Abstract
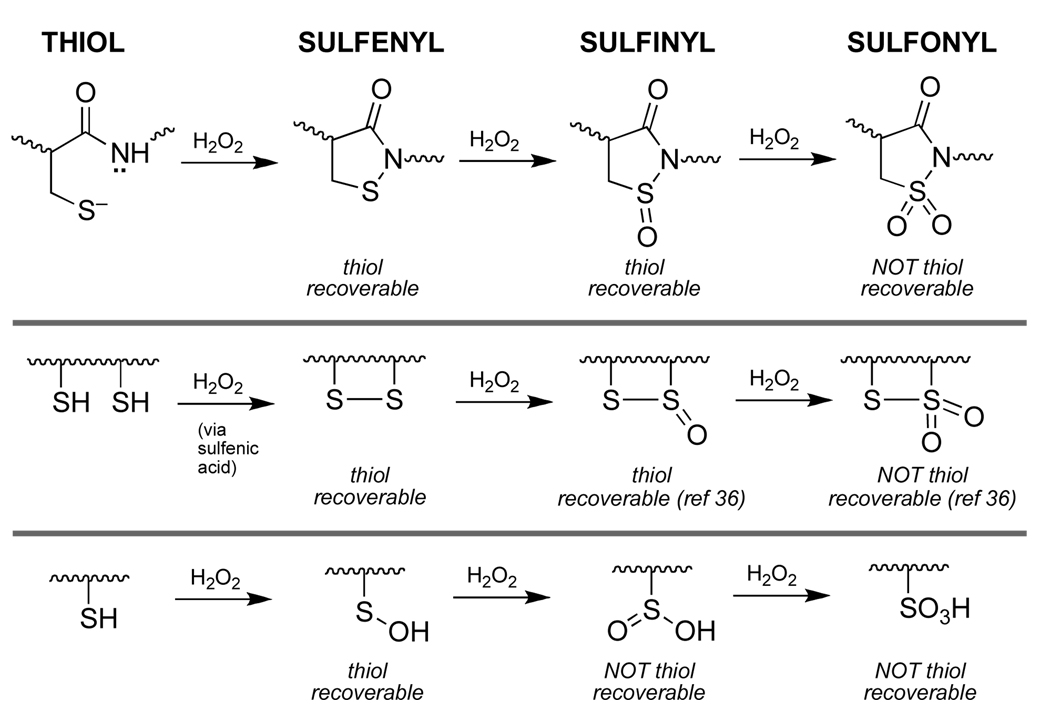
Model reactions offer a chemical mechanism by which formation of a sulfenyl amide residue at the active site of the redox-regulated protein tyrosine phosphatase PTP1B protects the cysteine redox switch in this enzyme against irreversible oxidative destruction. The results suggest that “overoxidation” of the sulfenyl amide redox switch to the sulfinyl amide in proteins is a chemically reversible event, because the sulfinyl amide can be easily returned to the native cysteine thiol residue via reactions with cellular thiols.
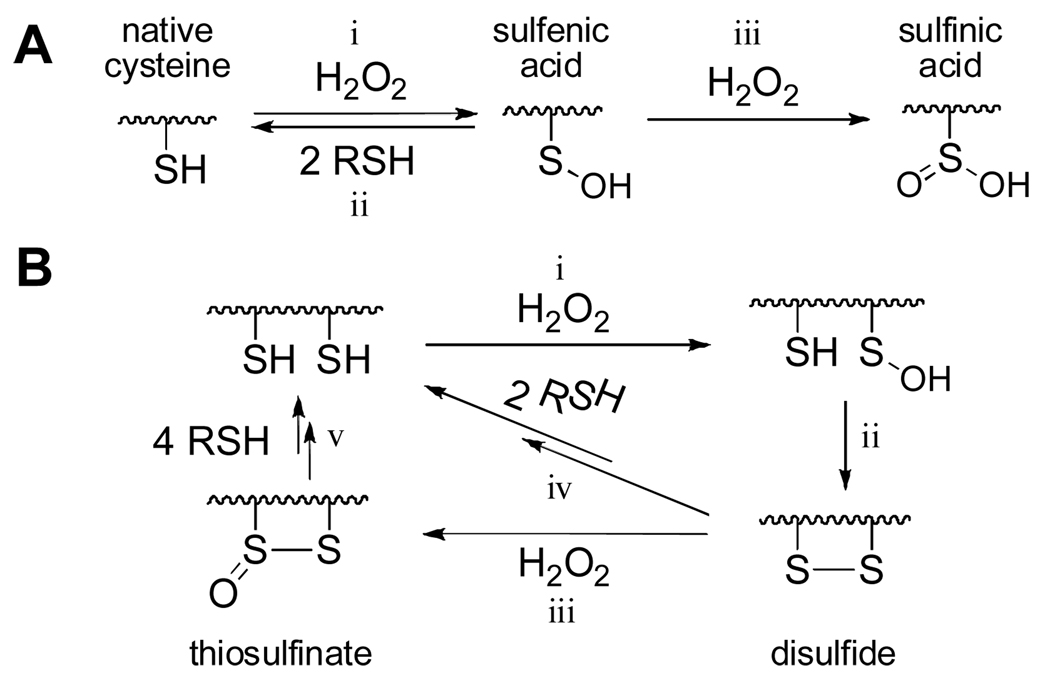
Intracellular concentrations of hydrogen peroxide (H2O2) increase under conditions of oxidative stress and during some normal signal transduction processes.1–3 An important mechanism by which cells issue temporary responses to such transitory increases in H2O2 levels involves reversible oxidation of cysteine residues on critical “sensor” proteins.4,5 The ability of cysteine residues to serve as reversible redox switches relies upon the unique ability of the γ–sulfur atom in this amino acid to cycle easily between (at least) two oxidation states under physiological conditions. Specifically, oxidation of a cysteine thiol by H2O2 yields a sulfenic acid residue (reaction i, Scheme 1A) that can, over time, be returned to the native thiol by reactions with biological thiols (reaction ii, Scheme 1A).4–8
Scheme 1.
Protein sulfenic acid residues also have the potential to undergo further reaction with hydrogen peroxide to generate the corresponding sulfinic acid (reaction iii, Scheme 1A).4–9 This reaction is irreversible, except in the case of some peroxiredoxins8 and, therefore, yields an overoxidized, “broken” redox switch. Alternatively, in some proteins, the initially-formed sulfenic acid intermediate undergoes reaction with a neighboring “back door” cysteine thiol to generate a disulfide linkage (reaction ii, Scheme 1B).10–13 Rudolph and Sohn provided evidence that, at least in the context of the phosphatase Cdc25B, disulfide formation protects the enzyme against irreversible overoxidation.10,11 There are at least two possible mechanisms underlying this protection. First, the disulfide may be relatively resistant to further oxidation (reaction iii, Scheme 1B).10,11 Second, if “overoxidation” does occur, the resulting thiosulfinate likely could be converted cleanly back to the native cysteine residues by reactions with biological thiols (reaction v, Scheme 1B).14
Protein tyrosine phosphatases (PTPs) are important targets of intracellular H2O2.15–17 These cysteine-dependent enzymes catalyze the removal of phosphoryl groups from tyrosine residues on their protein substrates.15–17 Accordingly, PTPs work in tandem with protein tyrosine kinases to regulate critical signal transduction cascades by modulating the phosphorylation status and, in turn, the functional properties of proteins involved in these pathways.15–17 The catalytic activity of some PTPs is subject to redox regulation as part of normal cell signaling processes.15–17 For example, the enzyme PTP1B, a major negative regulator of the insulin signaling pathway, is inactivated by a burst of H2O2 that is produced upon binding of insulin to its cell-surface receptor.18,19 Subsequent reactions with cellular thiols slowly return the enzyme to its active form.18,19 This transient oxidative inactivation of PTP1B serves as a “timing device” that increases phosphorylation levels on the insulin receptor and insulin receptor substrates, thus potentiating cellular responses for a defined period of time following insulin stimulation.18,19
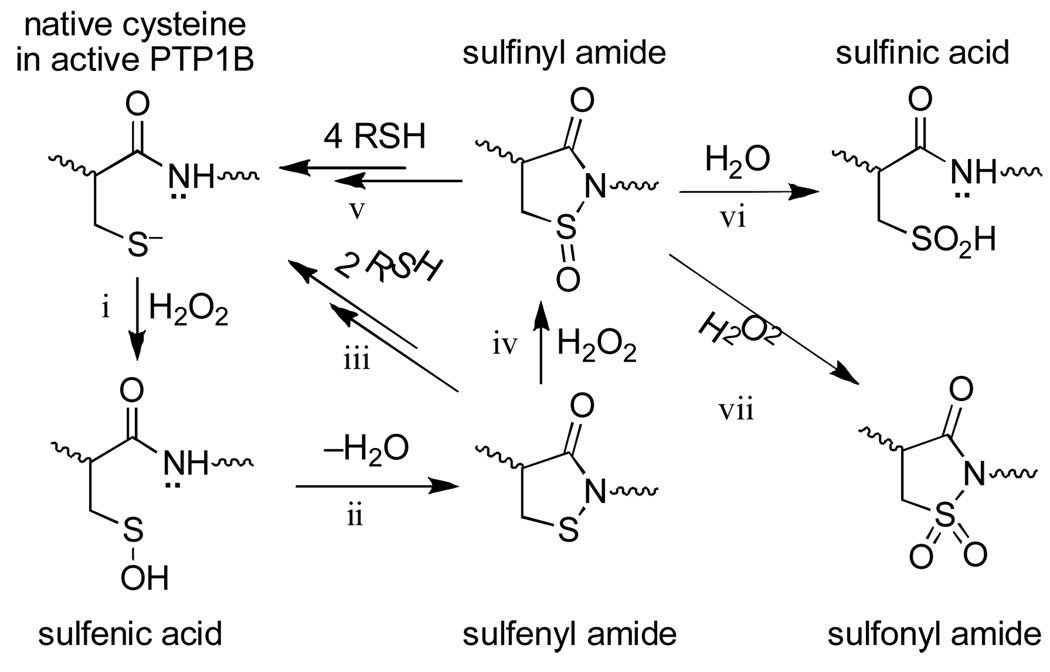
For some time, it was widely assumed that redox regulation of PTPs involved either sulfenic acid or disulfide intermediates, as shown in Scheme 1. However, recent studies in the context of PTP1B revealed a new mechanism for redox regulation of PTP activity in which the initially-formed sulfenic acid undergoes reaction with the neighboring amide nitrogen to yield a cyclic sulfenyl amide (known, more formally, as an isothiazolidin-3-one, reaction ii, Scheme 2).20–22 As required for a functional redox switch, reactions with biological thiols can convert the sulfenyl amide back to the catalytically active thiol form of the enzyme (reaction iii, Scheme 2).20–22 Importantly, sulfenyl amide formation subsequently has been observed in other proteins and it has been suggested that this posttranslational cysteine modification can occur in cells.23,24
Scheme 2.
Like other cysteine-based redox switches, the sulfenyl amide residue has the potential to undergo “overoxidation” to sulfinyl and sulfonyl derivatives (reactions iv and vii, Scheme 2). Therefore, complete understanding of this redox switch requires consideration of the reactivity of these higher oxidation states under physiologically relevant conditions. In the work described here we employed small organic molecules to model the reactivity of protein sulfinyl and sulfonyl amides that are potential “overoxidation” products of the sulfenyl amide redox switch. Classic25–28 and modern29,30 studies have employed model compounds to define the inherent reactivity of critical functional groups found at enzyme active sites. Such studies provide insight regarding the chemical mechanisms of enzyme catalysis and lay a foundation for understanding how protein microenvironments alter inherent organic reactivities to achieve observed enzyme function.
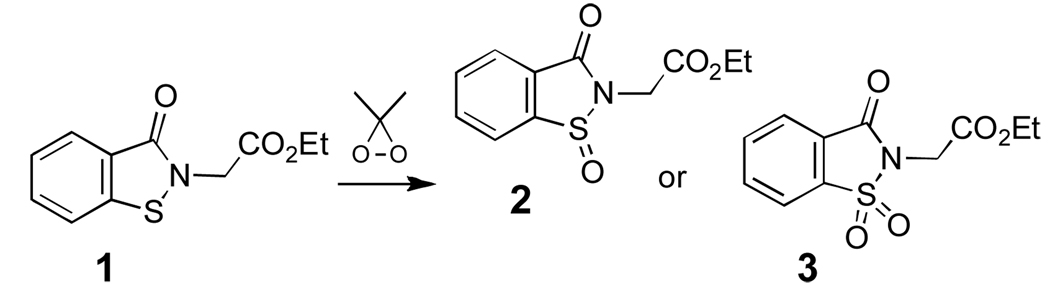
The present studies build upon our previous use of compound 1 to model the reactivity of the protein sulfenyl amide residue found in PTP1B.22 For these studies, it may be important that the pKa of the corresponding thiol (5) and, thus, the leaving group ability of the sulfur residue in 1 resembles that of the catalytic cysteine residue in PTP enzymes.22
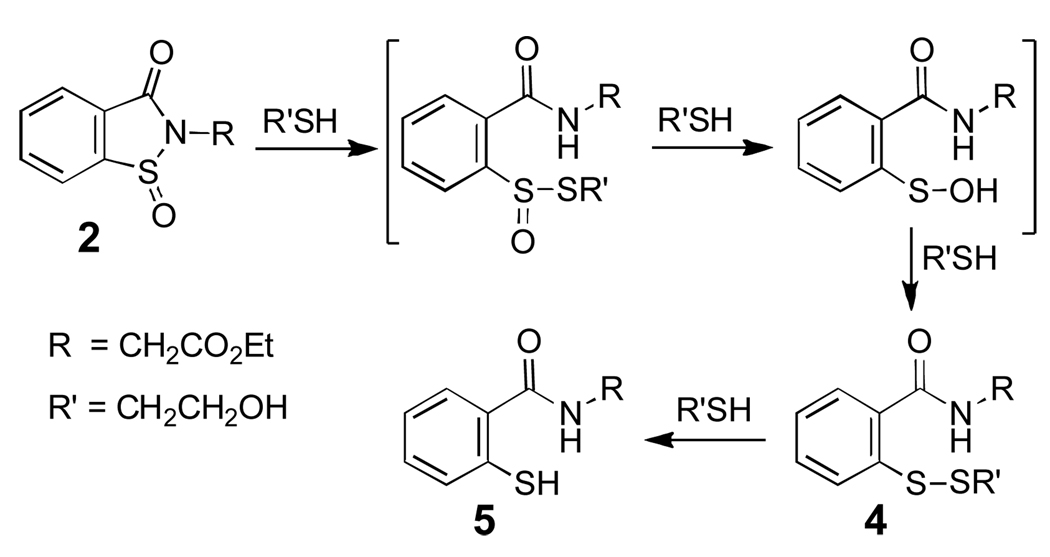
The “overoxidized” sulfenyl amide model compounds 2 and 3 for use in the present studies were prepared by oxidation of 1 22 with dimethyldioxirane (Scheme 3).31 We first examined the reactivity of the sulfinyl amide 2 with thiol. Incubation of 2 (10 mM) with 2-mercaptoethanol (150 mM) in aqueous sodium phosphate buffer (300 mM, pH 7) for 2 h gave the thiol derivative 5 in high isolated yield (85%).32 Similar results were obtained when 2 was mixed with thiol in methylene chloride containing catalytic amounts of triethylamine as a base. These reactions presumably proceed via the thiosulfinate, sulfenic acid, and disulfide intermediates shown in Scheme 4.33 Consistent with this idea, HPLC analysis at early times in the reaction between 2 (50 µM) and 2-mercaptoethanol (500 µM) in sodium phosphate buffer (50 mM, pH 7, containing 30% acetonitrile by volume) revealed an intermediate whose retention time and mass match that of the disulfide 4 at m/z 316 for (M+H)+ (Fig. 1). Further, when the reaction was conducted using only two equivalents of thiol, HPLC analysis showed the disulfide to be a major final product alongside unreacted starting material and 5. The thiol, 2-mercaptoethanol, is converted to the corresponding disulfide in this reaction.
Scheme 3.
Scheme 4.
Figure 1.
Reaction of 2 with 10 equiv 2-mercaptoethanol in aqueous buffer: A) Compound 2 alone in buffer B) 1 min after addition of thiol C) 25 min after addition of thiol. Compound 2 (2.5 µL of a 10 mM stock in CH3CN) was added to a mixture containing sodium phosphate buffer (50 µL, 500 mM, pH 7.0), water (275 µL), 2-mercaptoethanol (25 µL of a 10 mM stock in water), and acetonitrile (147.5 µL) at 25 °C (final concentrations: 2, 50 µM; buffer, 50 mM, pH 7.0; thiol, 500 µM; acetonitrile, 30% by volume). The disappearance of 2 was monitored at 254 nm. Aliquots (40 µL) from the reaction mixture were injected onto a C-18 Varian Microsorb-MV column, 100 Å sphere size, 5 µm pore size, 25 cm length, 4.6 mm i.d. eluted with a solvent system composed of water with 0.5% acetic acid v/v (A) and acetonitrile (B), at a flow rate of 0.8 mL/min. The column was eluted with 70:30 A/B for 4 min and then ramped to 50:50 A/B over 4 min, held at 50:50 A/B for 7 min, and then ramped back to 70:30 A/B over the next 3 min. The peak at 10.2 min was identified as the mixed disulfide 4. The identity of 4 was confirmed by co-injection with an authentic standard22 and by LC/MS analysis which showed that the compound displays an m/z of 316 corresponding to that expected for the [M+H]+ ion of 4. The early peaks in the chromatogram correspond to buffer salts, 2-mercaptoethanol, and the disulfide of 2-mercaptoethanol.
The reaction of 2 (50 µM) with excess thiol (1 mM) in sodium phosphate buffer (50 mM, pH 7, containing 50% acetonitrile by volume) occurs with a pseudo-first-order rate constant of 5.5 ± 0.2 × 10−3 s−1 (t1/2 ~ 2 min). This corresponds to an apparent second-order rate constant of 5.5 ± 0.2 M−1 s−1 (Fig. 2). These results offer the prediction that “overoxidation” of the sulfenyl amide redox switch to the sulfinyl amide in proteins is a chemically reversible event, because the sulfinyl amide can be easily returned to the native cysteine thiol residue via reactions with thiols (reaction v, Scheme 2).
Figure 2.
A representative plot for the disappearance of 2 in the presence of thiol. Compound 2 (2.5 µL of a 10 mM stock in CH3CN) was added to a mixture containing sodium phosphate buffer (50 µL, 500 mM, pH 7.0), water (150 µL), 2-mercaptoethanol (50 µL of a 10 mM stock) and acetonitrile (247.5 µL) at 25 °C. The mixture (final concentrations: 2, 50 µM; buffer, 50 mM, pH 7.0; thiol, 1 mM; acetonitrile, 50% by volume) was vortex mixed and the disappearance of 2 (a is the peak area at time = t and a0 is the peak area at time = 0) monitored by reverse phase HPLC at regular time intervals as described in the legend for Figure 1. From the slope of the plot, a pseudo-first-order rate constant of 5.5 × 10−3 s−1 (t1/2 = 2 min) at 1 mM thiol was obtained. This corresponds to an apparent second-order rate constant of 5.5 ± 0.2 M−1 s−1.
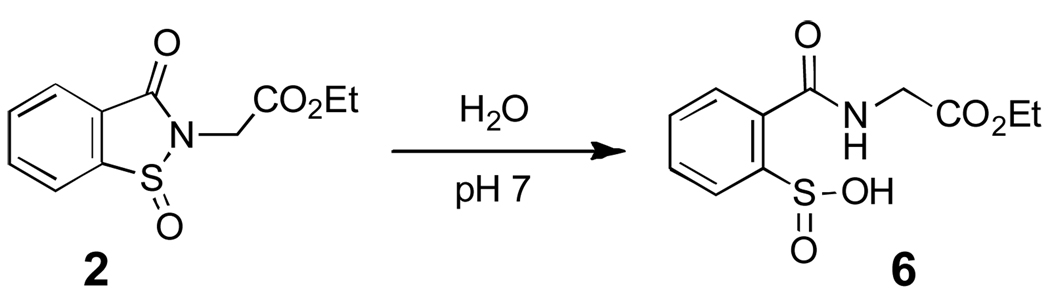
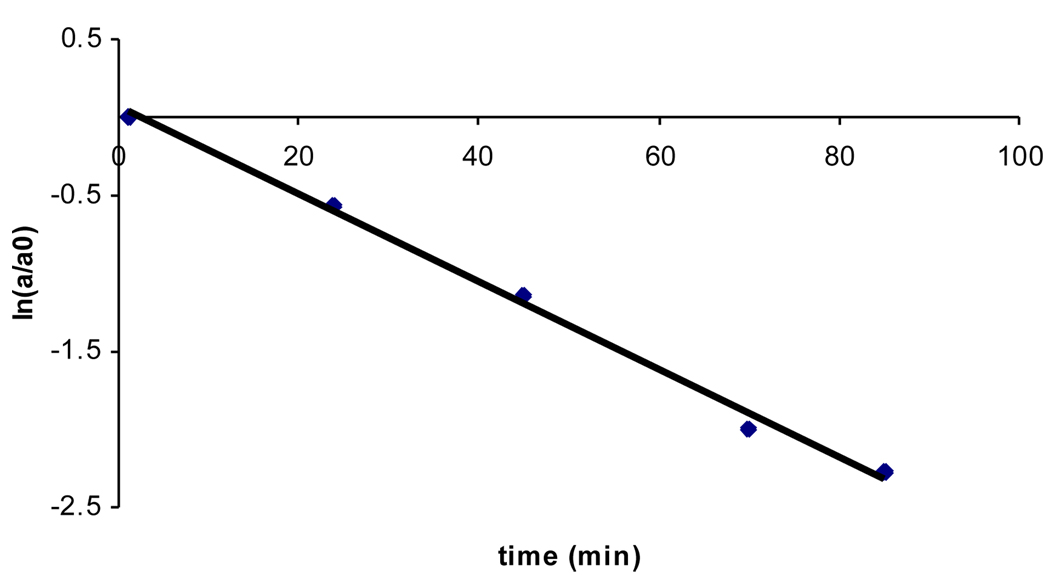
In the absence of thiol, the sulfinyl amide 2 undergoes a relatively slow reaction with water to yield the corresponding sulfinic acid derivative 6 in 88% yield (Scheme 5, aqueous sodium phosphate buffer, 50 mM, pH 7, 12 h, 24 °C).34 Compound 6 was characterized as its methyl ester derivative 7 following treatment of the reaction mixture with excess methyl iodide.35 The hydrolysis of 2 (50 µM) in sodium phosphate buffer (50 mM, pH 7, containing 50% acetonitrile by volume) occurs with a pseudo-first-order rate constant of 4.5 ± 0.2 × 10−4 s−1, corresponding to a half-life of 26 min (Fig. 3). In contrast, the parent sulfenyl amide 1 is stable under these conditions (no significant decomposition observed over 24 h). These results forecast that protein sulfinyl amide residues can undergo chemically irreversible hydrolysis to the sulfinic acid (reaction vi, Scheme 2); however, rate measurements in the context of this model compound suggest that if water and physiological concentrations of thiol (1–10 mM) enjoy equal access to the sulfinyl amide, thiol-mediated recovery of enzyme activity will be approximately 10–100 times faster than irreversible loss of activity due to hydrolysis. It is interesting to note that thiosulfinates may be similarly labile to hydrolysis.36,37
Scheme 5.
Figure 3.
Representative plot for the disappearance of 2 in the absence of thiol. Compound 2 (2.5 µL of a 10 mM stock in CH3CN) was incubated at 25 °C in a solution composed of sodium phosphate buffer (50 µL, 500 mM, pH 7.0), water (200 µL) and acetonitrile (247.5 µL). The mixture (final concentrations: 2, 50 µM; buffer, 50 mM, pH 7.0; acetonitrile, 50% by volume) was vortex mixed and the disappearance of compound 2 (a is the peak area at time = t and a0 is the peak area at time = 0) analyzed by reverse phase HPLC as described in the legend of Figure 1. The pseudo-first-order rate constant of 0.027 ± 0.001 min−1 was obtained from the slope of the plot. This corresponds to a half-life of 26 min for the hydrolysis of 2 under these conditions.
Finally, we examined the reactivity of the sulfonyl amide 3.38 HPLC analysis revealed that this compound is stable in aqueous sodium phosphate buffer (50 mM, pH 7, containing 40% acetonitrile by volume) either in the presence or absence of the thiol, 2-mercaptoethanol. This suggests that exhaustive oxidation of the sulfenyl amide to the sulfonyl amide (Scheme 2, reaction vii) likely represents chemically irreversible destruction of the sulfenyl amide redox switch.
In conclusion, these studies offer chemical insight regarding possible functional roles of sulfenyl amide formation in redox-switched proteins. Sulfenyl amide formation has the potential to protect a single-cysteine redox switch against irreversible overoxidation in much the same way that disulfide formation protects dithiol redox switches from oxidative destruction. In both cases, further oxidation to the sulfinyl form yields an intermediate that readily can be resolved by reactions with biological thiols to regenerate the native cysteine residues (Scheme 6). Thus, irreversible oxidative destruction of these switches requires conversion to the sulfonyl derivatives which presumably requires relatively harsh oxidizing conditions (Scheme 6). In contrast, a sulfenic acid redox switch has no failsafe mechanism. The sulfenic acid group is prone to further oxidation4–9 and, in this case, conversion to the sulfinyl oxidation state represents irreversible destruction of the redox switch.
Scheme 6.
Acknowledgements
We thank the National Institutes of Health for financial support of this work (CA 83925 and CA 119131).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Autréaux BD, Toledano MB. Nature Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 2.Janssen-Heininger YMW, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Free Rad. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee SG. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 4.Poole LB, Karplus PA, Claiborne A. Ann. Rev. Pharm. Tox. 2004;44:3325–3347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 5.Winterbourn CC, Hampton MB. Free Rad. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Brandes N, Schmitt S, Jakob U. Antioxidants Redox Signaling. 2008;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddie KG, Carroll KS. Curr. Opin. Chem. Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Jacob C, Holme AL, Fry FH. Org. Biomol. Chem. 2004;2:1953–1956. doi: 10.1039/b406180b. [DOI] [PubMed] [Google Scholar]
- 9.Turell L, Botti H, Carballal S, Ferrer-Sueta G, Souza JM, Durán R, Freeman BA, Radi R, Alvarez B. Biochemistry. 2008;47:358–367. doi: 10.1021/bi701520y. [DOI] [PubMed] [Google Scholar]
- 10.Sohn J, Rudolph J. Biochemistry. 2003;42:10060–10070. doi: 10.1021/bi0345081. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-Y, Willard D, Rudolph J. Biochemistry. 2009;48:1399–1409. doi: 10.1021/bi801973z. [DOI] [PubMed] [Google Scholar]
- 12.Lee S-R, Yang K-S, Kwon J, Lee C, Jeong W, Rhee SG. J. Biol. Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 13.Tsai SJ, Sen U, Zhao L, Greenleaf WB, Dasgupta J, Fiorillo E, Orrú V, Bottini N, Chen XS. Biochemistry. 2009;48:4838–4845. doi: 10.1021/bi900166y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kice JL, Rogers TE. J. Am. Chem. Soc. 1974;96:8015–8019. [Google Scholar]
- 15.Tonks NK. Nature Rev. Cell. Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 16.Denu JM, Tanner KG. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 17.Tonks NK. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Mahedev K, Zilbering A, Zhu L, Goldstein BJ. J. Biol. Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 19.Meng T-C, Buckley DA, Galic S, Tiganis T, Tonks NK. J. Biol. Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 20.Salmeen A, Anderson JN, Myers MP, Meng T-C, Hinks JA, Tonks NK, Barford D. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 21.van Montfort RLM, Congreeve M, Tisi D, Carr R, Jhoti H. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 22.Sivaramakrishnan S, Keerthi K, Gates KS. J. Am. Chem. Soc. 2005;127:10830–10831. doi: 10.1021/ja052599e. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Groen A, Lemeer S, Jans A, Slijper M, Roe SM, den Hertog J, Barford D. Biochemistry. 2007;46:709–719. doi: 10.1021/bi061546m. [DOI] [PubMed] [Google Scholar]
- 24.Lee J-W, Soonsanga S, Helmann JD. Proc. Nat. Acad. Sci. 2007;104:8743–8748. doi: 10.1073/pnas.0702081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender ML, Turnquest BW. J. Am. Chem. Soc. 1957;79:1656–1663. [Google Scholar]
- 26.Breslow R. J. Am. Chem. Soc. 1958;80:3719–3726. [Google Scholar]
- 27.Westheimer FH, Bender ML. J. Am. Chem. Soc. 1962;84:4908–4909. [Google Scholar]
- 28.Bruice TC. Prog. Bioorg. Chem. 1976;4:1–87. [Google Scholar]
- 29.Wennemers H, Raines RT. Curr. Opin. Chem. Biol. 2008;12:690–691. doi: 10.1016/j.cbpa.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt DA, Pesavento RP, van der Donk WA. Org. Lett. 2005;7:2735–2738. doi: 10.1021/ol050916g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Synthesis of ethyl 2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetate (1). A solution of 1,2-benzisothiazol-3-one (Aldrich, 1 mmol, 150 mg) in methylene chloride (5 mL) was added to a dry flask under an atmosphere of nitrogen. To this was added triethylamine (10 mol %, 14 µL) and ethyl bromoacetate (1.2 equiv, 133 µL) and the mixture was allowed to stir at room temperature for 2 h. The reaction was monitored by thin layer chromatography and additional aliquots of triethylamine (14 µL) were added every 2 h until all starting material was consumed. When the reaction was complete, the mixture was dried by rotary evaporation, the residue taken up in water (15 mL), and the pH adjusted to 9. This mixture was extracted with ethyl acetate (3 × 15 mL), the combined organic layers dried over sodium sulfate, filtered and evaporated under reduced pressure. Purification by column chromatography (95:5, hexane:ethyl acetate) afforded the desired compound 1 as an off-white solid (67%, 160 mg). All spectral data for this material matched that reported previously.22 Synthesis of ethyl 2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetate 1-oxide (2). To a stirred solution of 1 (20 mg, 0.085 mmol) in dry acetone (2 mL) was added a cold solution of dimethyldioxirane22 in acetone (approximately 3 mL dropwise from a Pasteur pipette). After about 1 min the reaction was dried by rotary evaporation, redissolved in CDCl3, and analyzed by 1H-NMR. If necessary, the mixture was dried, redissolved in dry acetone, and an additional aliquot of cold dimethyldioxirane sufficient to complete the reaction was added. The acetone and any remaining DMD was then evaporated under reduced pressure to yield 2 as colorless oil (25 mg, 94%). Rf = 0.38 (3:2 hexane/ethyl acetate). 1H-NMR (CDCl3, 300 MHz) δ 1.30 (3H, t, J = 7 Hz), 4.25 (2H, q, J = 7.2 Hz), 4.37 (1H, d, J = 18 Hz), 4.80 (1H, d, J = 18 Hz), 7.81 (1H, t, J = 8 Hz), 7.87 (1H, t, J = 8 Hz), 7.97 (1H, d, J = 8 Hz); 8.07 (1H, d, J = 8 Hz); 13C-NMR (CDCl3, 75.47 MHz) δ 167.64, 165.18, 145.90, 134.45, 133.25, 127.55, 126.36, 125.25, 62.03, 41.24, 14.01. HRMS (ESI) calcd for C11H12NO4S [M+H]+ 254.0487, found 254.0493.
- 32.Generation of 2-(2-mercaptobenzamido)acetate (5) by reaction of compound 2 with 2-mercaptoethanol in aqueous buffer. To a stirred solution of 2 (15 mg, 0.059 mmol) in acetonitrile (1.78 mL) was added a mixture of sodium phosphate buffer (3.56 mL, 500 mM, pH 7.0), water (538 µL) and 2-mercaptoethanol (62.2 µL of a 14.3 M solution in water). The resulting colorless solution was stirred at 25 °C (final concentrations: 2, 10 mM; buffer, 300 mM, pH 7.0; thiol, 150 mM; acetonitrile, 30% by volume). The starting material disappeared in less than 5 min as indicated by the TLC. The reaction mixture was stirred for further 2 h and acidified to pH 2 using dilute hydrochloric acid. The aqueous solution was extracted with diethyl ether (3 × 5 mL), the combined organic extract washed with water, dried over anhydrous sodium sulfate, filtered, and then evaporated to yield a colorless oil that was purified by flash column chromatography (7:3 ethyl acetate/hexane) to obtain 5 (11 mg, 80%). Rf = 0.61 (7:3 ethyl acetate/hexane). 1H-NMR (CDCl3, 300 MHz) δ 1.32 (3H, t, J = 7 Hz), 4.24 (4H, m), 4.74 (1H, s), 6.57 (1H, s, broad), 7.17 (1H, m), 7.31 (2H, m), 7.53 (1H, dd, J = 7.6 Hz, 1.0 Hz); 13C-NMR (CDCl3, 75.4 MHz) δ 169.79, 168.45, 133.37, 132.16, 131.07, 130.94, 128.12, 125.21, 61.76, 41.83, 14.14. HRMS (ESI) calcd for C11H14NO3S [M+H]+ 240.0694, found 240.0706.
- 33.Clarke V, Cole ER. Phos. Sulfur Silicon. 1994;91:45–52. [Google Scholar]
- 34.For a mechanistic study on the hydrolysis of simple sulfinamides, see: Okuyama T, Lee JP, Kazunobu O. J. Am. Chem. Soc. 1994;116:6480–6481.
- 35.Hydrolysis of 2 yields the sulfinic acid, ethyl 2-(2-(methoxysulfinyl) benzamido)acetate (6). To a stirred solution of 2 (20 mg, 0.08 mmol) in a mixture of water (632.4 µL) and acetonitrile (948.6 µL) was added sodium phosphate buffer (1581 µL, 500 mM, pH 7) and the resulting solution stirred at 25 °C for 12 h (final concentrations: 2, 25 mM; buffer, 250 mM, pH 7.0; acetonitrile, 30% by volume). The resulting product was derivatized without purification by addition of methyl iodide (1 mL), followed by stirring at 25 °C for 24 h. The mixture was extracted with ethyl acetate (3 × 6 mL), the combined organic extracts dried over anhydrous sodium sulfate, filtered, and then evaporated to yield a yellow oil that was purified by flash column chromatography (3:2 hexane/ethyl acetate) to give 7 as a white solid (20 mg, 89 %). Rf = 0.25 (3:2 hexane/ethyl acetate). 1H-NMR (CDCl3, 250 MHz) δ 1.32 (3H, t, J = 7 Hz), 3.36 (3H, s), 4.25 (4H, m), 6.63 (1H, s), 7.65 (3H, m), 8.11 (1H, m); 13C-NMR (CDCl3, 62.9 MHz) δ 169.37, 168.05, 138.29, 136.39, 133.72, 130.53, 129.66, 128.61, 61.75, 45.16, 41.95, 14.09. HRMS (ESI) calcd for C12H16NO5S [M+H]+ 286.0749, found 286.0755.
- 36.Kice JL, Rogers TE. J. Am. Chem. Soc. 1974;96:8009–8015. [Google Scholar]
- 37.Nagy P, Ashby MT. Chem. Res. Toxicol. 2007;20:1364–1372. doi: 10.1021/tx700168z. [DOI] [PubMed] [Google Scholar]
- 38.Synthesis of ethyl 2-(3-oxobenzo[d]isothiazol-2(3H)-yl)acetate 1,1-oxide (3). To a stirred solution of 122 (20 mg, 0.085 mmol) in dry acetone (2 mL) was added an excess of freshly prepared dimethyldioxirane22 in acetone dropwise. The solution was allowed to warm to room temperature and an additional aliquot of dimethyl dioxirane equal in volume to the initial amount was added. After 12 h of stirring at 25 °C the solution was evaporated to yield 3 as a colorless oil (22 mg, 98%). Rf = 0.43 (3:2 hexane/ethyl acetate). 1H-NMR (CDCl3, 300 MHz) δ 1.30 (3H, t, J = 7 Hz), 4.27 (2H, q, J = 7.2 Hz), 4.45 (2H, s), 7.90 (3H, m), 8.10 (1H, dd, J = 7.65 Hz, 0.9 Hz); 13C-NMR (CDCl3, 75.47 MHz) δ 165.85, 158.77, 137.78, 135.05, 134.47, 127.10, 125.47, 121.21, 62.27, 39.11, 14.04. HRMS (ESI) calcd for C11H12NO5S [M+H]+ 270.0436, found 270.0446.