Abstract
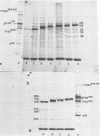
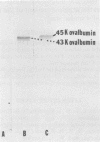
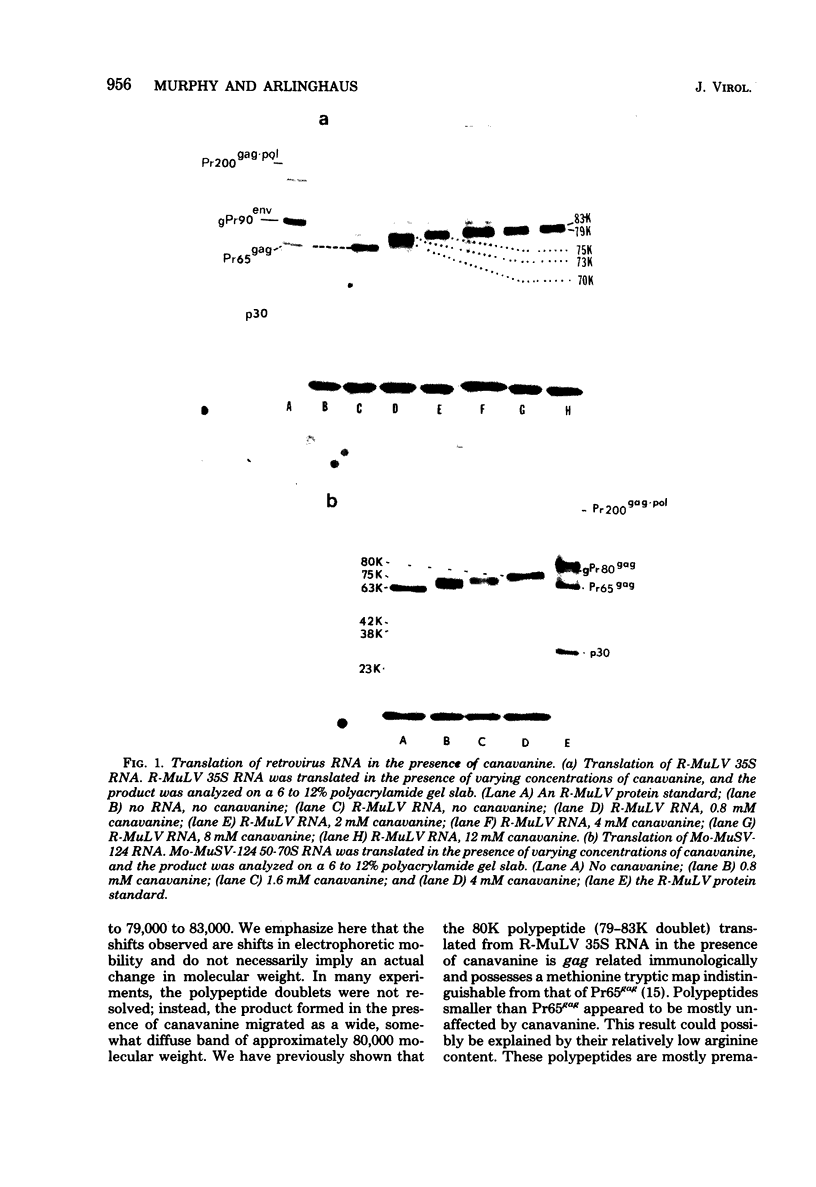
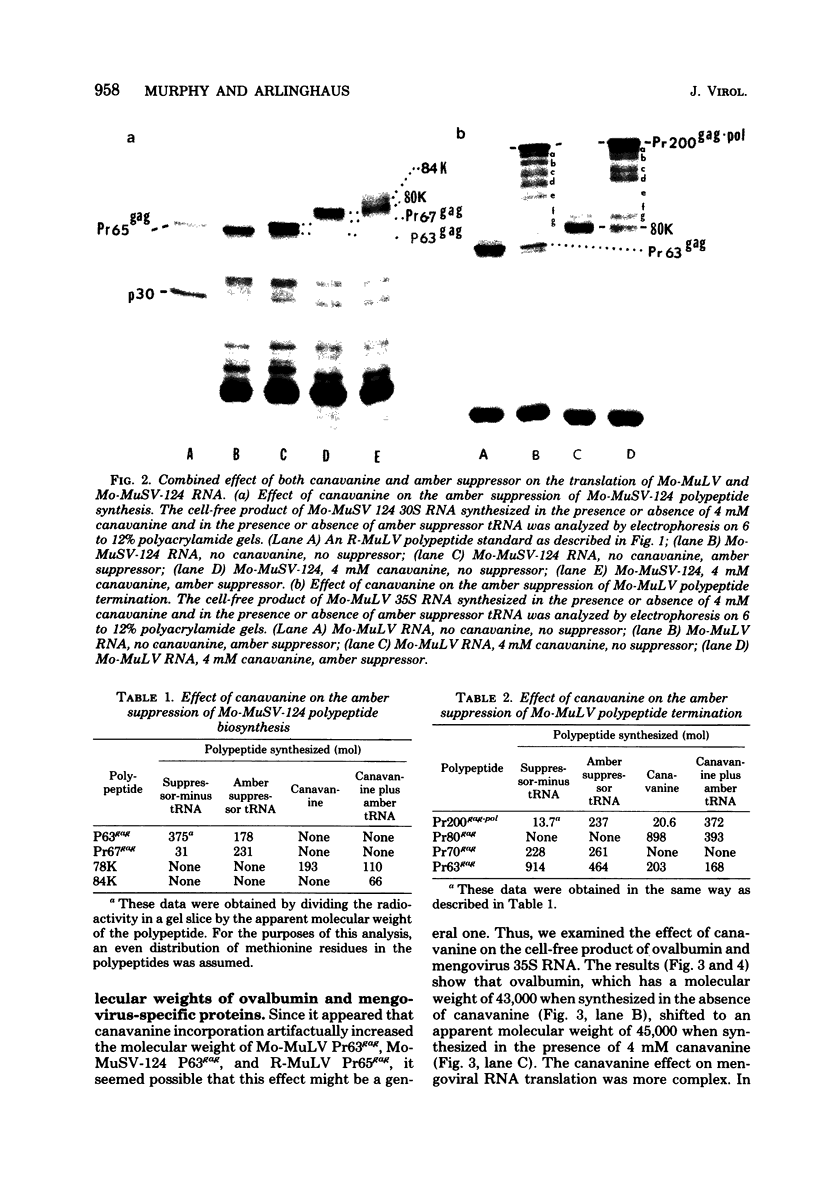
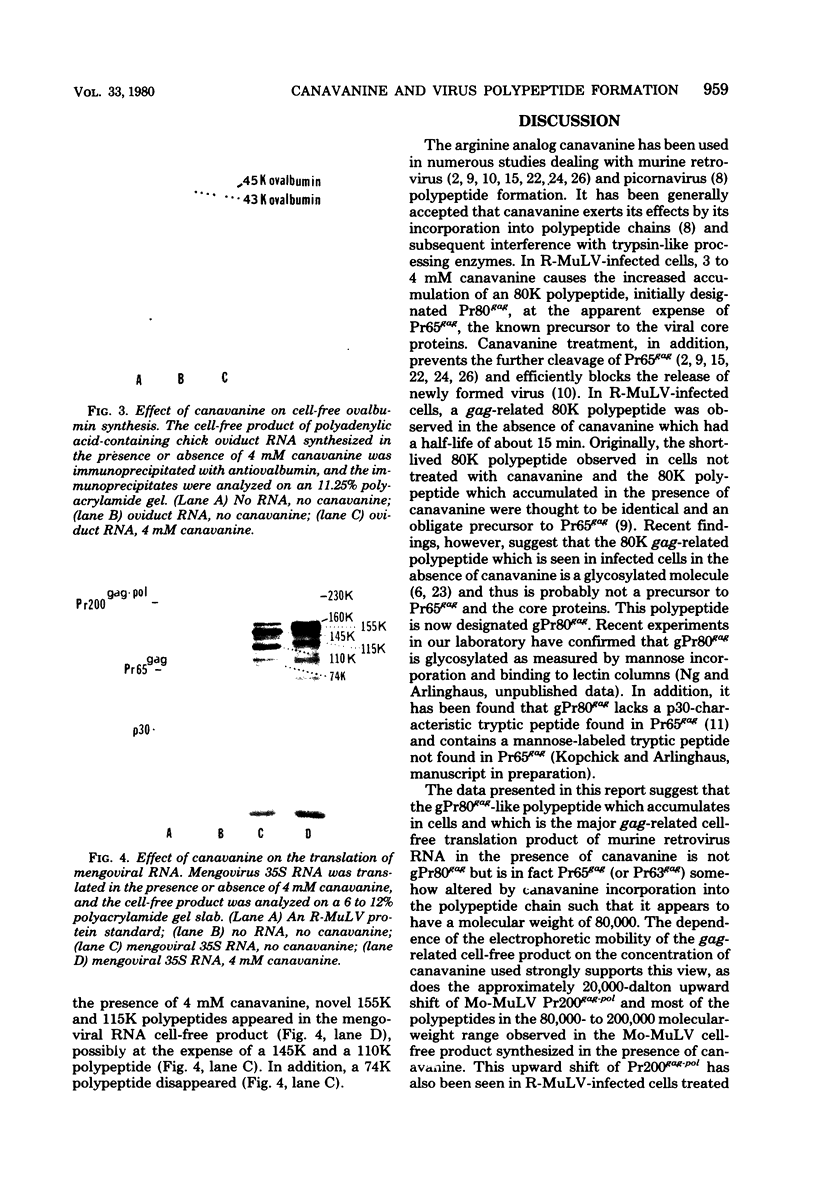
Canavanine is an arginine analog which is widely used to inhibit proteolytic processing of viral polyproteins. Certain results obtained with canavanine have suggested that it may have other effects. Therefore, we examined the effects of canavanine on the cell-free synthesis of murine retrovirus proteins. It was found that the electrophoretic mobility of the major gag-related cell-free product of both Rauscher murine leukemia virus (R-MuLV) and Moloney murine sarcoma virus 124 (Mo-MuSV-124) RNA was dependent on the concentration of canavanine used during translation. As the canavanine concentration was increased up to 4 mM, the apparent size of the major gag-related polypeptide also increased from 65,000 (R-MuLV RNA) or 63,000 (Mo-MuSV-124 RNA) to approximately 80,000 daltons. Additional increases in the canavanine concentration up to 12 mM did not increase the size of the gag gene product beyond 80,000 daltons. This change in electrophoretic mobility appeared to be due to a substitution of canavanine for arginine residues in the polypeptides, not to a change in their actual size. If amber suppressor tRNA and canavanine were used together during translation of Mo-MuSV-124 RNA and Mo-MuLV RNA, the results were also in agreement with this proposal. Translation experiments done with ovalbumin mRNA and mengovirus 35S RNA indicated that canavanine incorporation caused a shift in the electrophoretic mobility of ovalbumin from 43,000 to 45,000 daltons and caused the appearance of two slightly larger polypeptides in the 155,000- and 115,000- dalton regions of the mengovirus RNA cell-free product.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Arlinghaus R. B. "gag" polyprotein precursors of Rauscher murine leukemia virus. Virology. 1977 Sep;81(2):284–297. doi: 10.1016/0042-6822(77)90145-3. [DOI] [PubMed] [Google Scholar]
- Ball J., McCarter J. A., Sunderland S. M. Evidence for helper independent murine sarcoma virus. I. Segregation of replication-defective and transformation-defective viruses. Virology. 1973 Nov;56(1):268–284. doi: 10.1016/0042-6822(73)90305-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brouwer J., Pluijms W. J., Warnaar S. O. Chemical characterization of Rauscher leukaemia virus proteins. J Gen Virol. 1979 Feb;42(2):415–421. doi: 10.1099/0022-1317-42-2-415. [DOI] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol. 1979 May;30(2):551–563. doi: 10.1128/jvi.30.2.551-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Ng V. L., Arlinghaus R. B. Inhibition of maturation of Rauscher leukemia virus by amino acid analogs. J Virol. 1978 Jan;25(1):408–412. doi: 10.1128/jvi.25.1.408-412.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick J. J., Karshin W. L., Arlinghaus R. B. Tryptic peptide analysis of gag and gag-pol gene products of Rauscher murine leukemia virus. J Virol. 1979 May;30(2):610–623. doi: 10.1128/jvi.30.2.610-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Cell-free synthesis of Rauscher murine leukemia virus "gag" and "gag-pol" precursor polyproteins from virion 35 S RNA in a mRNA-dependent translation system derived from mouse tissue culture cells. Virology. 1978 May 15;86(2):329–343. doi: 10.1016/0042-6822(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Tryptic peptide analyses of polypeptides generated by premature termination of cell-free protein synthesis allow a determination of the Rauscher leukemia virus gag gene order. J Virol. 1978 Dec;28(3):929–935. doi: 10.1128/jvi.28.3.929-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Kopchick J. J., Watson K. F., Arlinghaus R. B. Cell-free synthesis of a precursor polyprotein containing both gag and pol gene products by Rauscher murine leukemia virus 35S RNA. Cell. 1978 Feb;13(2):359–369. doi: 10.1016/0092-8674(78)90204-0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Salden M. H., Selten-Versteegen A. M., Bloemendal H. Translation of Rauscher murine leukemia viral RNA. A model for the function of virus-specific messenger. Biochem Biophys Res Commun. 1976 Sep 20;72(2):610–618. doi: 10.1016/s0006-291x(76)80084-8. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., August J. T. High molecular weight precursor polypeptides to structural proteins of Rauscher murine leukemia virus. J Mol Biol. 1976 Nov 15;107(4):459–477. doi: 10.1016/s0022-2836(76)80078-2. [DOI] [PubMed] [Google Scholar]
- Syrewicz J. J., Naso R. B., Wang C. S., Arlinghaus R. B. Purification of large amounts of murine ribonucleic acid tumor viruses produced in roller bottle cultures. Appl Microbiol. 1972 Sep;24(3):488–494. doi: 10.1128/am.24.3.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Dekker-Michielsen J. A., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus: synthesis, identification, and processing. Virology. 1976 Nov;75(1):113–129. doi: 10.1016/0042-6822(76)90011-8. [DOI] [PubMed] [Google Scholar]