Abstract
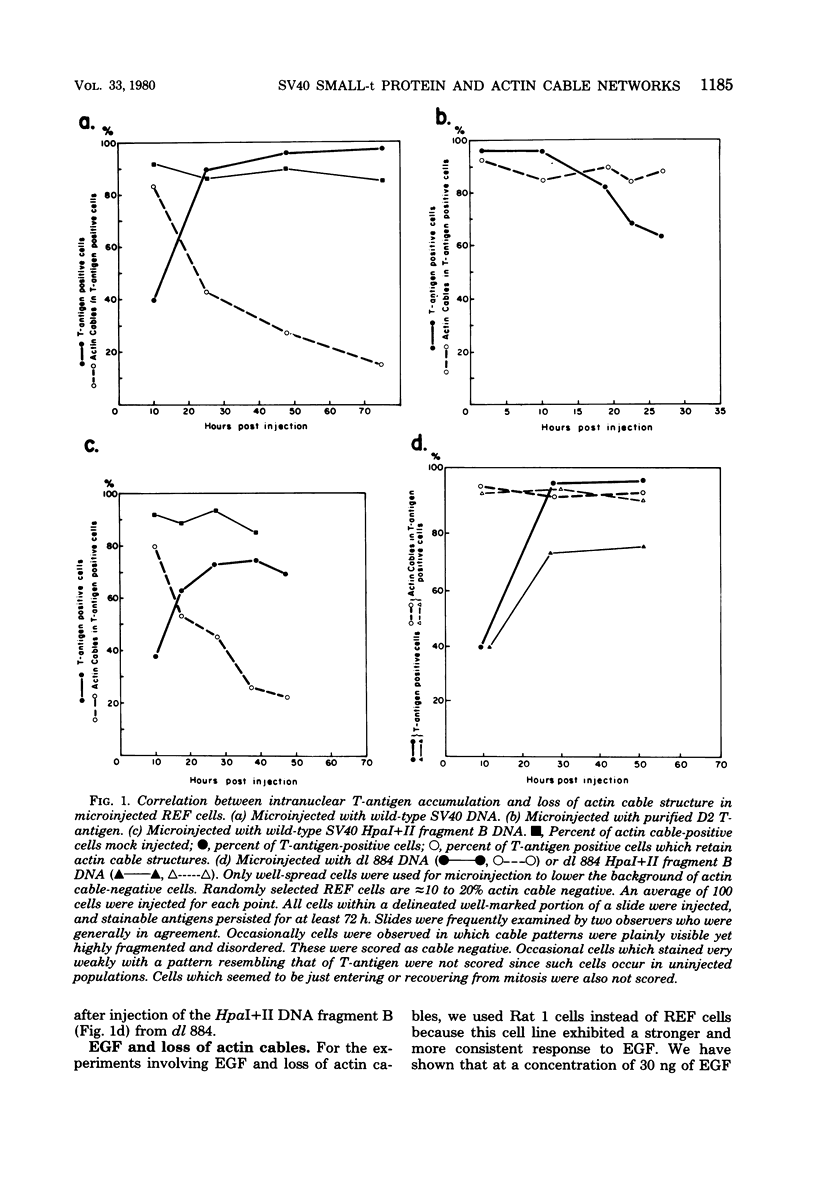
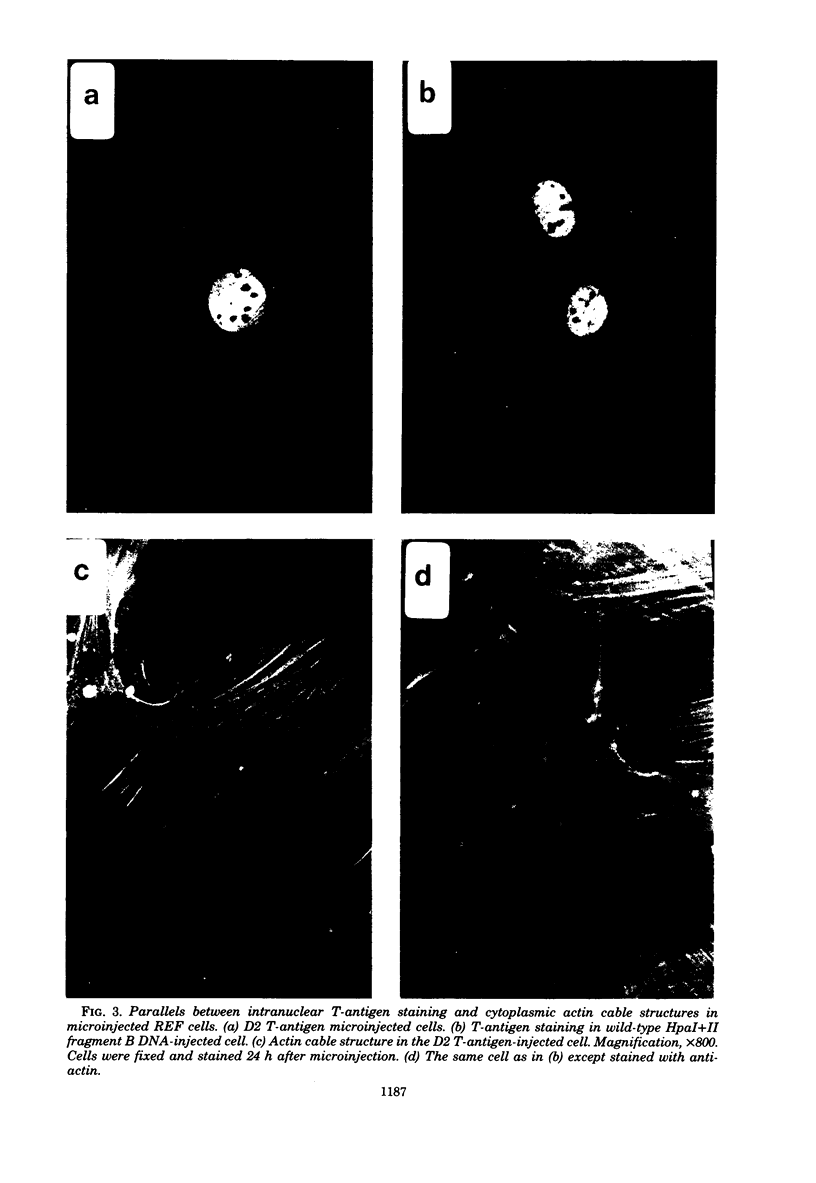
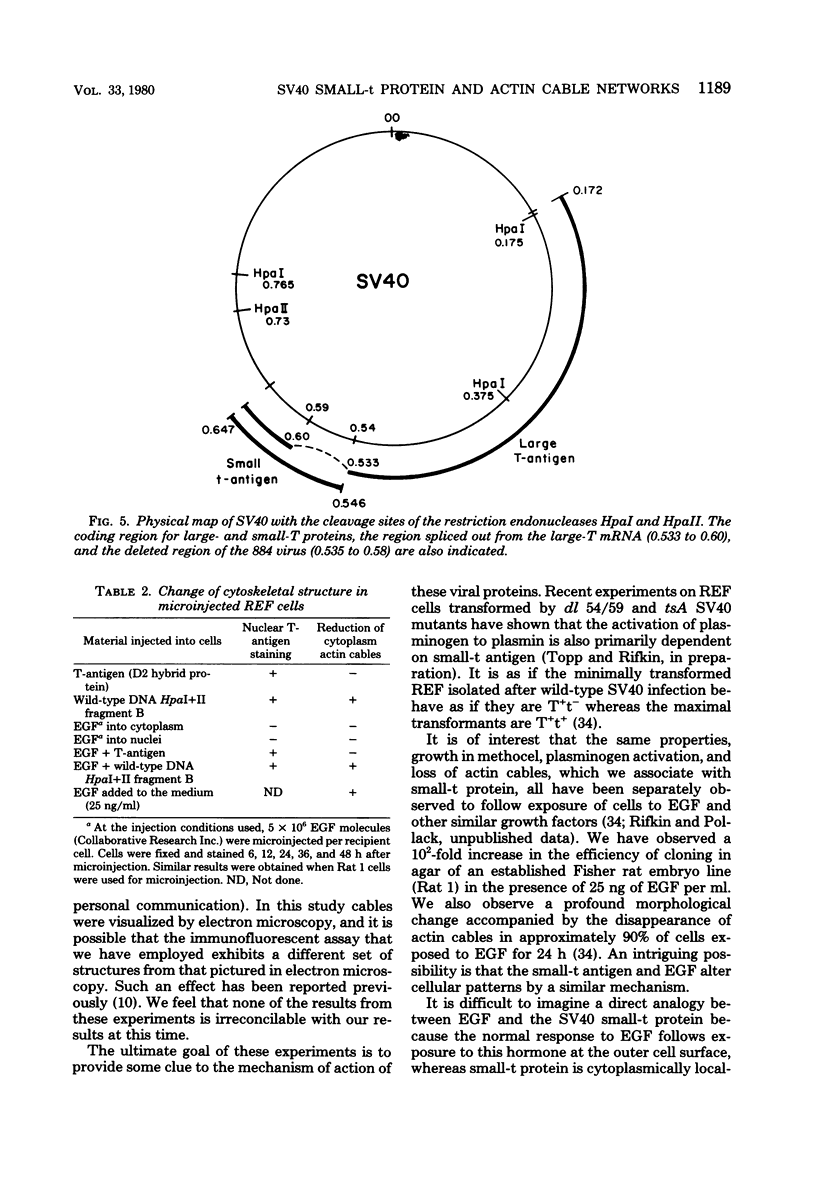
The ability of the two early simian virus 40 (SV40) coded proteins, the large and small T-antigens, to abortively induce the disappearance of cytoplasmic actin-containing networks in cultured cells has been studied in rat embryo fibroblasts after microinjection of intact SV40 DNA, DNA fragments from the early region of SV40, and a purified SV40 large T-antigen related protein (the D2 hybrid protein) isolated from cells infected with the adenovirus-SV40 hybrid virus Ad2+D2. Injection of either the 107,000-dalton D2 hybrid protein or SV40 DNA from the deletion mutant dl 884 SV40, which lacks part of the region (0.54 to 0.59) encoding small t-antigen, failed to cause any detectable change in the structure of actin cables in recipient cells over a period of 72 h. By contrast, injection of wild-type SV40 DNA or a DNA fragment containing the entire region coding for a small-t antigen leads to the disruption of actin cable networks within 24 h of injection. It appears likely that the SV40 small-t protein is necessary for the abortive loss of actin cables in injected cells. Epidermal growth factor also causes loss of actin cables in rat embryo fibroblasts or Rat 1 cells (an established rat embryo line), but only after exposure of the cells to epidermal growth factor in the culture medium and not after injection of epidermal growth factor into the cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971 Aug;67(2):359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Biological and molecular studies of the mitogenic effects of human epidermal growth factor. Symp Soc Dev Biol. 1978;(35):13–31. doi: 10.1016/b978-0-12-612981-6.50007-7. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G., Lewis J. B., Grodzicker T., Bothwell A. Characterization of a fused protein specified by the adenovirus type 2-simian virus 40 hybrid Ad2+ND1 dp2. J Virol. 1979 Apr;30(1):201–217. doi: 10.1128/jvi.30.1.201-217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Yerna M. J., Schloss J. A. Localization and organization of microfilaments and related proteins in normal and virus-transformed cells. J Supramol Struct. 1976;5(2):155–183. doi: 10.1002/jss.400050206. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grässman A. Mikrochirurgische Zellkerntransplantation bei Säugetierzellsn. Exp Cell Res. 1970 Jun;60(3):373–382. doi: 10.1016/0014-4827(70)90530-6. [DOI] [PubMed] [Google Scholar]
- Grässmann A., Grässmann M. Uber die Bildung von Melanin in Muskelzellen nach der direkten Ubertragung von RNA aus Harding-Passey-Melanomzellen. Hoppe Seylers Z Physiol Chem. 1971 Apr;352(4):527–532. [PubMed] [Google Scholar]
- Hassell J. A., Lukanidin E., Fey G., Sambrook J. The structure and expression of two defective adenovirus 2/simian virus 40 hybrids. J Mol Biol. 1978 Apr 5;120(2):209–247. doi: 10.1016/0022-2836(78)90065-7. [DOI] [PubMed] [Google Scholar]
- Heaysman J. E., Pegrum S. M. Early contacts between fibroblasts. An ultrastructural study. Exp Cell Res. 1973 Mar 30;78(1):71–78. doi: 10.1016/0014-4827(73)90039-6. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV40-transformed cells. II. Ultrastructural study. J Cell Biol. 1971 Sep;50(3):691–708. doi: 10.1083/jcb.50.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Paucha E., Mellor A., Harvey R., Smith A. E., Hewick R. M., Waterfield M. D. Large and small tumor antigens from simian virus 40 have identical amino termini mapping at 0.65 map units. Proc Natl Acad Sci U S A. 1978 May;75(5):2165–2169. doi: 10.1073/pnas.75.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. State of the viral DNA in rat cells transformed by polyoma virus. I. Virus rescue and the presence of nonintergrated viral DNA molecules. J Virol. 1976 May;18(2):436–444. doi: 10.1128/jvi.18.2.436-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Benjamin T. L. Cellular alterations dependent upon the polyoma virus Hr-t function: separation of mitogenic from transforming capacities. Cell. 1978 Jul;14(3):587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Thoenen H. Selective binding, uptake, and retrograde transport of tetanus toxin by nerve terminals in the rat iris. An electron microscope study using colloidal gold as a tracer. J Cell Biol. 1978 Apr;77(1):1–13. doi: 10.1083/jcb.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp W. C. Variable defectiveness for lytic growth of the dl 54/59 mutants of simian virus 40. J Virol. 1980 Mar;33(3):1208–1210. doi: 10.1128/jvi.33.3.1208-1210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena T. E., Borysenko J. Z., Karnovsky M. J., Berlin R. D. Effects of colchicine, cytochalasin B, and 2-deoxyglucose on the topographical organization of surface-bound concanavalin A in normal and transformed fibroblasts. J Cell Biol. 1974 Apr;61(1):70–82. doi: 10.1083/jcb.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollet J. J., Brugge J. S., Noonan C. A., Butel J. S. The role of SV40 gene A in the alteration of microfilaments in transformed cells. Exp Cell Res. 1977 Mar 1;105(1):119–126. doi: 10.1016/0014-4827(77)90157-4. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]