Abstract
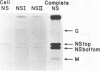
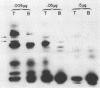
Vesicular stomatitis virus contains a phosphorylated NS protein which is necessary along with L protein and RNP template for transcription of mRNA. To further define the structure of the NS protein and its function in transcription and replication, virion NS was purified and separated into two different phosphrylated forms (NSI and NSII) on DEAE-cellulose columns. Cytoplasmic preparations of NS contained one phosphorylated species which eluted from the column in the same place as the virion NSI. When electrophoresed in sodium dodecyl sulfate-polyacrylamide gels containing urea, NSI and NSII each resolved into two components, whereas cell NS migrated as a single band. NSI and cell NS exhibited little activity in a reconstituted transcription assay, whereas the more highly phosphorylated NSII was very active in the same system. Addition of NSI or cell NS to a transcription system containing NSII resulted in even higher levels of activity, indicating that the various NS species might have different enzymatic functions.
Full text
PDF


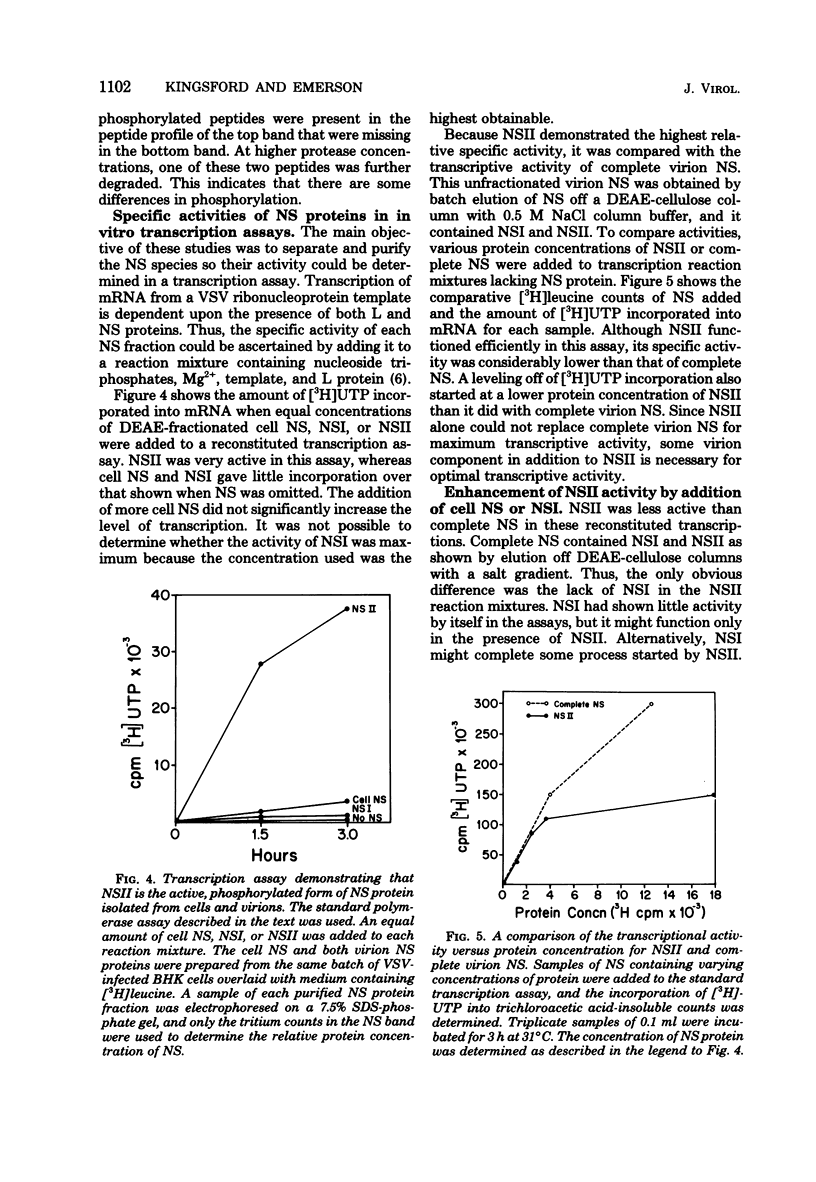
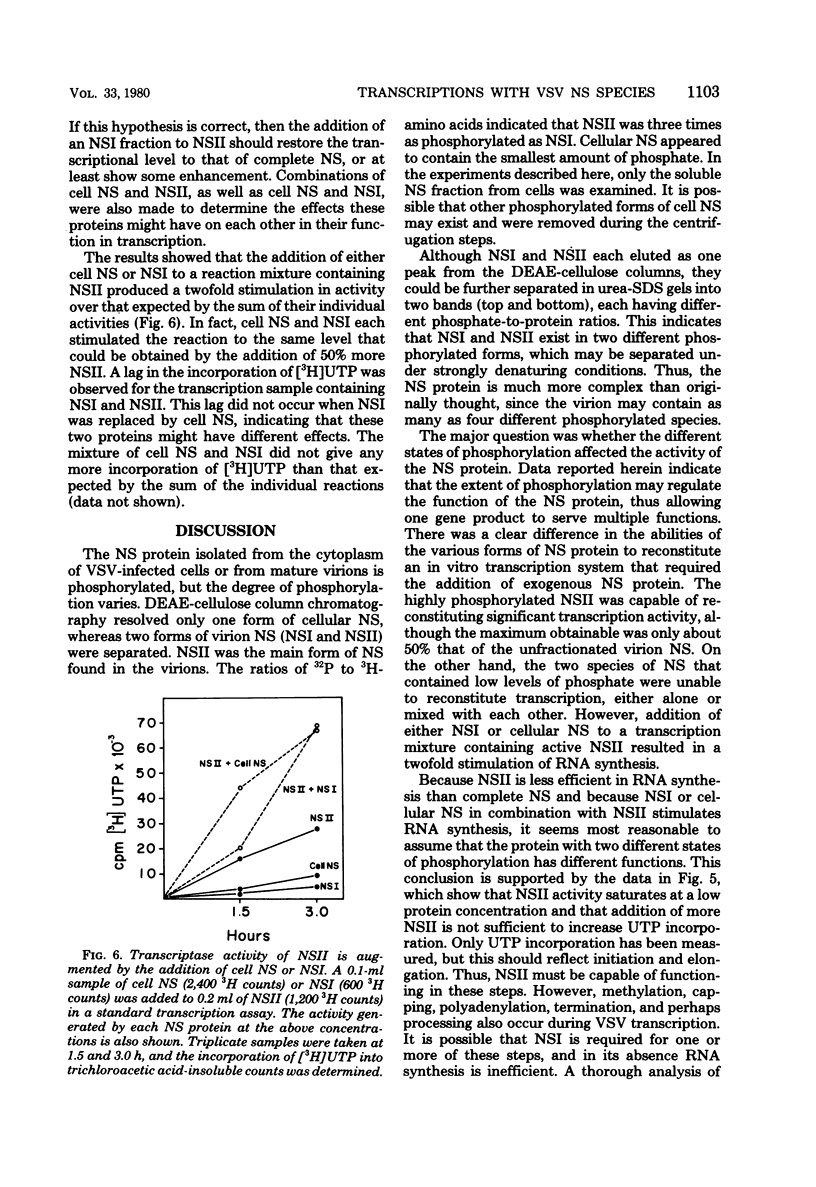


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. The nature of the RNA products synthesized in vitro by subviral components of visicular stomatitis virus. Virology. 1976 May;71(1):230–241. doi: 10.1016/0042-6822(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D., Pringle C. R., Szilágyi J. F. Temperature-sensitive mutants of complementation group E of vesicular stomatitis virus New Jersey serotype possess altered NS polypeptides. J Virol. 1979 Aug;31(2):325–333. doi: 10.1128/jvi.31.2.325-333.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Twiddy E., Gilden R. V. Protein kinase associated with RNA tumor viruses and other budding RNA viruses. Virology. 1972 Feb;47(2):536–538. doi: 10.1016/0042-6822(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng Y. H., Wold W. S., Sugawara K., Gilead Z., Green M. Adenovirus type 2 coded single-stranded DNA binding protein: in vivo phosphorylation and modification. J Virol. 1977 May;22(2):402–411. doi: 10.1128/jvi.22.2.402-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T., Watanabe Y. Role for nucleocapsid protein phosphorylation in the transcription of influenza virus genome. Nature. 1977 Jun 2;267(5610):460–462. doi: 10.1038/267460a0. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lesnaw J. A., Dickson L. R., Curry R. H. Proposed replicative role of the NS polypeptide of vesicular stomatitis virus: structural analysis of an electrophoretic variant. J Virol. 1979 Jul;31(1):8–15. doi: 10.1128/jvi.31.1.8-15.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnaw J. A., Reichmann M. E. RNA synthesis by temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. Virology. 1975 Feb;63(2):492–504. doi: 10.1016/0042-6822(75)90322-0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Postel E. H., Levine A. J. In vivo and in vitro phosphorylation of the adenovirus type 5 single strand-specific DNA-binding protein. Virology. 1977 Jun 1;79(1):144–159. doi: 10.1016/0042-6822(77)90341-5. [DOI] [PubMed] [Google Scholar]
- Mellon M. G., Emerson S. U. Rebinding of transcriptase components (L and NS proteins) to the nucleocapsid template of vesicular stomatitis virus. J Virol. 1978 Sep;27(3):560–567. doi: 10.1128/jvi.27.3.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Pal B. K., Roy-Burman P. Phosphoproteins: structural components of oncornaviruses. J Virol. 1975 Mar;15(3):540–549. doi: 10.1128/jvi.15.3.540-549.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B., Stevenson M. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. J Virol. 1971 Dec;8(6):836–841. doi: 10.1128/jvi.8.6.836-841.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Phosphorylation of murine type C viral p12 proteins regulates their extent of binding to the homologous viral RNA. Cell. 1977 Mar;10(3):489–496. doi: 10.1016/0092-8674(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutation on activity of the RNA transcriptase of vesicular stomatitis virus New Jersey. J Virol. 1979 Jun;30(3):692–700. doi: 10.1128/jvi.30.3.692-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Uryvayev L. Isolation of an infectious ribonucleoprotein from vesicular stomatitis virus containing an active RNA transcriptase. J Virol. 1973 Feb;11(2):279–286. doi: 10.1128/jvi.11.2.279-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]