cardiovasc Surg (Torino) 1988;29(1):32–36. [PubMed] [Google Scholar]
- 4.Kyriakides C, Austen WG, Jr, Wang Y, Favuzza J, Moore FD, Jr, Hechtman HB. Neutrophil mediated remote organ injury after lower torso ischemia and reperfusion is selectin and complement dependent. J Trauma. 2000;48(1):32–38. doi: 10.1097/00005373-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253(3 Pt 2):H699–703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Chen LE, Seaber AV, Urbaniak JR. S-nitroso-N-acetylcysteine protects skeletal muscle against reperfusion injury. Microsurgery. 1998;18(5):299–305. doi: 10.1002/(sici)1098-2752(1998)18:5<299::aid-micr1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Hobson RW, 2nd, Neville R, Watanabe B, Canady J, Wright JG, Belkin M. Role of heparin in reducing skeletal muscle infarction in ischemia-reperfusion. Microcirc Endothelium Lymphatics. 1989;5(3–5):259–276. [PubMed] [Google Scholar]
- 8.Woo YJ, Taylor MD, Cohen JE, Jayasankar V, Bish LT, Burdick J, Pirolli TJ, Berry MF, Hsu V, Grand T. Ethyl pyruvate preserves cardiac function and attenuates oxidative injury after prolonged myocardial ischemia. J Thorac Cardiovasc Surg. 2004;127(5):1262–1269. doi: 10.1016/j.jtcvs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Conrad MF, Albadawi H, Stone DH, Crawford RS, Entabi F, Watkins MT. Local administration of the Poly ADP-Ribose Polymerase (PARP) inhibitor, PJ34 during hindlimb ischemia modulates skeletal muscle reperfusion injury. J Surg Res. 2006;135(2):233–237. doi: 10.1016/j.jss.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Hua HT, Albadawi H, Entabi F, Conrad M, Stoner MC, Meriam BT, Sroufe R, Houser S, Lamuraglia GM, Watkins MT. Polyadenosine diphosphate-ribose polymerase inhibition modulates skeletal muscle injury following ischemia reperfusion. Arch Surg. 2005;140(4):344–351. doi: 10.1001/archsurg.140.4.344. discussion 351–342. [DOI] [PubMed] [Google Scholar]
- 11.Black JH, Casey PJ, Albadawi H, Cambria RP, Watkins MT. Poly adenosine diphosphate-ribose polymerase inhibitor PJ34 abolishes systemic proinflammatory responses to thoracic aortic ischemia and reperfusion. J Am Coll Surg. 2006;203(1):44–53. doi: 10.1016/j.jamcollsurg.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Qi WN, Allen DM, Chen LE, Seaber AV, Stamler JS, Urbaniak JR. Inhibition of iNOS with 1400W improves contractile function and alters nos gene and protein expression in reperfused skeletal muscle. Microsurgery. 2004;24(4):324–331. doi: 10.1002/micr.20029. [DOI] [PubMed] [Google Scholar]
- 13.Cunha MS, da Silva JC, Nakamoto HA, Ferreira MC. Study of warm ischemia followed by reperfusion on a lower limb model in rats: effect of allopurinol and streptokinase. Clinics. 2005;60(3):213–220. doi: 10.1590/s1807-59322005000300006. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm MP, Schlensak C, Hoh A, Knipping L, Mangold G, Rojas DD, Beyersdorf F. Controlled reperfusion using a simplified perfusion system preserves function after acute and persistent limb ischemia: a preliminary study. J Vasc Surg. 2005;42(4):690–694. doi: 10.1016/j.jvs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 15.Ouriel K, Veith F, Sasahara A. A comparions of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199804163381603. [DOI] [PubMed] [Google Scholar]
- 16.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54(3):375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 17.Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. Embo J. 1999;18(16):4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, Endres M. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med. 2001;7(3):255–260. [PubMed] [Google Scholar]
- 19.Jagtap P, Soriano FG, Virag L, Liaudet L, Mabley J, Szabo E, Hasko G, Marton A, Lorigados CB, Gallyas F, Jr, Sumegi B, Hoyt DG, Baloglu E, VanDuzer J, Salzman AL, Southan GJ, Szabo C. Novel phenanthridinone inhibitors of poly (adenosine 5′-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents. Crit Care Med. 2002;30(5):1071–1082. doi: 10.1097/00003246-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 20.McCormack MC, Kwon E, Eberlin KR, Randolph M, Friend DS, Thomas AC, Watkins MT, Austen WG., Jr Development of reproducible histologic injury severity scores: skeletal muscle reperfusion injury. Surgery. 2008;143(1):126–133. doi: 10.1016/j.surg.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Crawford RS, Hashmi F, Jones JE, Albadawi H, McCormack M, Eberlin K, Entabi F, Atkins M, Conrad MF, Austen WG, Watkins MT. A Novel Model of Acute Murine Hind Limb Ischemia. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00581.2006. [DOI] [PubMed] [Google Scholar]
- 22.Bonheur JA, Albadawi H, Patton GM, Watkins MT. A noninvasive murine model of hind limb ischemia-reperfusion injury. J Surg Res. 2004;116(1):55–63. doi: 10.1016/s0022-4804(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 23.Goldfarb RD, Marton A, Szabo E, Virag L, Salzman AL, Glock D, Akhter I, McCarthy R, Parrillo JE, Szabo C. Protective effect of a novel, potent inhibitor of poly(adenosine 5′-diphosphate-ribose) synthetase in a porcine model of severe bacterial sepsis. Crit Care Med. 2002;30(5):974–980. doi: 10.1097/00003246-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Szabo G, Bahrle S, Stumpf N, Sonnenberg K, Szabo EE, Pacher P, Csont T, Schulz R, Dengler TJ, Liaudet L, Jagtap PG, Southan GJ, Vahl CF, Hagl S, Szabo C. Poly(ADP-Ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ Res. 2002;90(1):100–106. doi: 10.1161/hh0102.102657. [DOI] [PubMed] [Google Scholar]
- 25.Yassin MM, Barros D’Sa AA, Parks G, Abdulkadir AS, Halliday I, Rowlands BJ. Mortality following lower limb ischemia-reperfusion: a systemic inflammatory response? World J Surg. 1996;20(8):961–966. doi: 10.1007/s002689900144. discussion 966–967. [DOI] [PubMed] [Google Scholar]
- 26.Yassin MM, Harkin DW, Barros D’Sa AA, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26(1):115–121. doi: 10.1007/s00268-001-0169-2. [DOI] [PubMed] [Google Scholar]
- 27.Abbruzzese TA, Albadawi H, Kang J, Patel VI, Yoo JH, Lamuraglia GM, Watkins MT. Enoxaparin does not ameliorate limb ischemia-reperfusion injury. J Surg Res. 2008;147(2):260–266. doi: 10.1016/j.jss.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Hua HT, Al-Badawi H, Entabi F, Stoner MC, Diamond RE, Bonheur JA, Houser S, Watkins MT. CXC chemokine expression and synthesis in skeletal muscle during ischemia/reperfusion. J Vasc Surg. 2005;42(2):337–343. doi: 10.1016/j.jvs.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 29.Wakai M, Winter D, Street J, O’Sullivan R, Wang J, Redmond H. Inosine Attenuates Tourniquet Induced Skeletal Muscle Reperfusion Injury. J Surg Res. 2001;99:311–315. doi: 10.1006/jsre.2001.6192. [DOI] [PubMed] [Google Scholar]
- 30.Szabo C, Biser A, Benko R, Bottinger E, Susztak K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes. 2006;55(11):3004–3012. doi: 10.2337/db06-0147. [DOI] [PubMed] [Google Scholar]
- 31.Mabley JG, Jagtap P, Perretti M, Getting SJ, Salzman AL, Virag L, Szabo E, Soriano FG, Liaudet L, Abdelkarim GE, Hasko G, Marton A, Southan GJ, Szabo C. Anti-inflammatory effects of a novel, potent inhibitor of poly (ADP-ribose) polymerase. Inflamm Res. 2001;50(11):561–569. doi: 10.1007/PL00000234. [DOI] [PubMed] [Google Scholar]
- 32.Rubin BB, Liauw S, Tittley J, Romaschin AD, Walker PM. Prolonged adenine nucleotide resynthesis and reperfusion injury in postischemic skeletal muscle. Am J Physiol. 1992;262(5 Pt 2):H1538–1547. doi: 10.1152/ajpheart.1992.262.5.H1538. [DOI] [PubMed] [Google Scholar]
- 33.Ariumi Y, Masutani M, Copeland TD, Mimori T, Sugimura T, Shimotohno K, Ueda K, Hatanaka M, Noda M. Suppression of the poly(ADP-ribose) polymerase activity by DNA-dependent protein kinase in vitro. Oncogene. 1999;18(32):4616–4625. doi: 10.1038/sj.onc.1202823. [DOI] [PubMed] [Google Scholar]
- 34.Ethier C, Labelle Y, Poirier GG. PARP-1-induced cell death through inhibition of the MEK/ERK pathway in MNNG-treated HeLa cells. Apoptosis. 2007;12(11):2037–2049. doi: 10.1007/s10495-007-0127-z. [DOI] [PubMed] [Google Scholar]
- 35.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112(7):1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem. 1998;179(1–2):169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- 37.Boyle EM, Jr, Kovacich JC, Hebert CA, Canty TG, Jr, Chi E, Morgan EN, Pohlman TH, Verrier ED. Inhibition of interleukin-8 blocks myocardial ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 1998;116(1):114–121. doi: 10.1016/S0022-5223(98)70249-1. [DOI] [PubMed] [Google Scholar]
- 38.Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89(8):684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- 39.Tuma RS. PARP inhibitors: will the new class of drugs match the hype? J Natl Cancer Inst. 2009;101(18):1230–1232. doi: 10.1093/jnci/djp315. [DOI] [PubMed] [Google Scholar]