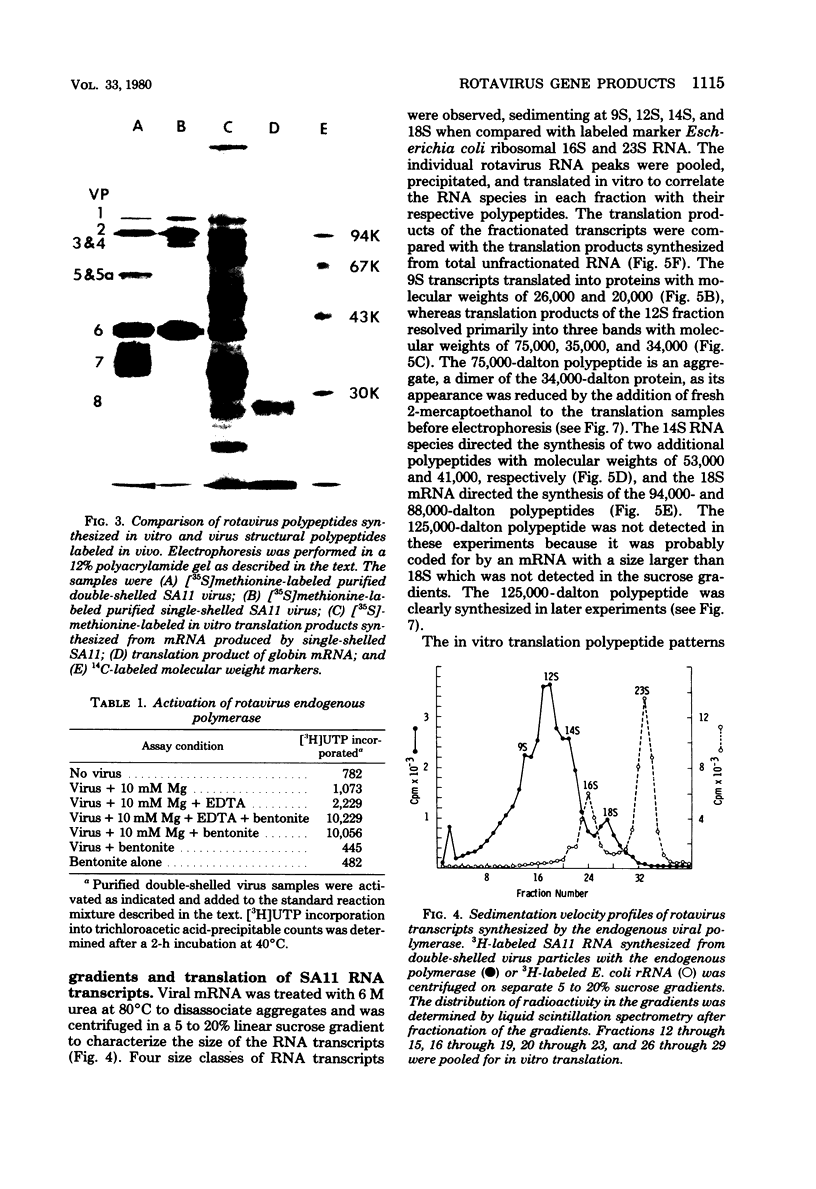
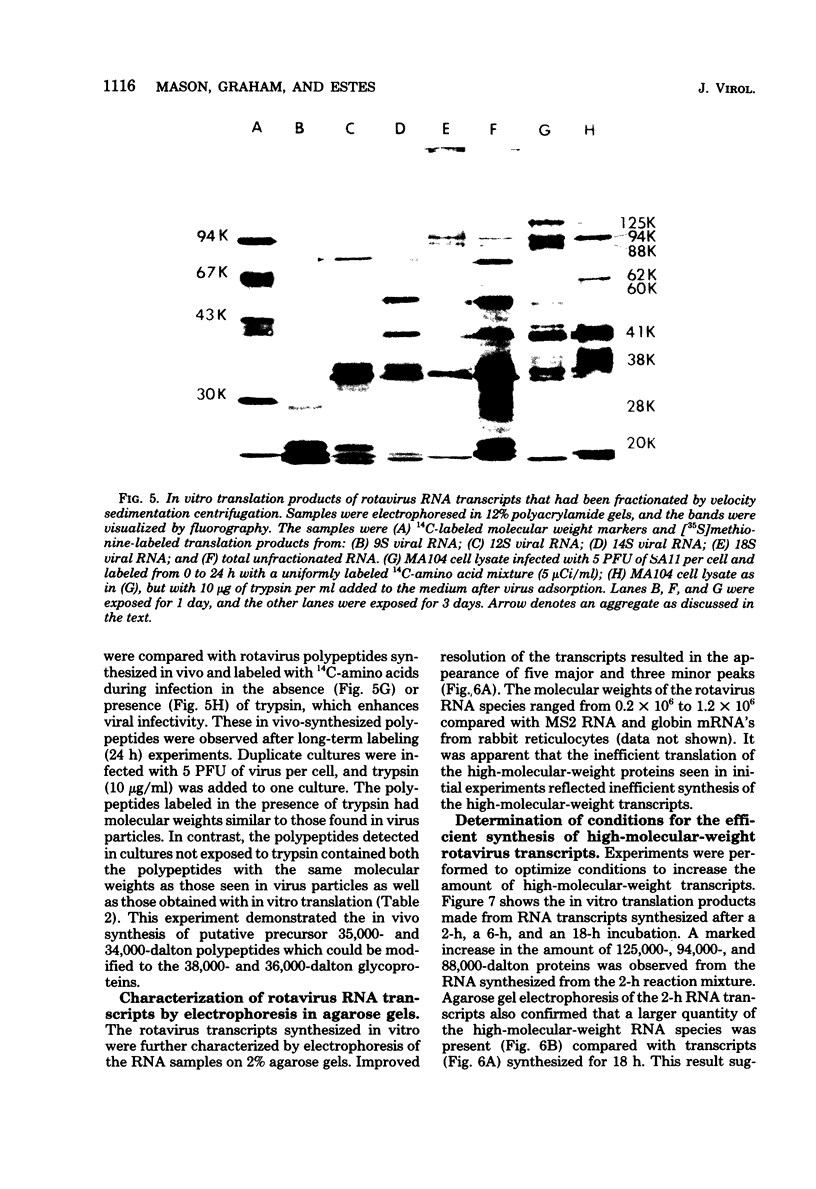
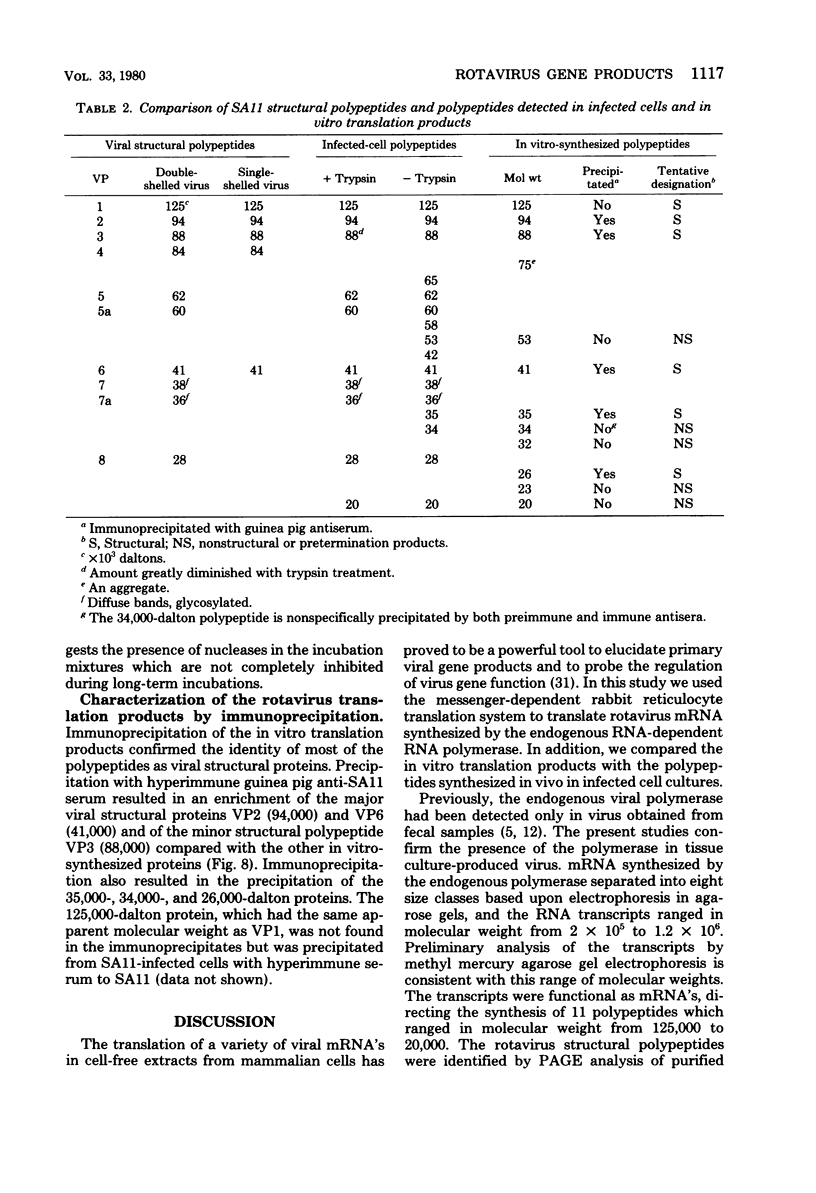
Abstract
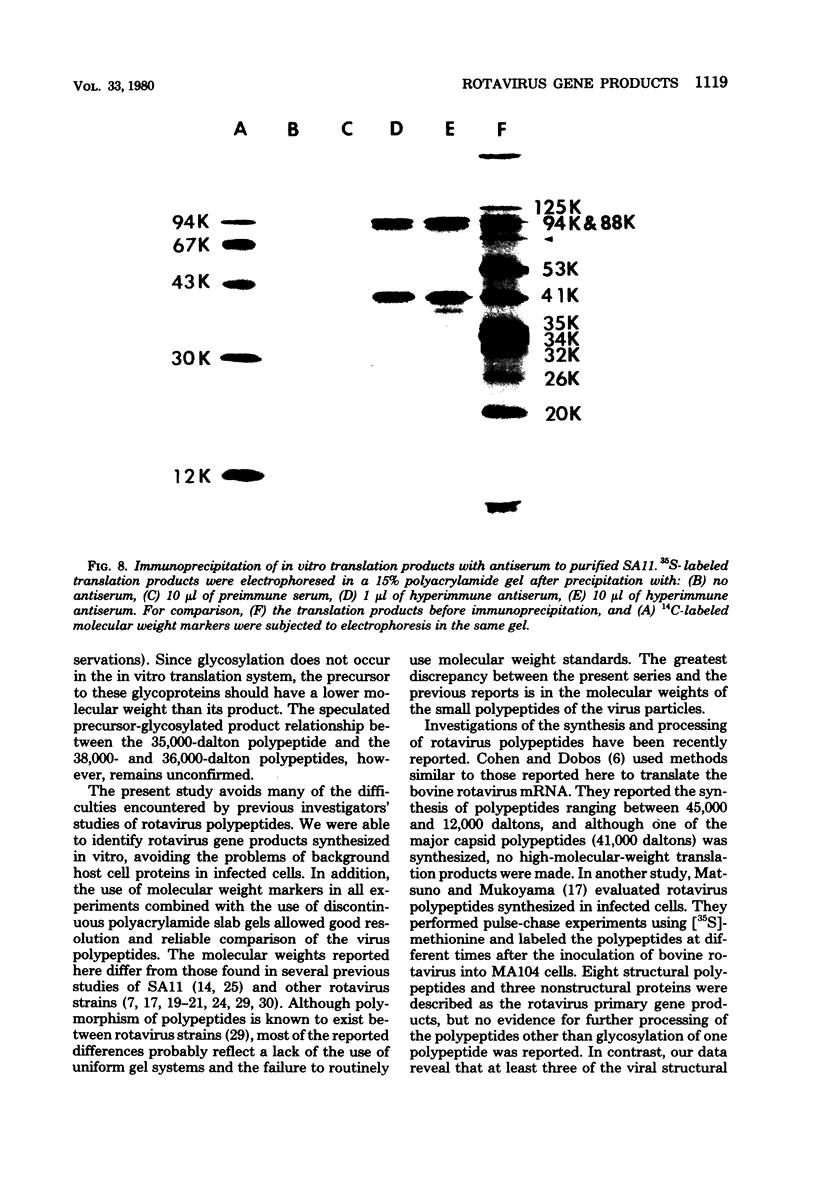
Rotavirus gene products were examined, with the simian rotavirus SA11 as a model. The endogenous viral RNA-dependent RNA polymerase associated with single-shelled virus particles or with activated double-shelled particles was used to synthesize viral RNA transcripts. Sedimentation velocity sucrose gradient analysis of the RNA transcripts revealed four peaks at 9S, 12S, 14S, and 18S, whereas agarose gel electrophoresis under partially denaturing conditions revealed eight groups of RNA species ranging in molecular weight from 2 x 10(5) to 1.2 x 10(6). The transcripts synthesized in vitro were active in an mRNA-dependent cell-free translation system derived from rabbit reticulocytes. The transcripts directed the synthesis of 11 polypeptides that had molecular weights ranging from 125,000 to 20,000 when analyzed by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. The products of in vitro translation were compared with polypeptides from purified virus and those synthesized in infected cells. Several of the polypeptides synthesized in vitro were designated as structural polypeptides by comparing the molecular weights determined by polyacrylamide gel electrophoresis analysis or by precipitation with hyperimmune serum prepared against purified virus. Three of the viral structural polypeptides (VP4, -5, and -5a) were not synthesized in vitro as primary gene products, demonstrating that processing must occur for the production of some structural polypeptides. Other in vitro-synthesized polypeptides were tentatively identified as either precursors to the viral glycoproteins or nonstructural polypeptides.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburg B. C., Graham D. Y., Estes M. K. Ultrastructural study of rotavirus replication in cultured cells. J Gen Virol. 1980 Jan;46(1):75–85. doi: 10.1099/0022-1317-46-1-75. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Dobos P. Cell free transcription and translation of rotavirus RNA. Biochem Biophys Res Commun. 1979 Jun 13;88(3):791–796. doi: 10.1016/0006-291x(79)91477-3. [DOI] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Gerba C. P., Smith E. M. Simian rotavirus SA11 replication in cell cultures. J Virol. 1979 Sep;31(3):810–815. doi: 10.1128/jvi.31.3.810-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Notter M. F., Menegus M. A., Steinhoff M. C. RNA polymerase associated with human rotaviruses in diarrhea stools. J Virol. 1978 May;26(2):544–546. doi: 10.1128/jvi.26.2.544-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Garon C. F., Wyatt R. G., Mebus C. A., van Kirk D. H., Chanock R. M., Kapikian A. Z. Differentiation of human and calf reoviruslike agents associated with diarrhea using polyacrylamide gel electrophoresis of RNA. Virology. 1976 Oct 1;74(1):86–92. doi: 10.1016/0042-6822(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Theodore T. S. Polypeptides of simian rotavirus (SA-11) determined by a continuous polyacrylamide gel electrophoresis method. J Gen Virol. 1979 May;43(2):463–466. doi: 10.1099/0022-1317-43-2-463. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A. Polypeptides of bovine rotavirus. J Gen Virol. 1979 May;43(2):309–316. doi: 10.1099/0022-1317-43-2-309. [DOI] [PubMed] [Google Scholar]
- McNulty M. S., Allan G. M., McFerran J. B. Cell culture studies with a cytopathic bovine rotavirus. Arch Virol. 1977;54(3):201–209. doi: 10.1007/BF01314786. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Palmer E. L., Martin M. L. Biochemical characterization of infantile gastroenteritis virus (IGV). J Gen Virol. 1977 Mar;34(3):485–497. doi: 10.1099/0022-1317-34-3-485. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M. Isolation and characterization of purified rat casein messenger ribonucleic acids. Biochemistry. 1976 Nov 30;15(24):5263–5271. doi: 10.1021/bi00669a011. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Estes M. K., Graham D. Y., Gerba C. P. A plaque assay for the simian rotavirus SAII. J Gen Virol. 1979 Jun;43(3):513–519. doi: 10.1099/0022-1317-43-3-513. [DOI] [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Biochemical studies on a reovirus-like agent (rotovirus) from lambs. J Virol. 1977 Mar;21(3):1215–1218. doi: 10.1128/jvi.21.3.1215-1218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]