Abstract
Studies using chemical inhibitors have suggested that the Ca2+-sensitive serine/threonine kinase Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a key regulator of both insulin- and contraction-stimulated glucose uptake in skeletal muscle. However, due to nonspecificity of these inhibitors, the specific role that CaMKII may play in the regulation of glucose uptake is not known. We sought to determine whether specific inhibition of CaMKII impairs insulin- and/or contraction-induced glucose uptake in mouse skeletal muscle. Expression vectors containing green fluorescent protein conjugated to a CaMKII inhibitory (KKALHRQEAVDCL) or control (KKALHAQERVDCL) peptide were transfected into tibialis anterior muscles by in vivo electroporation. After 1 wk, muscles were assessed for peptide expression, CaMK activity, insulin- and contraction-induced 2-[3H]deoxyglucose uptake, glycogen concentrations, and changes in intracellular signaling proteins. Expression of the CaMKII inhibitory peptide decreased muscle CaMK activity ∼35% compared with control peptide. Insulin-induced glucose uptake was not changed in muscles expressing the inhibitory peptide. In contrast, expression of the inhibitory peptide significantly decreased contraction-induced muscle glucose uptake (∼30%). Contraction-induced decreases in muscle glycogen were not altered by the inhibitory peptide. The CaMKII inhibitory peptide did not alter expression of the glucose transporter GLUT4 and did not impair contraction-induced increases in the phosphorylation of AMP-activated protein kinase (Thr172) or TBC1D1/TBC1D4 on phospho-Akt substrate sites. These results demonstrate that CaMKII does not regulate insulin-stimulated glucose uptake in skeletal muscle. However, CaMKII plays a critical role in the regulation of contraction-induced glucose uptake in mouse skeletal muscle.
Keywords: Ca2+/calmodulin-dependent protein kinase II, Ca2+ signaling, exercise, metabolism
type 2 diabetes is the most common form of diabetes, accounting for 90–95% of all diagnosed cases in the United States ((8). In patients with type 2 diabetes, the intracellular signaling mechanisms that regulate insulin-induced increases in skeletal muscle glucose uptake are impaired (2). Importantly, non-insulin-dependent mechanisms, including those regulated by exercise or muscle contraction, remain intact (25). Thus, uncovering the signaling pathways governing exercise/contraction-mediated increases in muscle glucose uptake is an important step toward the development of new pharmaceutical treatments for individuals with type 2 diabetes.
Increases in intracellular Ca2+ levels are a fundamental part of the molecular signals underlying muscle contraction. Not surprisingly, this has led many investigators to hypothesize that Ca2+ signaling may regulate contraction-mediated metabolic events, including glucose transporter 4 (GLUT4) translocation and muscle glucose uptake (16, 17, 37). Evidence in support of this hypothesis first emerged from studies using the sarcoplasmic reticulum Ca2+ store-releasing agent caffeine. Low concentrations of caffeine (3.0 mM) stimulated muscle glucose uptake (15, 18, 35) independent of detectable force production or alterations in high-energy phosphates [i.e., ATP levels (43)], demonstrating a distinct role for Ca2+ in the regulation of glucose uptake. Identification of the downstream signals mediating Ca2+-dependent muscle glucose uptake has proven challenging, although studies using the Ca2+/calmodulin competitive inhibitors KN-62 {1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine} or KN-93 {N-[2-N-(4-chlorocinnamyl)-N-methylaminomethyl]phenyl-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide phosphate salt} have suggested that Ca2+/calmodulin-dependent protein kinases (CaMKs) may be important in this process. There are several CaMKs expressed in skeletal muscle, including CaMK kinase (CaMKK)α and -β (28, 33, 37), CaMKIα, -β, and -δ (23, 30, 36), and CaMKIIβM, -δ, and -γ (32, 33), and all of these isoforms require Ca2+/calmodulin for activation. Importantly, CaMKII is not phosphorylated by CaMKK, but instead it autophosphorylates upon Ca2+/calmodulin binding (19, 21). Thus, CaMKII is not a substrate of CaMKK and exists in a distinct signaling pathway directly regulated by Ca2+/calmodulin.
Incubation of rodent muscles with KN-62 or KN-93 inhibited caffeine- and muscle contraction-induced increases in glucose uptake (24, 41) and CaMKII phosphorylation (41), suggesting that Ca2+ stimulates glucose uptake via CaMKII. Intriguingly, studies have also shown that insulin stimulates the phosphorylation of CaMKII (40) and that treatment of rodent muscles with KN-62 significantly impairs both insulin-induced increases in CaMKII phosphorylation (40) and glucose uptake (5, 40). Collectively, these data suggest either that CaMKII is a convergence point linking both insulin and contraction stimuli to glucose uptake or that there is a nonspecific effect of KN-62/93 on muscle glucose uptake that is coincident with inhibition of CaMKII. Importantly, to date, no studies have specifically inhibited CaMKII signaling and examined insulin- or contraction-induced muscle glucose uptake.
Recent work from our group and others has now raised the possibility that another CaMK, CaMKKα, may be a downstream signal linking increases in intracellular Ca2+ levels to stimulation of muscle glucose uptake (24, 37). Targeted expression of a constitutively active form of CaMKKα in skeletal muscle increased glucose uptake via a mechanism that did not require changes in AMP-activated protein kinase (AMPK) activity (37), consistent with early work demonstrating a Ca2+-sensitive glucose uptake pathway that is not dependent on high-energy phosphates (43). In addition, inhibition of CaMKK signaling with the chemical CaMKK inhibitor STO-609 (7-Oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid-acetic acid) impaired contraction- but not insulin-induced muscle glucose uptake (24, 37), linking CaMKK to only the contraction-induced glucose uptake pathway. Thus, it is possible that the inhibitory effect of the Ca2+/calmodulin competitive inhibitors KN-62/93 on contraction-induced glucose uptake may not be mediated via CaMKII but may instead be mediated via CaMKK.
The data linking CaMKII to both insulin- and contraction-induced muscle glucose uptake rely solely on studies that utilized the Ca2+/calmodulin-competitive inhibitors KN-62/93. Since these inhibitors have been shown to inhibit other Ca2+/calmodulin-sensitive and non-Ca2+/calmodulin-sensitive kinases (6, 7), the role that CaMKII may play in the regulation of muscle glucose uptake is still unknown. Therefore, the goal of this study was to specifically inhibit CaMKII signaling to definitively determine whether the CaMKII isoform plays a role in the regulation of insulin- and/or contraction-induced skeletal muscle glucose uptake.
MATERIALS AND METHODS
Animals.
All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Joslin Diabetes Center and in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Mice were housed in cages at 21–22°C with a 12:12-h light-dark cycle. LabDiet rodent chow (Purina Mills) and water were available ad libitum. Female ICR mice (6–8 wk) were obtained from Taconic Laboratories.
Skeletal muscle incubations and 2-[3H]deoxyglucose uptake.
Muscle incubation experiments were performed as described previously (37). Briefly, mice were fasted overnight and euthanized by cervical dislocation. The extensor digitorum longus and soleus muscles were removed and placed in Krebs-Ringer bicarbonate (KRB) solution containing (in mM) 117 NaCl, 4.7 KCl, 2.5 CaCl2·2H2O, 1.2 KH2PO4, 1.2 MgSO4·7H2O, and 24.6 NaHCO3 supplemented with 2 mM pyruvic acid and either DMSO (0.1%) or 10 μM KN-62 (Sigma-Aldrich) for 50 min. For insulin experiments, muscles were incubated in KRB containing insulin (50 mU/ml; Eli Lilly) for 20 min. For contraction experiments, muscles were electrically stimulated to contract for 10 min (Grass S88 stimulator settings: train rate = 1/min; train duration = 10 s; pulse rate = 100 pulses/s; duration = 0.1 ms; volts = 100 V). Force production was monitored using an isometric force transducer (Kent Scientific), and the converted digital signal was captured by a data acquisition system (iWorx114; CB Sciences) and analyzed with software (Labscribe; CB Sciences).
For glucose uptake, muscles were incubated in KRB buffer containing 1.5 μCi/ml 2-[3H]deoxyglucose, 1 mM deoxyglucose, 0.45 μCi/ml [14C]mannitol, 7 mM cold mannitol, and the appropriate amount of DMSO, KN-62, and/or insulin. Muscles were frozen in liquid N2, solubilized in 1 N NaOH at 80°C, and neutralized with 1 N HCl. Samples were centrifuged at 11,000 g for 1 min. Aliquots were removed for scintillation counting of the [3H] and [14C] labels, and 2-[3H]deoxyglucose uptake was calculated.
For immunoblot analyses, muscles were pulverized and then homogenized in ice-cold buffer containing (in mM) 20 Tris·HCl, pH 7.5, 5 EDTA, 10 Na4P2O7, 100 NaF, 2 NaVO4, 0.01 leupeptin, 3 benzamidine, 1 phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 1% Nonidet P-40. Samples were rotated end over end for 1 h at 4°C and then centrifuged at 13,000 g for 30 min. Protein content in muscle lysates was assessed via the Bradford method (4).
Transfection of plasmid DNA into skeletal muscle using in vivo electroporation.
Expression vectors containing green fluorescent protein (GFP) conjugated to the CaMKII inhibitory (KKALHRQEAVDCL) or the CaMKII control peptide (KKALHAQERVDCL) (44) were transfected into mouse tibialis anterior muscles using in vivo electroporation, as described previously (37). Mice were allowed 1 wk to express the peptides prior to additional experiments. For insulin stimulation, the control peptide was transfected into the muscle of one leg while the inhibitory peptide was transfected into the contralateral leg. For contraction experiments, both legs were transfected with the same peptide, and one of the legs was contracted. In our laboratory, this procedure has resulted in >85% of skeletal muscle fibers expressing exogenous protein (10).
In vivo skeletal muscle 2-[3H]deoxyglucose uptake.
In vivo muscle glucose uptake was measured as described previously (27). Briefly, mice were fasted overnight and anesthetized with pentobarbital sodium (100 mg/kg ip). After 30 min, blood was taken from the tail to assess basal glucose and background radioactivity. For in vivo insulin stimulation, a bolus of 20% glucose (1 g glucose/kg body wt) plus 2-[3H]deoxyglucose (0.33 μCi [3H]/g body wt) was administered retroorbitally. This method has previously been shown by our group to increase blood insulin levels ∼3.5-fold 5 min postinjection (27). For in situ contraction, electrodes were attached to the peroneal nerve of one leg. Immediately before the contraction, a bolus of 0.9% NaCl plus 2-[3H]deoxyglucose (0.33 μCi [3H]/g body wt) was administered retroorbitally. The muscle was contracted for 15 min (Grass S88 stimulator settings: train rate = 0.5 trains/s, train duration = 200 ms; pulse rate = 100 pulses/s, pulse duration = 0.1 ms). Blood samples were taken 5, 10, 15, 25, 35, and 45 min after the 3H injection for glucose and 3H measurements. Mice were euthanized by cervical dislocation and muscles frozen in liquid N2. Muscles were pulverized and homogenized in ice-cold lysis buffer containing (in mM) 20 Tris·HCl, pH 7.5, 5 EDTA, 10 Na4P2O7, 100 NaF, 2 NaVO4, 0.01 leupeptin, 3 benzamidine, 1 phenylmethylsulfonyl fluoride, and 10 μg/ml aprotinin. Accumulation of muscle radioactivity was assessed in an aliquot of muscle homogenate using a perchloric acid precipitation procedure modified from Ferre et al. (9), and the rate of glucose uptake was calculated as described previously (27). A separate portion of the muscle homogenate was mixed with 1% Nonidet P-40 and rotated end over end at 4°C for 1 h. Samples were centrifuged at 13,000 g for 30 min, and the supernatant was removed. Total protein content in the lysates was determined using the Bradford method (4) and then used in maximal CaMK activity assays, CaMKII immunoblots, or fluorescent analyses for GFP peptide expression.
Immunoblot analysis.
Immunoblots were performed using standard procedures (37). Primary antibodies were obtained from sources as follows: phospho-AMPK (Thr172) from Biosource International; phospho-Akt (Thr308), phospho-Akt substrate (PAS), phospho-CaMKII (Thr286/287), and TBC1D1 from Cell Signaling Technology; GLUT4 from Chemicon International; CaMKII from Santa Cruz Biotechnology; and TBC1D4 from Upstate Biotechnology. AMPKα1/α2 antibody was custom-generated by Covance (11).
Fluorescence quantification of GFP peptides.
The amount of GFP peptide in the mouse muscles was assessed as fluorescence (excitation 485 nm and emission 528 nm), using a Synergy Mx microplate reader (BioTek Instruments). The peptide concentration was corrected for muscle protein concentration, as described previously (44).
Kinase activity assays.
Kinase activity assays using recombinant CaMKKα, CaMKIδ, CaMKIIγ, and protein kinase D (PKD)3 protein were performed using methods adapted from Ishida et al. (22). Briefly, 50 ng of recombinant protein (SignalChem) was added to kinase reaction buffer consisting of (in mM) 10 HEPES, pH 7.4, 1 EGTA, pH 8.0, 0.1 sodium pyrophosphate, 2 CaCl2, 0.002 calmodulin, 5 MgCl2, 0.1 ATP, 0.02 substrate peptide, and 0.2 mCi/ml [32P]ATP and incubated 30°C for 30 min. The substrate peptides were LKB1tide (Jena Bioscience) for CaMKK, Ziptide (Upstate) for CaMKI, and syntide-2 (Sigma-Aldrich) for CaMKII and PKD. CaMK activity assays using skeletal muscle lysates were performed using methods adapted from Zhang et al. (44). Briefly, lysate (20 μg) was added to kinase reaction buffer (described above) containing 0.02 mM syntide-2 and either 0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, or 10 μM of nontagged, control, or CaMKII inhibitory peptide (EZ Biolabs). Importantly, for muscles transfected with GFP peptides, no recombinant control or inhibitory peptide was added. Reactions were incubated at 30°C for 5 min. For all assays, reaction products were spotted on Whatman P81 filter paper and washed for 5 × 20 min in 0.1% phosphoric acid and 10 min in acetone. Radioactivity was assessed by liquid scintillation counting of the 32P label. CaMK activity was assessed by the incorporation of 32P into the substrate peptide.
Measurement of skeletal muscle glycogen levels.
Muscle glycogen content was assessed by a hexokinase enzymatic reaction, as described previously (3, 34, 40). Briefly, muscles were pulverized, weighed, and then hydrolyzed in 2.0 N HCl at 95°C for 2 h. Samples were neutralized with 2.0 N NaOH, vortexed, and then centrifuged at 13,000 g. Muscle glucose levels were assessed using hexokinase reagent (CIMA Scientific).
Statistical analysis.
The data are presented as means ± SE. Statistical significance was defined as P < 0.05 and determined by t-tests or by two-way analysis of variance and Student-Newman-Keuls post hoc analysis. The number of muscles utilized to determine significance is indicated in the figure legends.
RESULTS
Effects of KN-62 on glucose uptake, force production, and glycogen content in mouse muscle.
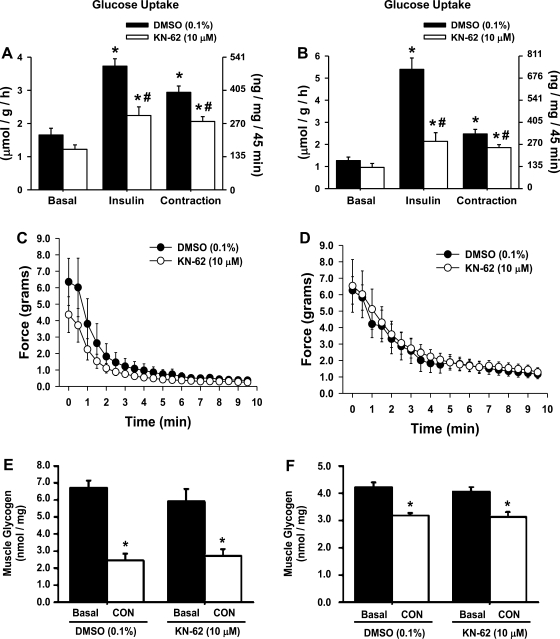
Studies have implicated CaMKII signaling in the regulation of muscle glucose uptake via the use of the chemical inhibitors KN-62/93 (5, 24, 40, 41). Since this work has involved several different model systems and different concentrations of the inhibitors, the first step of this study was to determine whether KN-62 impairs insulin- and/or contraction-induced glucose uptake in mouse muscle using our procedures. Isolated extensor digitorum longus and soleus muscles were treated with 10 μM KN-62 and then stimulated by insulin or contraction. As shown in Fig. 1, A and B, treatment of mouse muscle with KN-62 significantly impaired both insulin- and contraction-induced glucose uptake. The decrease in contraction-induced glucose uptake was not due to impairment in force production, since neither muscle exhibited a significant decrease in contractile force (Fig. 1, C and D). Muscle glycogen content, which is inversely related to muscle glucose uptake (13), was also not affected by KN-62 treatment (Fig. 1, E and F), suggesting that glucose uptake was not impaired via alterations in muscle glycogen metabolism.
Fig. 1.
Effects of the Ca2+/calmodulin-competitive inhibitor KN-62 on glucose uptake, glycogen content, and force production in mouse extensor digitorum longus and soleus muscles. A, C, and E: extensor digitorum longus muscles. B, D, and F: soleus muscles. A and B: pretreatment of muscles with KN-62 (10 μM) for ≥50 min inhibited muscle glucose uptake in response to both insulin (50 mU/ml, 20 min) and contraction (CON; 10 min) in mouse extensor digitorum longus and soleus muscles; n = 5–9 muscles/group. C and D: KN-62 did not significantly alter muscle force production in either the extensor digitorum longus or soleus muscles (n = 6–12 muscles/group). E and F: KN-62 did not affect basal or CON-induced decreases in muscle glycogen content; n = 3–4 muscles/group. Statistical significance was defined as P < 0.05. *Vs. basal; #vs. DMSO.
Effects of KN-62 on intracellular signaling in mouse muscle.
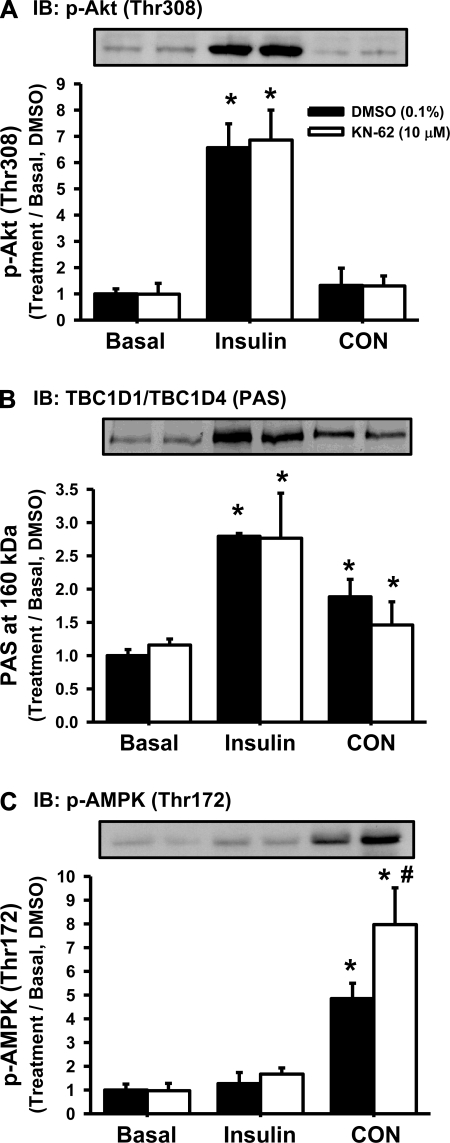
Insulin stimulates muscle glucose uptake via an intracellular signaling cascade that involves phosphorylation of the serine/threonine kinase Akt (Thr308) and phosphorylation of the Rab-GTPase TBC1D4 on phospho-Akt substrate (PAS) motif sites (20, 27). In contrast, the signaling mechanisms underlying contraction-stimulated muscle glucose uptake are less clear, although studies have implicated both phosphorylation of the AMPK (Thr172) (12, 14, 29) and TBC1D1/TBC1D4 (PAS) (1, 27) in this process. To assess whether KN-62 impaired insulin-induced Akt and/or TBC1D1/TBC1D4 signaling or contraction-induced AMPK and/or TBC1D1/TBC1D4 signaling, immunoblots were performed. As shown in Fig. 2, A and B, neither the insulin-induced phosphorylation of Akt (Thr308) nor the phosphorylation of TBC1D1/TBC1D4 (PAS) was affected by KN-62 treatment. In addition, neither the contraction-induced phosphorylation of AMPK (Thr172) nor the phosphorylation of TBC1D1/TBC1D4 (PAS) was impaired by KN-62. Interestingly, the contraction-induced phosphorylation of AMPK (Thr172) was significantly higher in muscles treated with KN-62. Thus, KN-62 does not impair insulin- or contraction-induced glucose uptake via a decrease in signaling through Akt, AMPK, or TBC1D1/TBC1D4 (PAS).
Fig. 2.
KN-62 impaired the phosphorylation of key intracellular signaling proteins that regulate glucose uptake in mouse skeletal muscle. Pretreatment of extensor digitorum longus muscles with KN-62 for ≥50 min did not inhibit the insulin-stimulated phosphorylation of Akt (Thr308; A), the phosphorylation of TBC1D1/TBC1D4 on phospho-Akt substrate (PAS) motif sites (B), or the CON-induced phosphorylation of AMP-activated protein kinase (AMPK; Thr172; C) (n = 4–6 muscles/group). Statistical significance was defined as P < 0.05. *Vs. basal; #vs. DMSO. IB, immunoblot.
Specific inhibition of CaMKII using the CaMKII inhibitory peptide autocamtide-3-derived peptide inhibitor.
KN-62 inhibits CaMKII but also inhibits other kinases (6, 7). Since KN-62 impaired both insulin- and contraction-induced glucose uptake in mouse muscle, the next step was to determine whether these effects could be attributed to specific inhibition of CaMKII. For this step, we utilized the CaMKII inhibitory peptide autocamtide-3-derived peptide inhibitor (AC3-I), a nonphosphorylatable 13-amino acid peptide that inhibits CaMKII activity by preventing CaMKII autophosphorylation (22). AC3-I exhibits nearly identical homology with the inhibitory domain of all three major isoforms of CaMKII found in skeletal muscle, i.e., CaMKIIβM, CaMKIIδ, and CaMKIIγ (Supplemental Fig. S1; Supplemental Material for this article can be found at the AJP-Endocrinology and Metabolism web site) (33) and thus should inhibit all of these isoforms equally. For this reason, the inhibitory peptide was the preferred method for inhibiting CaMKII in this study.
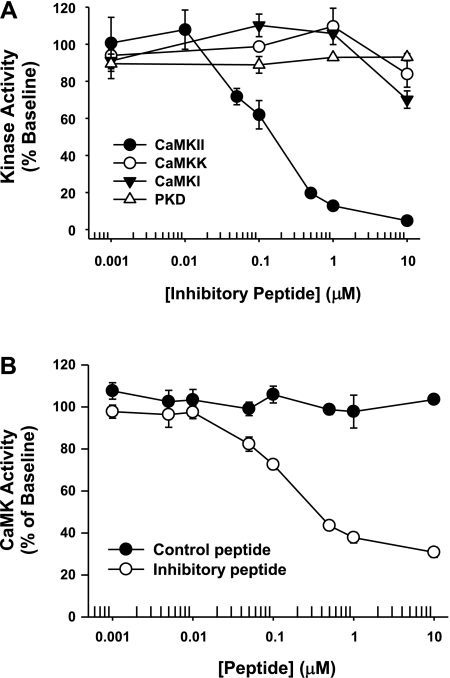
Before the effects of the inhibitory peptide on glucose uptake were examined, it was first necessary to determine whether the peptide exhibited a nonspecific inhibitory effect on CaMKK, since recent studies have implicated CaMKK in the regulation of muscle glucose uptake (24, 37). To assess this possibility, recombinant CaMKK or CaMKII protein was incubated with increasing concentrations of recombinant inhibitory peptide. As shown in Fig. 3A, maximal CaMKII inhibition was achieved with ∼1 μM inhibitory peptide, whereas no inhibition of CaMKK was observed until peptide concentrations reached ≥10 μM. Similar results were obtained for two other members of the CaMK superfamily, CaMKI and PKD (Fig. 3A). Thus, the inhibitory peptide is an effective means for selectively inhibiting CaMKII but not CaMKK, CaMKI, or PKD activity.
Fig. 3.
The CaMKII inhibitory peptide autocamtide-3-derived peptide inhibitor (AC3-I) is a specific tool for inhibiting CaMKII activity. A: 50 ng of recombinant CaMKKα, CaMKIδ, CaMKIIγ, or protein kinase D (PKD)3 protein was incubated with increasing concentrations of recombinant CaMKII inhibitory peptide in the presence of ∼1 mM Ca2+ and 2 μM calmodulin. The IC50 for CaMKII in the assay was ∼150 nM, whereas the inhibitory peptide did not inhibit CaMKK or CaMKI activity until peptide concentrations reached ≥10 μM. No inhibitory effect was observed for PKD (n = 2–4 replicates/peptide concentration). B: 20 μg of mouse tibialis anterior muscle protein was incubated with increasing concentrations of recombinant CaMKII inhibitory peptide or the control peptide in the presence of ∼1 mM Ca2+ and 2 μM calmodulin. The IC50 for CaMKII in the muscle assay was ∼150 nM (n = 4 muscles/peptide/concentration).
The CaMKII inhibitory peptide does not covalently bind to CaMKII but instead acts as a competitive, reversible inhibitor (22). Thus, to assess the ability of the peptide to inhibit CaMKII activity, kinase activity assays would need to be performed in the absence of immunoprecipitation procedures, as has been described previously (44). To test whether this approach would be effective at assessing CaMKII activity in mouse muscle, muscle lysate was incubated in the presence of Ca2+/calmodulin and increasing concentrations of recombinant control or inhibitory peptide. As shown in Fig. 3B, the control peptide did not affect total muscle CaMK activity at any of the concentrations tested. In contrast, addition of inhibitory peptide resulted in a dose-dependent decrease in total muscle CaMK activity that reached a maximum effect at ∼1 μM, a concentration consistent with that observed for maximal inhibition of recombinant CaMKII protein (Fig. 3A). Importantly, maximal inhibition of CaMKII activity resulted in only an ∼60% inhibition of total muscle Ca2+/calmodulin-stimulated kinase activity. Thus, ∼40% of the Ca2+/calmodulin-stimulated kinase activity in muscle is emanating from other kinases that can phosphorylate the substrate peptide syntide-2. Collectively, these findings suggest that this approach can be used to assess inhibition of CaMKII activity by the inhibitory peptide in mouse skeletal muscle.
Effect of the CaMKII inhibitory peptide on muscle glucose uptake.
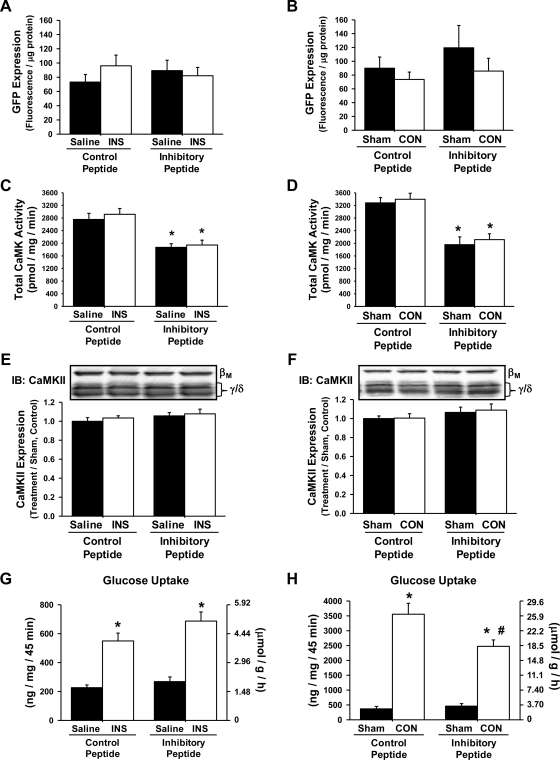
To determine whether CaMKII regulates insulin- and/or contraction-stimulated glucose uptake in skeletal muscle, mouse tibialis anterior muscles were transfected with expression vectors containing GFP-CaMKII inhibitory or GFP-CaMKII control peptide using in vivo electroporation. After 1 wk, in vivo muscle 2-[3H]deoxyglucose uptake was assessed in response to a physiological insulin response or electrical stimulation of the peroneal nerve to elicit muscle contraction. Importantly, GFP peptide expression, total CaMK activity, and CaMKII protein expression were also assessed in the exact same muscles in which glucose uptake was assessed. As shown in Fig. 4, A and B, expression of the peptides was not different among the treatment groups, and this level of CaMKII inhibitory peptide expression resulted in an ∼35% decrease in total CaMK activity compared with muscle expressing control peptide (Fig. 4, C and D). This decrease in total CaMK activity was not due to decreases in CaMKII protein expression (Fig. 4, E and F) because no difference was detected in the expression of CaMKIIβM or CaMKIIγ/δ. Insulin-induced glucose uptake was not decreased by expression of the inhibitory peptide (Fig. 4G). In contrast, contraction-induced muscle glucose uptake was impaired significantly (∼30%) by the inhibitory peptide (Fig. 4H). Collectively, these findings establish a role for the CaMKII isoform in the regulation of contraction-induced, but not insulin-induced, glucose uptake in skeletal muscle.
Fig. 4.
The green fluorescent protein (GFP)-CaMKII inhibitory peptide inhibits CON-induced but not insulin (INS)-induced glucose uptake into mouse tibialis anterior muscle. Mouse tibialis anterior muscles were transfected with expression vectors containing either the GFP-CaMKII inhibitory peptide or the GFP control peptide. After 1 wk, mice were anesthetized and then stimulated by CON or by injection of a glucose bolus to elicit a physiological INS response. Muscles were harvested to assess peptide expression, CaMK activity, CaMKII protein expression, and muscle 2-[3H]deoxyglucose uptake. A and B: peptide expression was assessed as fluorescence and was not significantly different among the treatment groups. C and D: total CaMK activity was assessed in muscles expressing the GFP inhibitory or GFP control peptide via [32P]ATP kinase assays. Expression of the inhibitory peptide decreased total muscle CaMK activity by ∼35% compared with the GFP control peptide muscles. E and F: CaMKII protein expression was not altered by expression of the GFP-CaMKII inhibitory peptide. G and H: INS-induced muscle glucose uptake was not affected by expression of the GFP-CaMKII inhibitory peptide, whereas CON-induced muscle glucose uptake was impaired ∼30%. Statistical significance was defined as P < 0.05. *Vs. saline or sham; #vs. control peptide (n = 6–14 muscles/group).
Effects of in vivo insulin and contraction stimulation on CaMKII phosphorylation.
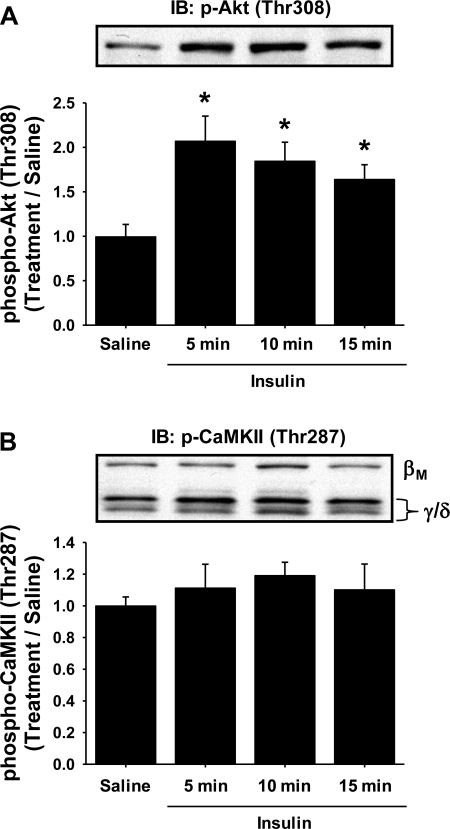
The finding that expression of the GFP-CaMKII inhibitory peptide inhibited contraction-induced but not insulin-induced glucose uptake in mouse skeletal muscle was a bit surprising since a previous study had shown that stimulation of isolated rat skeletal muscle by insulin (100 μU/ml) resulted in a significant (∼30%) increase in CaMKII (Thr286) phosphorylation (40). To determine whether the in vivo insulin stimulation could increase CaMKII (Thr287) phosphorylation in mouse muscle, separate experiments were performed in which mice were given a retroorbital injection of glucose (1 g glucose/kg body wt) to induce a physiological insulin response and the tibialis anterior muscles were harvested 5, 10, or 15 min later. As shown in Fig. 5, A and B, this protocol significantly increased Akt (Thr308) phosphorylation at 5, 10, and 15 min, with no significant increase in CaMKII (Thr287) phosphorylation. Thus, in vivo insulin stimulation does not result in a significant increase in CaMKII phosphorylation in mouse muscle.
Fig. 5.
In vivo insulin stimulation does not stimulate CaMKII in mouse muscle. Mice were injected with a glucose bolus to elicit a physiological insulin response, and tibialis anterior muscles were harvested 5, 10, and 15 min later to assess protein phosphorylation. In vivo insulin stimulation significantly increased Akt (Thr308) phosphorylation (A) but did not increase CaMKII (Thr287) phosphorylation (B). Statistical significance was defined as P < 0.05. *Vs. saline (n = 6 muscles/group).
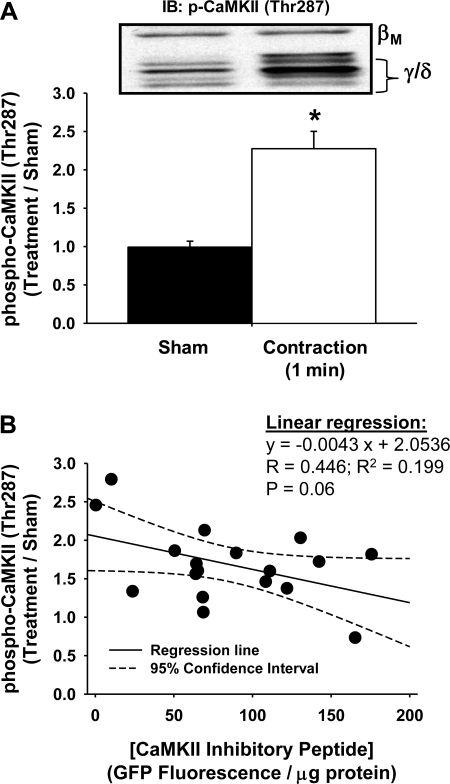
Previous studies in rat skeletal muscle have shown that CaMKII (Thr286) phosphorylation is increased in response to muscle contractile activity in vivo and ex vivo (31, 41). To confirm that the contraction protocol utilized for the in vivo contraction-induced muscle glucose uptake experiments could increase CaMKII (Thr287) phosphorylation in mouse skeletal muscle, separate experiments were performed in which the peroneal nerve of one leg was electrically stimulated for 1 min to induce contraction of the tibialis anterior muscle. As shown in Fig. 6A, this protocol resulted in an approximately twofold increase in CaMKII (Thr287) phosphorylation. Thus, utilizing the same protocol that we utilized to assess the effects of the CaMKII inhibitory peptide on in vivo contraction-induced glucose uptake, we observed a significant increase in CaMKII phosphorylation.
Fig. 6.
The GFP-CaMKII inhibitory peptide inhibits in vivo CON-stimulated CaMKII phosphorylation in mouse muscle. A: mice were anesthetized, and the tibialis anterior muscle from 1 leg was stimulated to CON in vivo for 1 min. Muscles were harvested to assess protein phosphorylation. In vivo CON stimulation significantly increased CaMKII (Thr287) phosphorylation ∼2-fold. Statistical significance was defined as P < 0.05. *Vs. sham (n = 10 muscles/group). B: mouse tibialis anterior muscles were transfected with expression vectors containing the GFP-CaMKII inhibitory peptide. After 1 wk, mice were anesthetized, and the tibialis anterior muscle from 1 leg was stimulated to contract in vivo for 1 min. Muscles were harvested to assess protein phosphorylation. Expression of the GFP-CaMKII inhibitory peptide dose-dependently inhibited the CON-induced phosphorylation of CaMKII (Thr287; n = 18 muscles).
The effect(s) of the GFP-CaMKII inhibitory peptide on contraction-induced CaMKII (Thr287) phosphorylation could not be assessed in the exact same muscles in which glucose uptake was assessed, since the optimal experimental time frame to assess muscle glucose uptake was ∼44 min beyond the peak of CaMKII activation. Thus, to assess the effects of the GFP-CaMKII inhibitory peptide on contraction-induced CaMKII (Thr287) phosphorylation, separate experiments were performed in which the GFP-CaMKII inhibitory peptide was electroporated into both tibialis anterior muscles. After 1 wk, the peroneal nerve of one leg was electrically stimulated to induce contraction of the tibialis anterior muscle for 1 min. The contralateral leg was sham operated as a control. As shown in Fig. 6B, expression of the GFP-CaMKII inhibitory peptide resulted in a concentration-dependent decrease in the contraction-induced phosphorylation of CaMKII (Thr287), demonstrating that the GFP-CaMKII inhibitory peptide is preventing the contraction-induced activation of CaMKII. Thus, collectively, these results provide more evidence suggesting a role for CaMKII in the regulation of contraction-induced but not insulin-induced glucose uptake in mouse skeletal muscle.
Effects of the CaMKII inhibitory peptide on muscle glycogen and intracellular signaling.
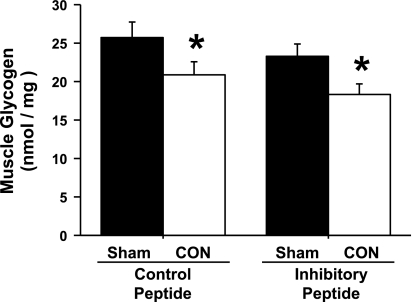
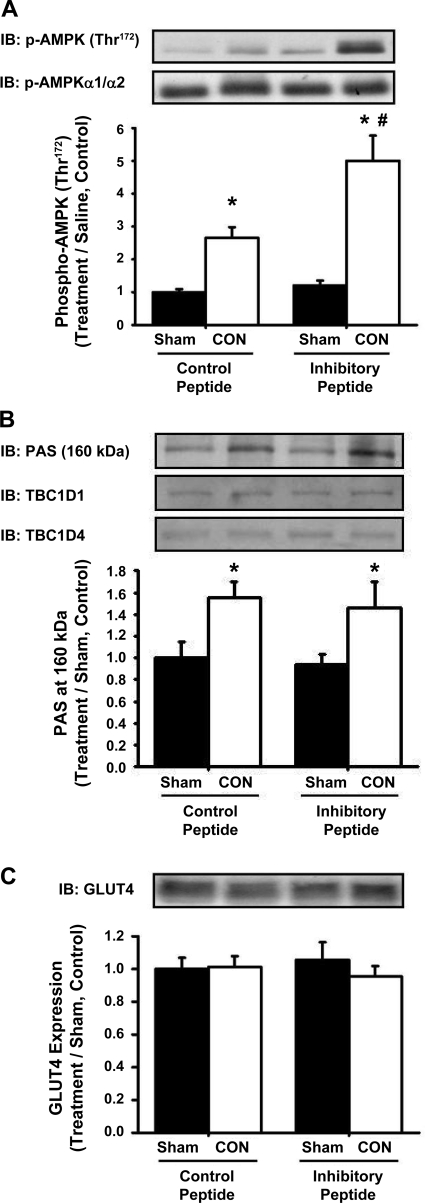
To investigate the possible intracellular mechanisms that may underlie the inhibition of contraction-induced glucose uptake by expression of the CaMKII inhibitory peptide, muscle glycogen content and contraction-induced signaling via AMPK, TBC1D1/TBC1D4, and GLUT4 were assessed. As shown in Fig. 7, contraction resulted in a significant decrease in muscle glycogen levels that was not different between the control and inhibitory peptide groups. In muscles expressing the control peptide, contraction increased AMPK (Thr172) phosphorylation ∼2.5-fold, and intriguingly, this phosphorylation was even greater (∼5-fold above sham, control peptide) in muscles expressing the CaMKII inhibitory peptide (Fig. 8A). Expression of the CaMKII inhibitory peptide had no effect on contraction-stimulated TBC1D1/TBC1D4 (PAS) phosphorylation (Fig. 8B). The protein expression of AMPKα1/α2, TBC1D1, TBC1D4, and GLUT4 was not altered by expression of the inhibitory peptide (Fig. 8, A and B, representative blots, and Fig. 8C). Collectively, these results demonstrate that the mechanism underlying the decrease in contraction-induced glucose uptake elicited by expression of the CaMKII inhibitory peptide is not due to alterations in muscle glycogen metabolism, AMPK (Thr172) or TBC1D1/TBC1D4 (PAS) phosphorylation, or GLUT4 protein expression.
Fig. 7.
The GFP-CaMKII inhibitory peptide does not impair CON-induced alterations in skeletal muscle glycogen metabolism. Mouse tibialis anterior muscles were transfected with DNA vectors containing either the GFP-CaMKII inhibitory peptide or the GFP control peptide. After 1 wk, mice were anesthetized and then stimulated by CON for 15 min. Muscles were harvested to assess muscle glycogen levels. Statistical significance was defined as P < 0.05. *Vs. sham (n = 6–8 muscles/group).
Fig. 8.
The GFP-CaMKII inhibitory peptide does not inhibit CON-induced muscle glucose uptake via alterations in key intracellular signaling proteins. Mouse tibialis anterior muscles were transfected with DNA vectors containing either the GFP-CaMKII inhibitory peptide or the GFP control peptide. After 1 wk, mice were anesthetized and then stimulated by CON for 15 min. Muscles were harvested to assess signaling proteins by IB analysis. B: the CON-induced phosphorylation of AMPK (Thr172) was enhanced in muscles expressing the GFP-CaMKII inhibitory peptide compared with the control peptide. There was no difference in AMPKα1/α2 protein expression. B: The GFP-CaMKII inhibitory peptide did not affect the CON-induced phosphorylation of TBC1D1/TBC1D4 on PAS motif sites. TBC1D1 and TBC1D4 protein expression were not altered by expression of the inhibitory peptide. C: expression of the inhibitory peptide did not alter the protein expression of the glucose transporter GLUT4. Statistical significance was defined as P < 0.05. *Vs. sham; #vs. control peptide (n = 6–8 muscles/group).
DISCUSSION
Muscle contraction is a multifactorial process involving changes in cellular energy status, mechanical stretch, hypoxia, the generation of reactive oxygen species, elevations in intracellular Ca2+ levels, etc., and this diverse array of stimuli collectively activate a large number of signaling pathways in skeletal muscle that could regulate glucose uptake. This tremendous complexity has made the identification of signaling proteins that regulate contraction-induced glucose uptake in skeletal muscle challenging. In this study, we provide the first direct evidence to definitively establish a role for the Ca2+-sensitive kinase CaMKII in the regulation of contraction-induced glucose uptake in mouse skeletal muscle. Not only do these important findings provide insight into the mechanisms regulating contraction-induced glucose uptake, but they also support future studies designed to assess the possible therapeutic potential of CaMKII agonists as novel pharmaceutical treatments for type 2 diabetes.
Previous work in rodent muscle using the Ca2+/calmodulin competitive-inhibitors KN-62 and KN-93 had suggested that CaMKII was a convergence point linking both insulin and contraction to increases in muscle glucose uptake (5, 24, 40, 41). In this study, we also found that treatment of rodent muscle with KN-62 significantly impaired both insulin- and contraction-induced muscle glucose uptake (Fig. 1, A and B). However, specific inhibition of CaMKII with the inhibitory peptide only inhibited contraction-stimulated glucose uptake (Fig. 4H), demonstrating that the ability of KN-62 to inhibit insulin-induced glucose uptake is not via CaMKII. Importantly, the lack of an effect of the inhibitory peptide on insulin-induced glucose uptake is not due to inadequate inhibition of CaMKII, since the amount of peptide expression was similar between the insulin and contraction experiments (Fig. 4, A and B). Our findings are in contrast to a recent study done in 3T3-L1 adipocytes that demonstrated a significant inhibitory effect of both KN-62 and the CaMKII inhibitor tat-CN21 on insulin-induced glucose uptake (42). In adipocytes, CaMKII regulates insulin-induced glucose uptake through phosphorylation of the motor protein Myo1c (42). Consistent with our findings, the authors of the adipocyte study stated that CaMKII failed to phosphorylate Myo1c in rodent skeletal muscle (42). Thus, CaMKII is likely to play a different role in the regulation of glucose uptake in different insulin-responsive tissues.
The mechanism by which KN-62 inhibits insulin-induced glucose uptake is currently unknown, although data from this study demonstrate that it does not involve disruption of Akt or TBC1D1/TBC1D4 (PAS) phosphorylation. In addition, data from our group have also shown that KN-62 does not block glucose uptake via direct binding to cell surface glucose transporters (Supplemental Fig. S2) (39). Whether KN-62 affects other insulin-stimulated signaling proteins or GLUT4 via another mechanism will be the focus of future studies.
An intriguing finding of the current study was that both KN-62 and the CaMKII inhibitory peptide resulted in a significantly greater contraction-induced increase in AMPK (Thr172) phosphorylation (Figs. 2C and 8A). These results were in contrast to previous studies done in rat muscle that demonstrated no significant effect of KN-62 on the phosphorylation of AMPK (Thr172) following 10 min of contraction (24, 41) and perhaps represent a difference in KN-62 response between mouse and rat muscle. The mechanism for elevated AMPK phosphorylation is unknown, although it is possible that the decrease in contraction-induced glucose uptake elicited by CaMKII inhibition may result in greater cellular stress (i.e., increased AMP/ATP ratio). This in turn could lead to an activation of AMPK in an attempt to either stimulate more glucose uptake into the muscle or alter cellular metabolism to favor the utilization of fatty acids. Consistent with this hypothesis, the contraction-induced phosphorylation of acetyl-CoA carboxylase-1/2 (Ser79/212) was significantly enhanced in muscles expressing the GFP-CaMKII inhibitory peptide (Supplemental Fig. S3). Regardless of the mechanism, it is interesting that enhanced AMPK is associated with a decrease in contraction-stimulated glucose uptake.
Phosphorylation of TBC1D4 on PAS sites has been implicated in the regulation of contraction-stimulated glucose uptake (27), and recent work from our group also implicates TBC1D1 (PAS) phosphorylation in contraction-stimulated glucose uptake (1). However, in this study, treatment with KN-62 or expression of the inhibitory peptide (Figs. 2B and 8B) had no effect on TBC1D1/TBC1D4 (PAS) phosphorylation. This finding was a bit surprising since three of the four PAS antibody immunoreactive sites identified on TBC1D4 (27) were found to be potential CaMKII phosphorylation sites (i.e., Ser588, Thr642, and Ser751) based on the phosphorylation site program ScanSite (http://scansite.mit.edu). However, since AMPK has been shown to phosphorylate TBC1D1/TBC1D4 on PAS sites in mouse muscle (26), and phosphorylation of AMPK (Thr172) was enhanced by KN-62 and the inhibitory peptide (Figs. 2C and 8A), it is possible that signaling through AMPK could maintain TBC1D1/TBC1D4 (PAS) phosphorylation even in the absence of CaMKII-dependent phosphorylation. Given that TBC1D1 and TBC1D4 are large proteins (∼160 kDa) with numerous phosphorylation sites, it is likely that PAS phosphorylation is not the only phosphorylation important for protein function. Future studies will determine whether CaMKII directly phosphorylates TBC1D1 and/or TBC1D4 and, if so, defines the specific phosphorylation sites.
Treatment of mouse muscle with 10 μM KN-62 did not significantly affect muscle force production (Fig. 1, C and D). This result was in contrast to a recent study where KN-62 was used at a higher dose (25 μM) in rat extensor digitorum longus muscles (31), suggesting that this difference may be due to differences in species, inhibitor concentration, or contraction protocol. Force production was not assessed in the tibialis anterior muscles expressing the inhibitory peptide. This is a limitation of transfecting the tibialis anterior muscle, because the larger size and irregular shape of the muscle prevent adequate oxygen diffusion into the interior of the muscle during ex vivo incubation studies. Thus, we cannot completely negate the possibility that expression of the inhibitory peptide decreased muscle force production. However, none of the other parameters assessed that would be sensitive to decreases in force production [e.g., glycogen levels or AMPK (Thr172) phosphorylation (Figs. 7 and 8A)] were impaired in muscles expressing the inhibitory peptide. Thus, we do not believe that a decrease in force production is the explanation for the decreased contraction-induced glucose uptake with the inhibitory peptide.
In summary, we demonstrate that specific inhibition of CaMKII activity via expression of a GFP-CaMKII inhibitory peptide results in a significant decrease in contraction-induced, but not insulin-induced, glucose uptake in mouse skeletal muscle in vivo. This impairment in contraction-mediated glucose uptake was observed in the absence of alterations in muscle glycogen metabolism, decreases in AMPK (Thr172) or TBC1D1/TBC1D4 (PAS) phosphorylation, or decreases in GLUT4 expression, demonstrating a CaMKII-dependent regulation of glucose uptake that is independent of established insulin- or contraction-stimulated signaling pathways. Expression of AC3-I, the peptide that specifically inhibits CaMKII activity, will be an important tool for future studies aimed at further defining this novel signaling mechanism in skeletal muscle.
GRANTS
This work was supported by grants from the National Institutes of Health to L. J. Goodyear (R01-AR-42238, R-01-AR-45670), C. A. Witczak (F-32-AR-051663 and K99-AR-056298), and the Joslin Diabetes Center (T-32-DK-07260). Additional funds to support this work were provided by an American Diabetes Association mentor-based fellowship to L. J. Goodyear, the Danish Agency for Science Technology and Innovation to N. Jessen (Grant no. 271-07-0719), the Nakatomi Foundation (Japan) and the Naito Foundation (Japan) to T. Toyoda, and the Japan Society for the Promotion of Science (Grant no. KAKENHI 21240063) to N. Fujii.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björnholm M, Kawano Y, Lehtihet M, Zierath JR. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes 46: 524– 527, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 20: 586– 590, 1974 [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein, utilizing the principle of protein-dye binding. Anal Biochem 72: 248– 254, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Brozinick JT, Jr, Reynolds TH, Dean D, Cartee G, Cushman SW. 1-[N, O-bis-(5-isoquinolinesulphonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62), an inhibitor of calcium-dependent camodulin protein kinase II, inhibits both insulin- and hypoxia-stimulated glucose transport in skeletal muscle. Biochem J 339: 533– 540, 1999 [PMC free article] [PubMed] [Google Scholar]
- 6.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95– 105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enslen H, Sun P, Brickey D, Soderling SH, Klamo E, Soderling TR. Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem 269: 15520– 15527, 1994 [PubMed] [Google Scholar]
- 8.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26, Suppl 1: S5– S20, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Ferre P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaethetized rat. Biochem J 228: 103– 110, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii N, Boppart MD, Dufresne SD, Crowley PF, Jozsi AC, Sakamoto K, Yu H, Aschenbach WG, Kim S, Miyazaki H, Rui L, White MF, Hirshman MF, Goodyear LJ. Overexpression or ablation of JNK in skeletal muscle has no effect on glycogen synthase activity. Am J Physiol Cell Physiol 287: C200– C208, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun 273: 1150– 1155, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem 280: 39033– 39041, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hargreaves M. Muscle glycogen and metabolic regulation. Proc Nutr Soc 63: 217– 220, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369– 1373, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Henriksen EJ, Rodnick KJ, Holloszy JO. Activation of glucose transport in skeletal muscle by phospholipase C and phorbol ester. Evaluation of the regulatory roles of protein kinase C and calcium. J Biol Chem 264: 21536– 21543, 1989 [PubMed] [Google Scholar]
- 16.Holloszy JO, Constable SH, Young DA. Activation of glucose transport in muscle by exercise. Diabetes Metab Rev 1: 409– 424, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Holloszy JO, Hansen PA. Regulation of glucose transport into skeletal muscle. Rev Physiol Biochem Pharmacol 128: 99– 193, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO, Narahara HT. Enhanced permeability to sugar associated with muscle contraction: studies of the role of Ca++. J Gen Physiol 50: 551– 562, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol 41: 471– 505, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237– 252, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J 364: 593– 611, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun 212: 806– 812, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa Y, Tokumitsu H, Inuzuka H, Murata-Hori M, Hosoya H, Kobayashi R. Identification and characterization of novel components of a Ca2+/calmodulin-dependent protein kinase cascade in HeLa cells. FEBS Lett 550: 57– 63, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Jensen TE, Rose AJ, Jørgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab 292: E1308– E1317, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 48: 1192– 1197, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067– 2076, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478– 31485, 2006 [DOI] [PubMed] [Google Scholar]
- 28.McGee SL, Mustard KJ, Hardie DG, Baar K. Normal hypertrophy accompanied by phosphoryation and activation of AMP-activated protein kinase alpha1 following overload in LKB1 knockout mice. J Physiol 586: 1731– 1741, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab 273: E1107– E1112, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Picciotto MR, Czernik AJ, Nairn AC. Calcium/calmodulin-dependent protein kinase I. cDNA cloning and identification of autophosphorylation site. J Biol Chem 268: 26512– 26521, 1993 [PubMed] [Google Scholar]
- 31.Rose AJ, Alsted TJ, Kobbero JB, Richter EA. Regulation and function of Ca2+-calmodulin-dependent protein kinase II of fast-twitch rat skeletal muscle. J Physiol 580: 993– 1005, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol 553: 303– 309, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol 574: 889– 903, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein MW. Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Methods of Enzymatic Analysis, edited by Bergmeyer H. New York: Academic, 1963, p. 117 [Google Scholar]
- 35.Terada S, Muraoka I, Tabata I. Changes in [Ca2+]i induced by several glucose transport-enhancing stimuli in rat epitrochlearis muscle. J Appl Physiol 94: 1813– 1820, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ueda T, Sakagami H, Abe K, Oishi I, Maruo A, Kondo H, Terashima T, Ichihashi M, Yamamura H, Minami Y. Distribution and intracellular localization of a mouse homologue of Ca2+/calmodulin-dependent protein kinase Ibeta2 in the nervous system. J Neurochem 73: 2119– 2129, 1999 [PubMed] [Google Scholar]
- 37.Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes 56: 1403– 1409, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Witczak CA, Hirshman MF, Jessen N, Fujii N, Seifert MM, Brandauer J, Hotamisligil GS, Goodyear LJ. JNK1 deficiency does not enhance muscle glucose metabolism in lean mice. Biochem Biophys Res Commun 350: 1063– 1068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witczak CA, Jessen N, Goodyear LJ. The CaMK inhibitor, KN-62, prevents insulin-, contraction-, and AICAR-stimulated glucose uptake, but not via inhibition of Akt, AMPK, or PKC l/z phosphorylation (Abstract). The Physiologist 47: 283, 2004. [Google Scholar]
- 40.Wright DC, Fick CA, Olesen JB, Lim K, Barnes BR, Craig BW. A role for calcium/calmodulin kinase in insulin stimulated glucose transport. Life Sci 74: 815– 825, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 53: 330– 335, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Yip MF, Ramm G, Larance M, Hoehn KL, Wagner MC, Guilhaus M, James DE. CaMKII-mediated phosphorylation of the myosin motor Myo1c is required for insulin-stimulated GLUT4 translocation in adipocytes. Cell Metab 8: 384– 398, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Youn JH, Gulve EA, Holloszy JO. Calcium stimulates glucose transport in skeletal muscle by a pathway independent of contraction. Am J Physiol Cell Physiol 260: C555– C561, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 11: 409– 417, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.