Abstract
Ca+-induced Ca2+ release tightly controls the function of ventricular cardiac myocytes under normal and pathological conditions. Two major factors contributing to the regulation of Ca2+ release are the cytosolic free Ca2+ concentration and sarcoplasmic reticulum (SR) Ca2+ content. We hypothesized that the amount of Ca2+ released from the SR during each heart beat strongly defines the refractoriness of Ca2+ release. To test this hypothesis, EGTA AM, a high-affinity, slow-association rate Ca2+ chelator, was used as a tool to modify luminal SR Ca2+ content. An analysis of the cytosolic and luminal SR Ca2+ dynamics recorded from the epicardial layer of intact mouse hearts indicated that the presence of EGTA reduced the diastolic SR free Ca2+ concentration and fraction of SR Ca2+ depletion during each beat. In addition, this maneuver shortened the refractory period and accelerated the restitution of Ca2+ release. As a consequence of the accelerated restitution, the frequency dependence of Ca2+ alternans was significantly shifted toward higher heart rates, suggesting a role of luminal SR Ca2+ in the genesis of this highly arrhythmogenic phenomenon. Thus, intra-SR Ca2+ dynamics set the refractoriness and frequency dependence of Ca2+ transients in subepicardial ventricular myocytes.
Keywords: Ca2+-induced Ca2+ release, epicardium, myocyte, sarcoplasmic reticulum
the epicardium is the outermost muscular layer of the cardiac ventricle. This layer is involved in the genesis of several ventricular arrhythmias, such as Brugada syndrome and short Q-T syndrome (2–4). In both syndromes, changes in the duration of the action potential (AP) and the generation of “spike-and-dome” AP morphology are associated with intracellular Ca2+ dynamics (18, 31, 32). Therefore, Ca2+ release from the sarcoplasmic reticulum (SR) is not only the key event triggering cardiac muscle contraction but also regulates the electrical properties of the epicardial layer.
Ryanodine receptors [ryanodine receptor type 2 (RyR2)], which mediate SR Ca2+ release, are regulated by Ca2+ in a complex way. For instance, the open probability (Po) of RyR2 is modulated by the free Ca2+ concentration (28, 36) and by the rate of change of free Ca2+ in the cytosolic face of RyR2 (20, 25). The luminal protein calsequestrin (CSQ)2, which is a major Ca2+-binding protein inside the SR, has been also shown to play an important role in the luminal control of Ca2+ release (23, 24, 26, 42, 44). Therefore, one would expect that the characteristics of Ca2+ release can be modified by introducing an exogenous buffer. Indeed, Terentyev et al. (48) demonstrated that low-affinity Ca2+ buffers are able to regulate Ca2+ release from the SR by affecting the amplitude and regularity of spontaneous Ca2+-release events in isolated ventricular myocytes.
Despite the accumulation of experimental data, several problems remain unsolved. For example, the role of luminal Ca2+ content in controlling the magnitude and dynamics of Ca2+ release is still poorly understood. Our progress in finding an answer to this question depends on the ability to assess intra-SR Ca2+ dynamics. The measurement of intra-SR Ca2+ with fluorescent dyes is a relatively new technique previously used only in isolated cells (38, 45). As a result, Ca2+ dynamics in the SR have not yet been characterized in the intact heart.
We applied the pulsed local field fluorescence (PLFF) technique (33) to assess cytosolic and SR luminal Ca2+ dynamics in the epicardial layer of intact beating mouse hearts. EGTA AM was used as a high-affinity, slow-binding Ca2+ chelator to study the effect of Ca2+ buffering on intracellular Ca2+ dynamics. EGTA allows Ca2+ released to the cytosol through RyR2 to diffuse to distances greater than other Ca2+ chelators like BAPTA, a high-affinity, fast-binding Ca2+ buffer (47). Therefore, unlike BAPTA, EGTA does not prevent the activation of neighboring RyR2s (30), allowing the occurrence of regenerative Ca2+ release from the SR. Although the presence of EGTA is expected to reduce cytoplasmic Ca2+ transients by chelating Ca2+, an exogenous buffer may also affect the Ca2+ release process itself.
We hypothesized that the refractoriness of Ca2+ release depends on the amount of Ca2+ released from the SR during each heart beat. Therefore, we proposed that EGTA cannot only decrease Ca2+ release from the SR but also reduce the refractoriness of Ca2+ release due to a smaller degree of depletion of Ca2+ stores during each beat. The modification of the refractoriness of Ca2+ release will affect the development of Ca2+ alternans, which is potentially arrhythmogenic and is characterized by cyclic beat-to-beat variations in the amplitude of Ca2+ transients.
The main goal of this work was to define the mechanism of action of an exogenous buffer (EGTA AM) on cytosolic and intra-SR Ca2+ dynamics and on the refractoriness of Ca2+ release in the epicardial layer of an intact beating heart. The results described in this report imply that the luminal Ca2+ level plays a significant role in controlling SR Ca2+ release in the beating heart.
METHODS
Heart Preparation
Hearts were removed from young Swiss Webster mice (male, age: 3–7 wk old). The aorta was cannulated and connected to a horizontal Langendorff apparatus for constant perfusion with normal Tyrode solution (2 mM CaCl2, 140 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 0.33 mM Na2HPO4, 10 mM HEPES, and 10 mM glucose; pH 7.4). The temperature of the solution outside the heart was controlled with a Peltier unit. All of the solutions were equilibrated with 100' O2. This protocol was approved by the Institutional Animal Care and Use Committee of the University of California (Merced, CA).
Fluorophore Loading
Mag-fluo-4 AM (Invitrogen) was used to measure changes in the intra-SR Ca2+ concentration. The dye was dissolved in 45–60 μl DMSO with 2.5' Pluronic and added to 1 ml normal Tyrode solution. Perfusion with dye started after the spontaneous heart rate became regular (within 10 min after cannulation). After 1 h of perfusion at room temperature (21–23°C), the solution was switched to normal Tyrode solution and the temperature was increased to 37°C within 10 min. The temperature increase induced the washing out of mag-fluo-4 from the cytosol, allowing us to measure intra-SR Ca2+ signals [see Shannon et al. (45) for additional information on this technique]. In most cases, a downward fluorescence signal reflecting depletion of the SR was apparent even before the heart was warmed up, although some minor upward (cytosolic) component was still present. This upward component completely disappeared within 10–20 min after the temperature reached 37°C. After mag-fluo-4 was removed from the cytosol, a sufficient amount of dye remained inside the SR to generate detectable signals for at least 2 h.
Rhod-2 AM (Invitrogen) was used to measure Ca2+ signals in the cytosol. Di-8-ANEPPS (Invitrogen) was used to measure the membrane potential in the epicardial layer of the hearts. These dyes were prepared and loaded in the same way as mag-fluo-4 AM. The time of loading was 25–35 min.
Optical Setup
A modified version of our custom-made PLFF microscopy setup (33) was used to measure Ca2+ signals from the intact heart. A simplified scheme of the optical apparatus is shown in the Supplemental Material (Supplemental Fig. 1).1 Briefly, two solid-state YAG lasers were used as an illuminating source. A MGL-50B-1 CW Ng-YAG laser (Enlight Technologies, Branchburg, NJ) was used for the excitation of rhodamine-based dyes with green light (532 nm). For the excitation of fluorescein-based dyes, blue light (473 nm) was obtained from a MBL-10-3 CW Ng-YAG laser (Enlight Technologies). Both lasers were time multiplexed by two ferroelectric modulators (50075 Oriel optical shutters, Newport, Stratford, CT) and then optically mixed with ultrafast dichroic mirrors.
The excitation light pulses were focused by a standard microscope objective (×40, numerical aperture: 0.45) into a small multimode optical fiber for the transmission of the exciting light to the epicardial layers. Emitted light was carried back through the same fiber, filtered to eliminate the reflected excitation component, and then focused on an avalanche photodiode, which was connected to an integrating current-to-voltage converter controlled by a digital signal processor (DSP 320, Texas Instruments). Headstage units and their corresponding high-voltage power supply were manufactured by IonOptix (Milton, MA). Fluorescence signals were digitized at a sampling frequency of 5 MHz and filtered to a bandwidth of 500 kHz. The acquisition system was controlled by an Athlon-based PC with a custom-designed, G-based software program (LabView).
One end of the optical fiber was gently placed on the tissue. This effectively allowed synchronous movement of the end of the fiber together with the heart surface. Such procedures considerably attenuated the motion artifacts generated by the beating hearts. The motion of the hearts was noticeably decreased upon the addition of EGTA AM (Invitrogen) to the solution used to load the hearts with dye. The final concentration of EGTA AM in the loading solution varied from 35.5 to 211 μM in different experiments. After the pacemaker cells of the sinoatrial and atrioventricular nodes were electrically ablated using an ophthalmic bipolar pencil (Mentor Ophthalmics, Santa Barbara, CA), hearts were continuously paced at various rates by an electrical stimulator (ISOSTIM A320R, World Precision Instruments, Sarasota, FL) controlled by a PC.
Drugs and Chemicals
All reagents and chemicals were purchased from Sigma Chemical (St. Louis, MO) unless otherwise indicated.
Statistical Analysis
Data are expressed as means ± SD; N indicates the number of independent experiments in each separate series. The total amount of animals used in this study was 60 animals. Statistical significance was tested using ANOVA. Differences were considered to be significant if P values were <0.05.
Mathematical Modeling of EGTA Treatment
A diffusion-reaction theoretical model was developed to provide a conceptual framework to increase the rigorousness of our interpretation. To simulate the myoplasmic Ca2+ transient during a twitch, a multicompartment unidimensional diffusion model was used (8, 9, 40, 48, 54). A detailed description of this model is provided in the Supplemental Material. One innovative aspect of the model is that it incorporates the published Markovian scheme of RyR2 gating (43). Single RyR2 gating is regulated by both cytosolic and luminal (i.e., inside the SR) Ca2+ levels. The RyR2-CSQ interaction is thought to be involved in the luminal Ca2+ regulation of RyR2. AP-like depolarizations activated an influx of Ca2+ across the surface membrane, elevating the free Ca2+ level in the diadic space (space between the T-tubule and SR membranes). This Ca2+ influx activates RyR2 channels, initiating the Ca2+-induced Ca2+ release.
Here, we present the details of the model, which allowed us to predict how an exogenous Ca2+ buffer (EGTA) may impact sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) activity and, ultimately, intracellular Ca2+ dynamics. Local changes in cytosolic and luminal Ca2+ concentrations were simulated using a one-dimensional diffusion-reaction model of Ca2+ dynamics in the myoplasm and SR luminal spaces derived from our previous models of Ca2+ handling (9, 48, 54). The geometry of the model consist of two adjacent sections, representing the myoplasm and SR luminal spaces of a sarcomere, all surrounded by an extracellular compartment. Each of these sections was sliced into a number (N) of diffusionally connected subcompartments. Ca2+ exchange between the cytosolic and luminal sections occurs only via RyRs (located inside the SR membrane between the first cytosolic and luminal compartments) and SERCA pumps (placed between the cytosolic and luminal compartments in the central region of the model). SR Ca2+ efflux is governed by the Ca2+ gradient across the SR membrane and by the SR Ca2+-release channel's Po, as described by a Markov model. The model accurately predicts 1) the measured rates of Ca2+-dependent activation, deactivation, adaptation, and inactivation; 2) the existence of modal gating; and 3) luminal regulation by the Ca2+-CSQ complex. The basic features of the Markovian scheme describing RyR2 kinetic behavior are the four Ca2+-binding sites for activation, the three open states for different kinetic modes, the one Ca2+-dependent inactivation site, and the luminal Ca2+ regulation through the interaction between Ca2+-CSQ and the RyR channel. Under steady-state conditions, the opening of single RyR channels occurs in bursts. These bursts fall into two categories and are temporally clustered into distinct modes of RyR channel gating [i.e., high Po and low Po (14, 51)]. In addition, the luminal regulation stabilizes the channel in the open state and mimics the luminal regulatory effects described by Györke and Györke (21). Ca2+ influx to the SR was simulated by integrating kinetic models for SERCA identical to the ones we have previously described (48).
Ca2+ buffering inside the SR by CSQ was modeled as for a low-affinity, high-capacity Ca2+-binding protein. An allosteric model (34) was used to describe the interaction between Ca2+ and CSQ. Myoplasmic Ca2+ buffering was modeled by including several mobile buffers (i.e., ATP, EGTA, and a Ca2+ indicator) and one fixed buffer (troponin).
Extracellular Ca2+ influx is controlled by a voltage-dependent Ca2+ channel modeled as a sarcolemmal Hogdkin-Huxley-type channel (41) located between the first cytosolic compartment and the extracellular medium. The channel also has voltage- and Ca2+-dependent inactivation that is activated by a controlled membrane potential change. Ca2+ extrusion from the cytosol was modeled through a Na+/Ca2+ exchanger transport mechanism (41). The parameters used for the simulation are shown in Supplemental Table 1. Finally, all the equations were numerically integrated using a finite-difference approximation (Euler method). The initial values for the state variables were calculated using inversion of the Q-matrix procedure (10). The boundary conditions were established to satisfy the following equation:
where JinCa is the net Ca2+ influx and JoutCa is the net Ca2+ efflux from compartment 0 at all times and t is time. In addition, a Neumann's boundary condition (49) was implemented in the last cytosolic and luminal compartments. Finally, the code was written in G language (National Instruments), and simulations were run in a 36-node Athlon-MP-based cluster under the control of Linux. The differential equations were numerically integrated with time increments of 4 μs to generate a 20-s trace.
RESULTS
Ca2+ Dynamics in the Cytosol and SR Lumen
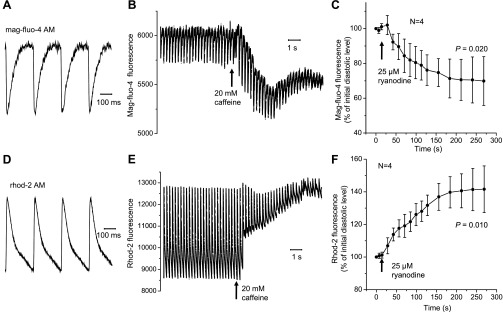
Measuring Ca2+ signals from intracellular organelles has always been a major challenge in cardiovascular physiology (6, 9, 22). Furthermore, up until now, it was unfeasible to evaluate the luminal Ca2+ dynamics at the whole organ level. Here, SR luminal Ca2+ dynamics at the subepicardial layer of an intact beating mouse heart were characterized using the fluorescent dye mag-fluo-4 AM. After the dye was effectively washed out of the cytosol at 37°C, the signal declined sharply in response to an electrical stimulation pulse and then slowly recovered to diastolic levels (Fig. 1A). The signal detected from the hearts loaded with rhod-2 AM changed in the opposite direction (Fig. 1D).
Fig. 1.
Changes in fluorescence from the sarcoplasmic reticulum (SR) lumen (A–C) and cytosol (D–F) measured with the Ca2+-sensitive dyes rhod-2 AM and mag-fluo-4 AM, respectively. Representative traces of luminal (A) and cytosolic (D) Ca2+ transients are shown. Diastolic (resting) levels of mag-fluo-4 (B) and rhod-2 (E) fluorescence changed in different directions in response to the application of caffeine. An analogous effect was observed in the presence of ryanodine. Changes in mag-fluo-4 (C) and rhod-2 (F) fluorescence reflect the release of Ca2+ from the SR and accumulation of Ca2+ in the cytosol, respectively. Data are means ± SD. All measurements were conducted at 4 Hz and 37°C.
To verify that the changes in the fluorescence signals observed in our experiments were due to changes in intra-SR and cytosolic Ca2+ content, Ca2+ release was modified by perfusing the hearts with Tyrode solution containing 20 mM caffeine, which causes the fast release of Ca2+ from the SR (Fig. 1, B and E). The application of caffeine for 10 s led to a decrease in both the diastolic level of mag-fluo-4 fluorescence and in the amplitude of Ca2+ transients [down to 92 ± 0.05' (N = 4, P = 0.012) and 51 ± 0.21' (N = 4, P = 0.003) of the initial values, respectively]. The amplitude of Ca2+ transients recorded with rhod-2 also decreased in the presence of caffeine [down to 18 ± 0.09' of the initial value (N = 4, P < 0.001)], whereas the diastolic level of rhod-2 fluorescence increased [up to 135 ± 0.09' (N = 4, P < 0.001)], reflecting the accumulation of Ca2+ in the cytosol (Fig. 1E). These data imply that mag-fluo-4, unlike rhod-2, detects the changes of free Ca2+ in a compartment that loses Ca2+ during caffeine pulses.
Another piece of evidence confirming the intra-SR origin of the mag-fluo-4 fluorescence was obtained in the experiments with 25 μM ryanodine, an alkaloid that binds specifically to RyR2 Ca2+-release channels located in the membranes of the SR and locks the channels in a long-dwell time subconductance state (50). The diastolic level of Ca2+ in the SR lumen slowly decreased in the presence of ryanodine (Fig. 1C). No additional decline in the fluorescence was observed when 20 mM caffeine pulses were applied after treatment with ryanodine (data not shown). Electrically induced changes in the mag-fluo-4 fluorescence signal disappeared almost completely after 10 min of ryanodine application (Supplemental Fig. 2). This experiment illustrates the time course of fractional binding of ryanodine to the RyR2 in an intact preparation and how ryanodine induces the depletion of luminal Ca2+. In contrast, the cytosolic Ca2+ level increased as a result of treatment with ryanodine (Fig. 1F). Since the results described above are in accordance with the mode of action of ryanodine, they support the assumption that rhod-2 and mag-fluo-4 fluorescence detect changes in cytosolic and intra-SR Ca2+, respectively.
Similar results were obtained in the presence of EGTA (Supplemental Figs. 2 and 3). Electrically stimulated mouse hearts still showed changes in the rhod-2 signal after 10 min of perfusion with ryanodine (Supplemental Fig. 3C). These remaining fluorescence transients can be attributed to the influx of external Ca2+ during the cardiac AP via voltage-dependent Ca2+ channels located in the sarcolemma. The difference in the amplitude between transients obtained in the presence and absence of ryanodine illustrates the magnitude of Ca2+ release from the SR in subepicardial cardiac myocytes. When an identical electrical stimulation protocol was used in a mag-fluo-4-loaded mouse heart, the fluorescence signal did not show any evident changes (i.e., AP-induced SR depletion) at the same time of perfusion with ryanodine (Supplemental Fig. 2C). This clearly indicates that mag-fluo-4 is located in a different ryanodine-sensitive intracellular compartment.
Thermodynamics of Intra-SR Ca2+ Dynamics
Rodents are homeothermic animals in which Ca2+ transport is sensitive to temperature. For example, the time course of relaxation of intracellular Ca2+ transients involves several temperature-dependent Ca2+ removal mechanisms [Na+/Ca2+ exchange, SR Ca2+ uptake through the SERCA pump, uptake of Ca2+ by the mitochondria, etc., and cytosolic buffering processes (mobile buffers, e.g., ATP, calmodulin, etc.) as well as fixed buffers (e.g., troponin C, etc.)]. Temperature can strongly modify the kinetics of all the transport mechanisms mentioned above. However, the relaxation phase of SR Ca2+ transients (depletion) is mainly governed by Ca2+ uptake by the SERCA pump and by the binding of Ca2+ to CSQ. Since the SERCA pump needs to hydrolyze ATP to transport Ca2+, measuring the temperature dependence of the luminal Ca2+ relaxation dynamics will allow us to examine the thermodynamic properties of the SERCA pump in vivo. Thus, to further understand the relationship between cytosolic and SR luminal Ca2+ dynamics, we studied the temperature dependence of these processes.
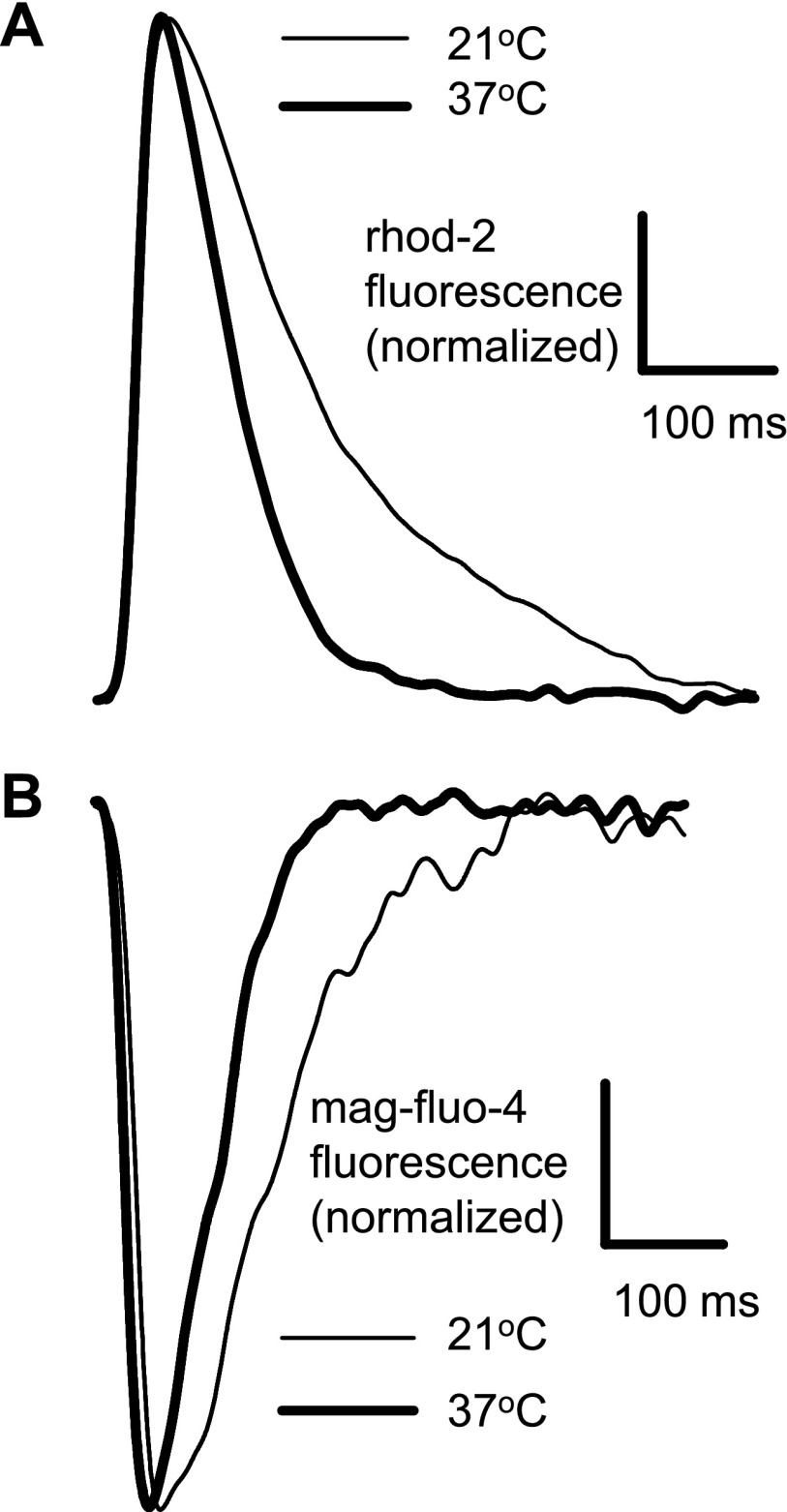
The increase in temperature noticeably accelerated the dynamics of Ca2+ in both the cytosol and SR (Fig. 2). The time to peak for rhod-2 signals (Fig. 2A) decreased from 28 ± 3 ms at 21°C to 19 ± 4 ms at 37°C (N = 4, P = 0.013). The time to nadir for mag-fluo-4 signals decreased from 34 ± 5 ms at 21°C to 22 ± 3 ms at 37°C (N = 4, P = 0.007). There were no statistical differences between the time to peak (rhod-2, cytosol) and time to nadir (mag-fluo-4, SR lumen) at any given temperature (P = 0.098 for 21°C and P = 0.234 for 37°C).
Fig. 2.
Cytosolic (rhod-2 AM; A) and luminal (mag-fluo-4 AM; B) Ca2+ transients recorded at 21 and 37°C. Hearts were paced at a frequency of 2 Hz during the recordings at both temperatures. No EGTA AM was added to the loading solution.
As shown in Fig. 2, the higher temperature was associated with a faster recovery of the Ca2+ signal to its diastolic level. The decay time constant decreased from 162 ± 35 ms at 21°C to 92 ± 4 ms at 37°C for the cytosolic signal (N = 4, P = 0.007). For the luminal signal, the magnitudes of the decay time constant were 120 ± 15 and 74 ± 7 ms at 21 and 37°C, respectively (N = 4, P = 0.001). Temperature coefficients (Q10 values) calculated on the basis of these data were not statistically different between cytosolic (1.42 ± 0.21) and luminal (1.35 ± 0.12) signals (N = 4, P = 0.593).
The similarity of the temperature dependence of the relaxation of Ca2+ transients in the cytosol and within the SR suggests that both processes are governed by the same Ca2+ transport mechanism.
Effect of EGTA on Cytosolic and Luminal Ca2+ Transients
The gain of Ca2+ release can determine both the time course and amplitude of cytosolic Ca2+ transients and the degree of depletion of the SR. In this study, the gain of Ca2+ release was modified by adding an exogenous cytosolic buffer to the Tyrode solution used to perfuse a mouse heart. Our working hypothesis was that the presence of a slow Ca2+ buffer like EGTA, which has a low association rate constant [5 × 106 M−1·s−1 (13)], would decrease the gain of Ca2+ release by buffering Ca2+ close to the cytosolic binding sites of the SERCA pump. Since the SERCA pump needs to bind two Ca2+ to be fully activated, a reduction of the free cytosolic Ca2+ concentration will reduce pump activity (5), promoting Ca2+ depletion of the SR. This will finally result in a decrease of the Ca2+ released from the SR. To test this hypothesis, mouse hearts previously loaded with the fluorescent dyes were perfused with Tyrode solution containing 211 μM EGTA AM, and changes in fluorescence were recorded.
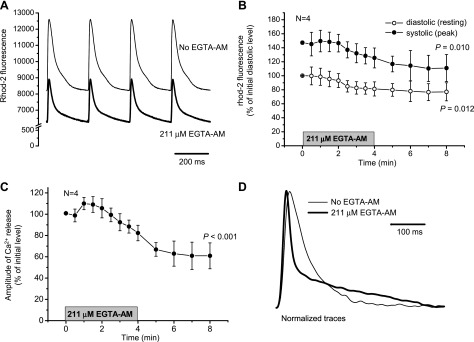
Figure 3A shows rhod-2 signals recorded before and after perfusion with the exogenous buffer. EGTA AM induced changes in both diastolic and systolic myoplasmic fluorescence. Average data representing the time course of these changes are shown in Fig. 3B. Diastolic and systolic levels of rhod-2 fluorescence were reduced by 23' (P = 0.012) and 25' (P = 0.010), respectively, whereas the amplitude of Ca2+ transients was reduced by 39' (P < 0.001; Fig. 3C). Moreover, the kinetics of Ca2+ transients were dramatically modified by the buffer. Note the two very distinct components of the relaxation process in hearts paced at 2 Hz after the 30-min load with EGTA AM (Fig. 3D).
Fig. 3.
Effect of 4-min perfusion with Tyrode solution containing 211 μM EGTA AM on Ca2+ dynamics in the cytosol measured with rhod-2 AM (A–C). Measurements were conducted at 37°C at a heart rate of 4 Hz. A: representative traces obtained before (thin line) and 4 min after (thick line) perfusion with EGTA AM. B: changes in the diastolic (resting) and systolic (peak of transient) levels of rhod-2 fluorescence during 4-min treatment with EGTA AM. C: reduction in the amplitude of Ca2+ transients after the addition of EGTA AM. The period of treatment with EGTA AM is indicated by the shaded bar. Data are means ± SD; N is the number of independent experiments (hearts). P values are the results of one-way ANOVA (initial vs. final levels of the parameters). D: typical traces of fluorescence from mouse hearts loaded with rhod-2 AM in the absence (thin line) and presence (thick line) of 211 μM EGTA AM (37°C, 2 Hz). Traces were normalized to the maximum amplitude of the release for the comparison of the kinetics.
The effect of EGTA on the amplitude and kinetics of cytoplasmic Ca2+ transients can be explained by several mechanisms. For example, EGTA may compete with cytosolic Ca2+ buffers (including rhod-2), reducing, in this way, the free Ca2+ concentration during Ca2+ release. A second possibility could be that EGTA competes with the cytosolic Ca2+-binding sites on RyR2. However, this option seems unlikely due to the slow association rate constant of EGTA (see the discussion). Finally, EGTA could decrease the free diastolic Ca2+ in the cytosol (Fig. 3B), thereby decreasing the activity of the SERCA pump, leading to a reduction in the intra-SR Ca2+ concentration (depletion) and, consequently, to a decrease in the amplitude of Ca2+ transients.
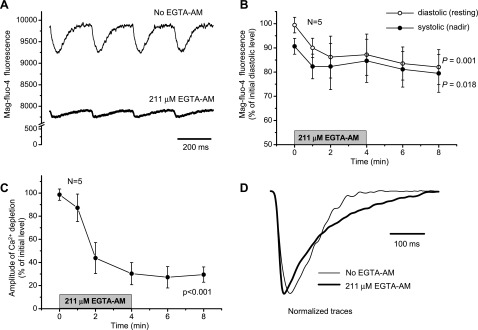
To test this last possibility, we evaluated the intra-SR Ca2+ dynamics by measuring the fluorescence of mag-fluo-4 when hearts were perfused with EGTA AM. A noticeable decline in diastolic and systolic intra-SR Ca2+ levels was observed (Fig. 4A). Figure 4B shows that the diastolic level of mag-fluo-4 fluorescence was decreased by 18' (P = 0.001), implying that the exogenous buffer depleted intra-SR Ca2+. Moreover, the amplitude of Ca2+ release from the SR was dramatically decreased [by 70' of the initial value (P < 0.001); Fig. 4C], which indicates that intra-SR Ca2+ content has a significant effect on Ca2+ release from the SR. The presence of EGTA also slowed down the rate of Ca2+ replenishment of the SR (Fig. 4D). These results support the hypothesis that EGTA decreases the free diastolic Ca2+ concentration in the cytosol, leading to a reduction in the intra-SR Ca2+ concentration (depletion) and, consequently, to a decrease in the amplitude of Ca2+ transients.
Fig. 4.
Effect of 4-min perfusion with Tyrode solution containing 211 μM EGTA AM on Ca2+ dynamics in the SR lumen measured with mag-fluo-4 AM (A–C). Measurements were conducted at 37°C at a pacing frequency of 4 Hz. A: representative traces obtained before (thin line) and 4 min after (thick line) perfusion with EGTA AM. B and C: changes in the diastolic (resting) and systolic (nadir) levels of mag-fluo-4 fluorescence (B) and decline in the amplitude of Ca2+ depletion (C) in response to 4-min treatment with 211 μM EGTA AM. The period of treatment with EGTA AM is indicated by the shaded bar. Data are means ± SD; N is the number of independent experiments (hearts). P values are the results of one-way ANOVA (initial vs. final levels of the parameters). D: typical traces of fluorescence from mouse hearts loaded with mag-fluo-4 AM in the absence (thin line) and presence (thick line) of 211 μM EGTA AM (37°C, 2 Hz). Traces were normalized to the maximum amplitude of the depletion to compare the kinetics.
Calibration of the Mag-fluo-4 Signal and the Effect of EGTA on Intra-SR Ca2+ Concentration
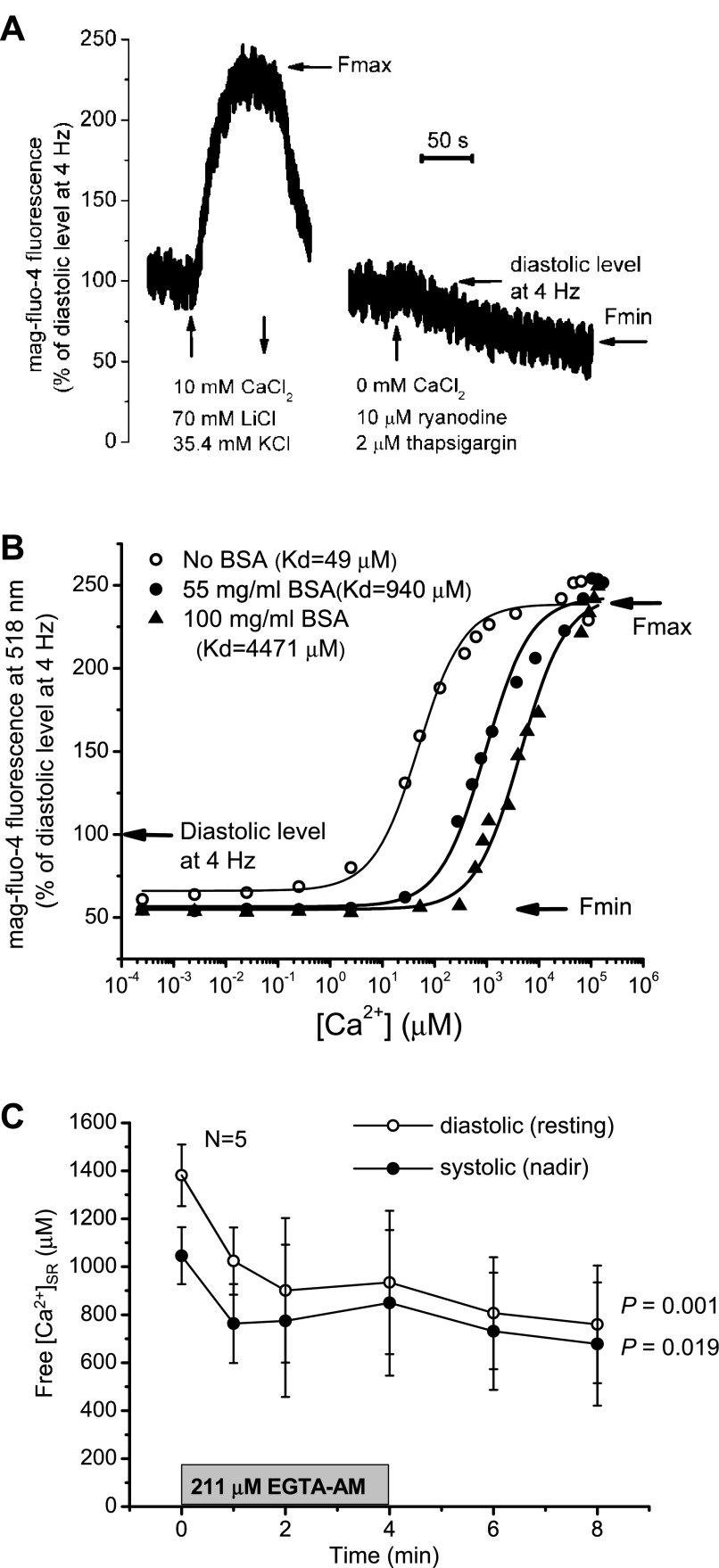
The procedure of in vivo calibration of a Ca2+ dye requires obtaining minimal (Fmin) and maximal fluorescence (Fmax) levels corresponding to Ca2+-free and Ca2+-bound dye, respectively. We conducted these experiments at a pacing frequency of 4 Hz and temperature of 37°C. Fmax was recorded in the presence of 10 mM CaCl2 in the extracellular perfusate. In addition to a higher Ca2+ concentration, Na+/Ca2+ exchanger activity was inhibited by replacing 70 mM NaCl with 70 mM LiCl in the Tyrode solution, allowing the accumulation of Ca2+ inside the cells. An additional 30 mM NaCl was replaced with 30 mM KCl to induce a depolarization of the cell membrane. The final concentrations of NaCl and KCl were equal to 40 and 35.4 mM, respectively. A typical recording is shown in Fig. 5A. Average Fmax calculated from four independent experiments (hearts) was 242 ± 20' of the diastolic (resting) value, which was recorded at 4 Hz before the application of high-Ca2+ Tyrode solution. These results imply that intra-SR Ca2+ concentrations do not saturate mag-fluo-4 fluorescence under our experimental conditions, i.e., before the application of high-Ca2+ solution. Note that changes in mag-fluo-4 fluorescence were reversible. Fmin was obtained by applying Tyrode solution without the addition of CaCl2 in the presence of 10 μM ryanodine (Calbiochem, La Jolla, CA) and 2 μM thapsigargin (Molecular Probes, Eugene, OR). Fmin was 54 ± 12' of the initial diastolic level at 4 Hz (N = 4). Values of Fmin and Fmax were corrected for bleaching.
Fig. 5.
Calibration of the fluorescent dye mag-fluo-4. A: representative recordings of fluorescence changes during the determination of maximum (Fmax) and minumum fluorescence (Fmin) in an intact mouse heart. B: dependence of the fluorescence at 518 nm on Ca2+ concentration in a cuvette in the presence and absence of albumin (BSA). Measurements were conducted at pH 6.8, 2 mM MgCl2, and 37°C. The y-axis was adjusted according Fmin and Fmax values obtained in vivo and calculated as a percentage of the initial diastolic level at 4 Hz and 37°C. C: changes in free intra-SR Ca2+ concentrations during EGTA AM treatment estimated using data from B as well as Fig. 4B.
To relate the changes in mag-fluo-4 fluorescence recorded from whole heart preparations to the actual concentrations of Ca2+, we performed in vitro calibrations of the dye. This was performed by dissolving the salt form of the dye with a solution containing 30 mM MOPS (pH 6.8), 100 mM KCl, and 2 mM MgCl2. To account for the effects of proteins present in the SR, either 55 or 100 mg/ml BSA (fraction V, U.S. Biological, Swampscott, MA) was added. Calcium Sponge S (BAPTA polystyrene, Invitrogen) was added into the buffer solutions (with or without BSA, 1/10 of the solution volume) to chelate the contaminant Ca2+. After a vigorous mix (vortexing), the solution was centrifuged at 10,000 rpm for 3 min, and the supernatant was carefully collected to be used in the experiments. Finally, mag-fluo-4 tetrapotassium salt (at a final concentration of 0.25 μM) was added. The fluorescence was excited at 473 nm and monitored at 37°C within the range of 475–630 nm using a spectrofluorometer (QuantaMaster 40, Photon Technology, Birmingham, NJ). The dependence of the maximum amplitude of fluorescence at 518 nm on the Ca2+ concentration was studied to determine the apparent dissociation constant (Kd).
In absence of BSA, the average measured Kd was 49 μM. These data correlate well with the numbers reported in Ref. 17 (70 and 81 μM), which were obtained at 22°C in the absence and presence of 1 mM MgCl2, respectively. The presence of BSA caused a significant shift of Kd values toward higher Ca2+ concentrations. Kd values of 940 and 4,471 μM were obtained in the presence of 55 and 100 mg/ml BSA, respectively. This effect was analogous to that observed for fluo-3 in the presence of aldolase (16).
The fluorescence values obtained in vitro were normalized using the diastolic fluorescence measured on intact hearts at 4 Hz and 37°C as a reference level (Fig. 5B). Minimal and maximal levels of fluorescence were adjusted to Fmin and Fmax obtained in vivo (54' and 242' of the diastolic level at 4 Hz, respectively). The fitting curve for the samples with 100 mg/ml BSA was used to estimate intra-SR free Ca2+ concentrations before and during the application of EGTA AM. As shown in Fig. 5C, 4-min perfusion with 211 μM EGTA AM caused a decrease in the diastolic free Ca2+ inside the SR from 1,381 ± 128 to 760 ± 245 μM. It should be noted that such estimations provide only approximate levels of intra-SR Ca2+ under the assumption that the obtained Fmax and Fmin values were close to the true values. Error sources include dye deterioration and washing out of the cells as well as variations in pH and SR content (Mg2+ and protein concentrations).
Mathematical Modeling of EGTA Treatment
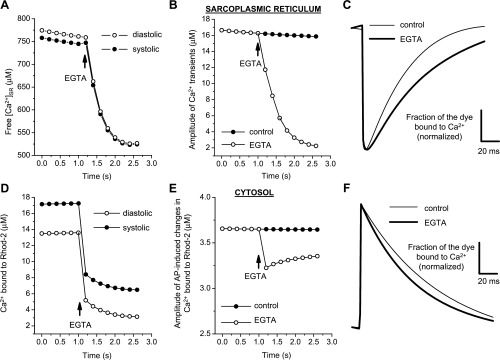
The experiments shown in Figs. 3D and 4D demonstrated that EGTA has a differential kinetic effect between fluorescent signals measured in the cytosol and SR. While EGTA accelerates the relaxation of cytosolic Ca2+ transients, the same buffer produced a slowness of SR Ca2+ uptake. To address this and other experimental observations, we used a mathematical model to simulate, in silico, the effect of EGTA treatment on free Ca2+ content in the SR and on the kinetics of Ca2+ transients.
The results of the calculations implied that the diastolic and systolic free Ca2+ concentration declines in both the SR (Fig. 6A) and cytosol (Fig. 6D) after the addition of 400 μM EGTA in the cytosol. The amplitude of intra-SR (Fig. 6B) and cytosolic (Fig. 6E) Ca2+ transients also decreased. In the presence of EGTA, the relaxation kinetics of Ca2+ transients were slowed down in the SR compartment (Fig. 6C) and accelerated in the cytosol (Fig. 6F). The decrease in the intra-SR Ca2+ concentration as well as the slowness of SR Ca2+ replenishment were due to the effects of EGTA in the cytosol and not a direct effect of EGTA in the SR. EGTA induced a decrease in the diastolic free Ca2+ concentration in the cytosol that promoted a decrease in SERCA pump activity that finally led to a reduction in the rate of SR Ca2+ uptake and to a decrease in the SR diastolic Ca2+ concentration. The results of these simulations are in agreement with the experimental data described above.
Fig. 6.
Results of the mathematical model that simulates changes in SR Ca2+ content (A–C) and in the cytosol (D–F) in response to the addition of an exogenous buffer (EGTA). A: effect of EGTA on free diastolic and systolic Ca2+ concentrations upon the addition of 400 μM EGTA in the cytosolic compartments. B and C: effects of EGTA on the amplitude and kinetics of Ca2+ transients in the SR. D and E: concentrations of rhod-2 bound to Ca2+ in the cytosol during diastole and systole as well as the changes and amplitude (difference between systole and diastole) when 400 μM EGTA was added to the cytosol. F: effect of EGTA on the kinetics of the fluorescent transient measured with rhod-2 in the cytosol. See the Supplemental Material for details.
Effect of EGTA on the Kinetics of Cytosolic and Luminal Ca2+ Transients
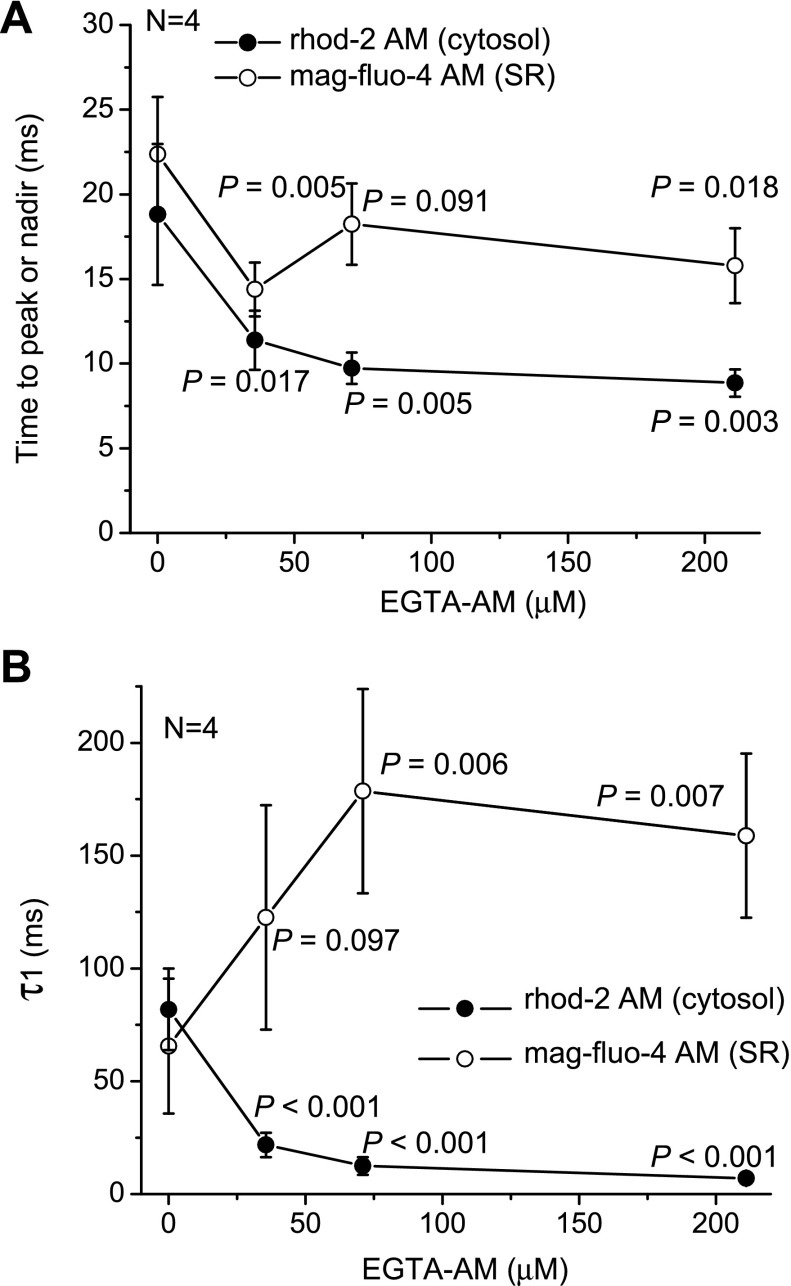
To further evaluate our working hypothesis and explore the effect of EGTA on the Ca2+ dynamics of cytosolic and intra-SR Ca2+ transients, we conducted experiments at several EGTA AM concentrations in the loading solution. A summary of the results is shown in Fig. 7. In response to the elevation of EGTA AM concentration, a decrease in the time to peak and time to nadir was observed for both cytosolic and luminal SR signals (Fig. 7A). However, the effect of 211 μM EGTA AM on the time to peak was much larger than the effect on the time to nadir [53' (P = 0.003) vs. 29' (P = 0.018), respectively]. This suggests that the effect of EGTA on the releasing phase of the cytosolic Ca2+ transient is mostly due to the competition between EGTA and cytosolic Ca2+ buffers and not to the release mechanism itself. The presence of EGTA also considerably modified the time course of the relaxation of cytosolic Ca2+ transients. Two distinct components were observed in the presence of the buffer: an initial phase accelerated by EGTA and a delayed (or second) phase, which was slowed down by the presence of the buffer (Fig. 3D). Consequently, hearts not treated with EGTA AM reached the diastolic levels of free Ca2+ in shorter times.
Fig. 7.
Changes in the kinetics of Ca2+ transients in the cytosol (●) and Ca2+ depletion in the SR lumen (○) as a result of increasing the EGTA AM concentration in the loading solution. A and B: time to peak (A) and faster time constant (τ1; B) obtained as a result of the fitting with a two-exponential decay function. Measurements were conducted at 37°C and at a pacing frequency of 2 Hz. Data are means ± SD; N is the number of independent experiments (4 for each combination of dye and EGTA AM concentration, total number: 32). P values are results of one-way ANOVA (no EGTA AM vs. different concentrations of EGTA AM).
Interestingly, the effect of increasing the concentration of EGTA AM on the time course of relaxation of SR luminal Ca2+ transients was dramatically different from that observed for cytosolic Ca2+ transients. The relaxation of the intra-SR signal was found to be slower in EGTA-treated hearts. As shown in Fig. 7B, the fastest time constant obtained as a result of fitting the relaxation kinetics with a two-exponential decline function (τ1) increased for intra-SR Ca2+ depletion by 142' (P = 0.007) in response to the elevation of EGTA AM concentration, whereas τ1, calculated for cytosolic Ca2+ transients, decreased by 91' (P < 0.001) in the presence of the buffer. The data for SR luminal Ca2+ transients (depletion) are in accordance with the previous observations that the kinetics of relaxation of cytosolic Ca2+ concentration during reuptake depend on the cytosolic Ca2+ level (5). The lower the level of free Ca2+ concentration in the cytosol, the slower will be the uptake kinetics. This is fully consistent with our working hypothesis (i.e., EGTA affects intra-SR Ca2+ loading).
Time Course of SR Ca2+ Replenishment
Restitution of Ca2+ depletions.
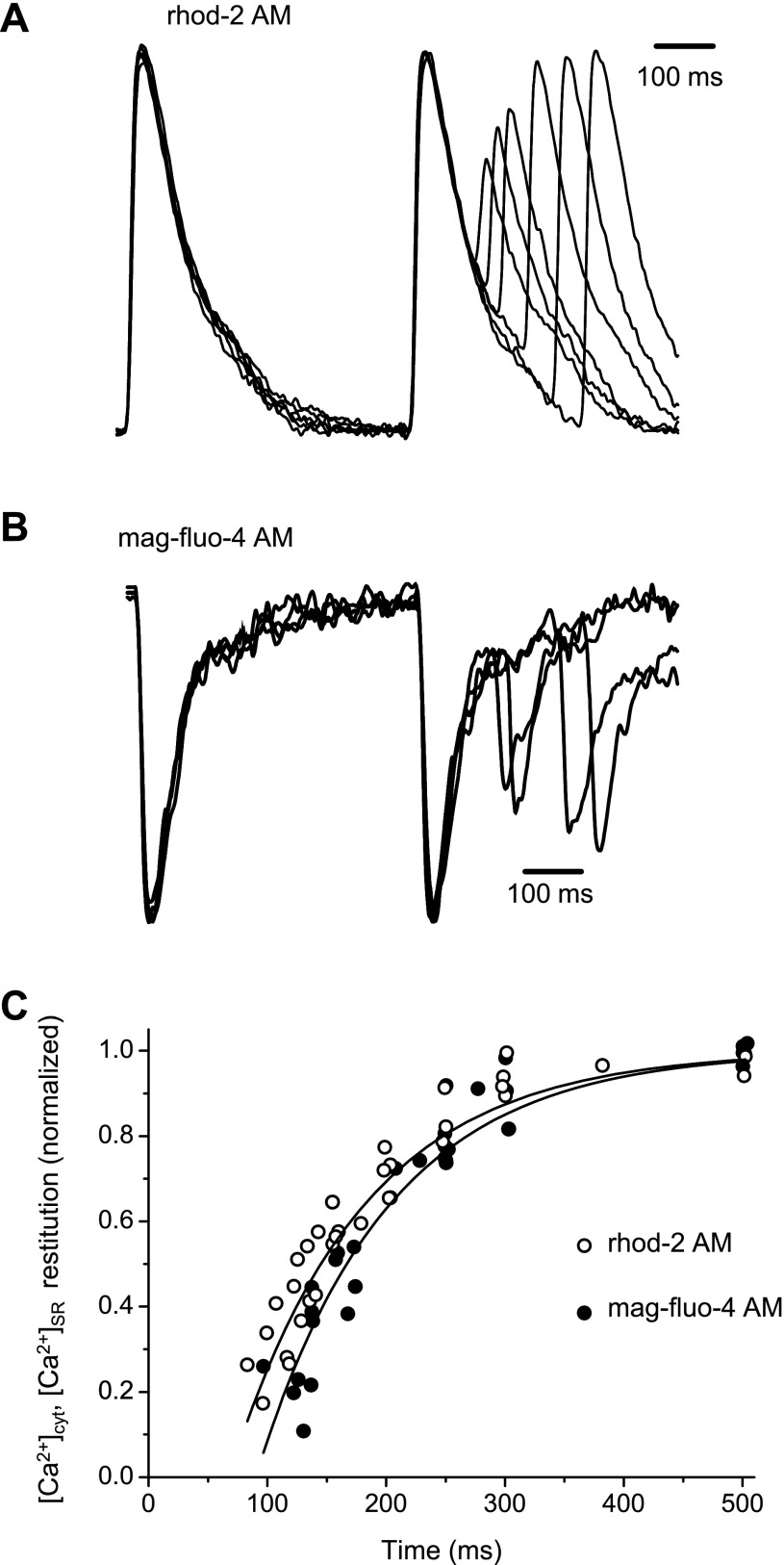
To understand the relationship between SR Ca2+ replenishment and Ca2+ release from this intracellular store, we used a time restitution protocol to evaluate the kinetics of both processes. The restitution of Ca2+ transients in the cytosol and SR lumen (depletion) was explored by applying an extrasystolic stimulus in hearts paced at 2 Hz at 37°C. The extrasystolic stimulus was generated by interspersing an extrastimulus, which was delayed with respect to the basal pacemaker stimulation (or stimulation frequency, 2 Hz).
Superimposed traces of cytosolic and SR lumen Ca2+ transients obtained as a result of applying extrasystolic stimuli at several delayed times between regular pacemaker stimulus pulses are shown in Fig. 8, A and B, respectively. Changes in the amplitude of the signals after an extrasystolic pulse were normalized to the amplitude of the previous Ca2+ transient/depletion trace measured in control hearts, where no exogenous EGTA AM was applied. In both cases, we observed that the shorter the time between electrical stimuli, the smaller the amplitude of the Ca2+ transient/depletion after the second pulse. Overall, the results of the restitution experiments are shown in Fig. 8C. No significant differences were found between the restitution of Ca2+ transients in the cytoplasm and in the lumen of the SR [exponential time constant of restitution: 97 ± 13 and 107 ± 14 ms for cytosolic and luminal Ca2+ transients, respectively (N = 4, P = 0.329)]. This suggests that the restitutions of cytosolic and luminal Ca2+ transients in the absence of exogenous buffer reflect kinetics of the same process (time-dependent recovery of Ca2+ release) monitored from two different sides (the cytosol and SR lumen).
Fig. 8.
Typical traces obtained during the measurement of restitution of Ca2+ transients in the cytosol (A) and Ca2+ depletions in the SR lumen (B). Changes in cytosolic and SR Ca2+ concentrations ([Ca2+]cyt and [Ca2+]SR, respectively) were normalized to the amplitude of the first peak, and the normalized traces were then superimposed. C: comparison of the time courses of restitution of Ca2+ release in the cytosol and Ca2+ depletion in the SR (N = 8, 4 heart preparations for each dye). Recordings were conducted at 37°C and at a pacing frequency of 2 Hz. No EGTA was added into the loading solution.
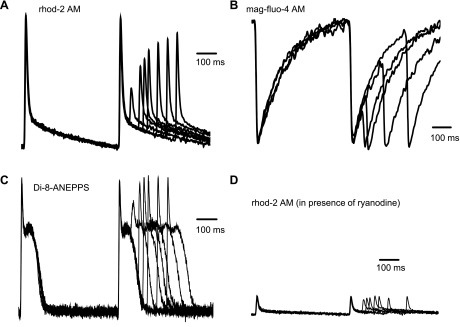
To evaluate how an increase in the cytosolic concentration of EGTA AM modified the relationship between SR Ca2+ replenishment and Ca2+ release from this intracellular store, we then performed restitution experiments in the presence of 211 μM EGTA AM. A typical family of traces of Ca2+ transients in the cytosol and SR lumen (depletion) was obtained when the same protocol as shown in Fig. 8 was applied in the presence of the exogenous buffer, as shown in Fig. 9, A and B. As in Fig. 8, the extrasystolic pulse induced a smaller than regular Ca2+ transient. This decrease in the amplitude of the Ca2+ transient evoked by the extrasystolic stimulus could be due to either an inability of the SR to release Ca2+ or a reduction in the influx of Ca2+ through the plasma membrane. To discriminate between these two possibilities, we performed experiments to find out if the refractoriness of Ca2+ transients was associated with the refractoriness of the AP. Figure 9C shows the restitution of optically recorded AP in hearts perfused with 211 μM EGTA AM. Interestingly, the restitution of the epicardial AP was much faster than that of Ca2+ transients in the cytosol and SR lumen (depletion). The amplitude of the AP was fully recovered after ∼100 ms, whereas the amplitude of the Ca2+ transient/depletion was not fully recovered at that time.
Fig. 9.
Typical traces of Ca2+ transients in the cytosol (A), Ca2+ depletions in the SR lumen (B), action potential (AP; C), and Ca2+ changes in the cytosol during the AP (D; measured in the presence of 25 μM ryanodine) when a restitution protocol was applied. The concentration of EGTA AM in loading solution was 211 μM. The changes in cytosolic and SR Ca2+ concentrations and AP were normalized to the amplitude of the first peak (or nadir in the case of intra-SR Ca2+ transients), and the normalized traces were then superimposed. Recordings were conducted at 37°C and at a pacing frequency of 2 Hz.
However, the refractoriness of Ca2+ transients in the cytosol and SR lumen could still depend on the recovery from inactivation of voltage-activated Ca2+ channels located in the plasma membrane. This hypothesis was evaluated by measuring cytosolic Ca2+ transients in hearts where SR Ca2+ release was completely abolished with 25 μM ryanodine (Fig. 9D). In the presence of ryanodine, Ca2+ transients reflected Ca2+ influx through the plasma membrane. The recovery of the amplitude of these transients during a restitution protocol was also faster than that of Ca2+ transients produced by Ca2+ released from the SR.
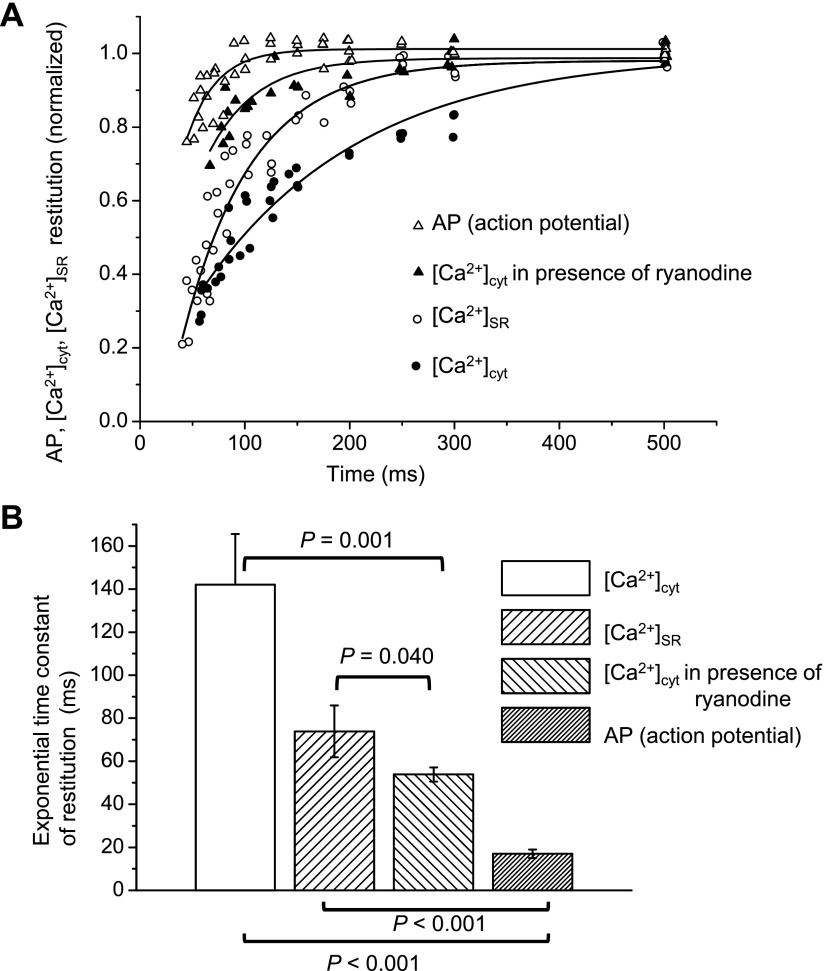
The comparison of the data collected for the various processes mentioned above is shown in Fig. 10A. This plot revealed that the kinetics of the recovery of AP and Ca2+ influx (in the presence of ryanodine) through the plasmalemma are noticeably faster than that of the intracellular Ca2+ release (see averaged values of time constants in Fig. 10B). This indicates that the time course of the recovery from refractoriness of cytosolic and SR Ca2+ transients is mostly defined by the release process from the SR and not by Ca2+ entering through the plasma membrane.
Fig. 10.
A: time courses of restitution of cytosolic Ca2+ transients, SR Ca2+ depletion, and AP obtained as shown in Fig. 9. The restitution of the AP was calculated as changes in the amplitude of the spike. B: comparison of the exponential time constants for the restitution process. P values are the results of one-way ANOVA for pairs identified by the horizontal brackets [total number of independent experiments (hearts): 15]. The concentration of EGTA AM in loading solution was 211 μM.
Effect of EGTA AM.
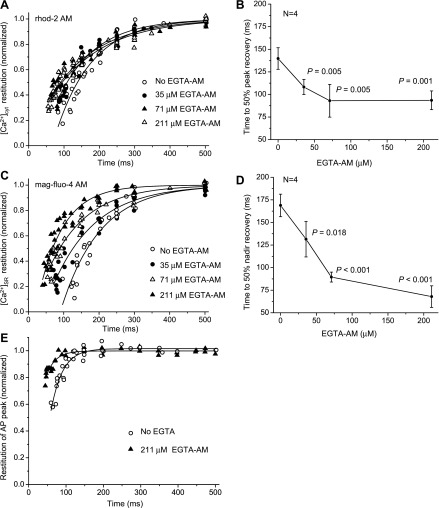
Evaluation of the time course of restitution is an adequate way to measure the refractoriness of the different steps involved in the Ca2+-release process. We performed experiments using several concentrations of EGTA AM in the loading solution to determine how an increase in the Ca2+ buffering capacity can modify the refractoriness of cytosolic and SR Ca2+ transients. Figure 11 shows that an increase in the EGTA AM concentration led to faster restitution kinetics of both cytosolic and SR Ca2+ transients. Time to 50' of peak and nadir recoveries for cytosolic and SR Ca2+ transients were 33' and 60' lower after treatment with 211 μM EGTA AM compared with control values (P < 0.001; Fig. 11, B and D, respectively). This suggests that reducing Ca2+ release (less SR Ca2+ depletion) during every beat can accelerate the restitution of Ca2+ release. Interestingly, the presence of EGTA AM also accelerated the restitution of the AP (Fig. 11E) from an average time constant of 28 ± 4 ms (control group) to 15 ± 1 ms (EGTA AM-treated group, P = 0.002). However, as shown in Fig. 10B, even in the presence of EGTA AM, the restitution of the AP was much faster than the restitution of intracellular Ca2+ release.
Fig. 11.
Restitution of Ca2+ transients in the cytosol (A and B) and SR lumen (C and D) obtained at several EGTA AM concentrations in the loading solution. Ca2+ transients were recorded at 37°C and at a pacing frequency of 2 Hz using rhod-2 AM (A and B) and mag-fluo-4 (C and D) as fluorescent Ca2+ indicators. Data were collected separately for each indicator during four independent experiments at different EGTA AM concentrations. Data in B and D are means ± SD. The total number of animals used was 32 animals; N is the number of animals used for each variant. P values are the results of one-way ANOVA (no EGTA AM vs. different concentrations of EGTA AM). E: time course of the restitution of the AP in the presence and absence of EGTA AM. The restitution of the AP was calculated as changes in the amplitude of the spike. Measurements were conducted using the potentiometric dye di-8-ANEPPS on mouse hearts perfused with 7.5 μM blebbistatin to prevent movement artifacts (37°C, 2 Hz). Data were collected during three to four independent experiments for each condition.
Ca2+ Alternans in the Presence of EGTA AM
Cytoplasmic Ca2+ alternans is an abnormality in Ca2+ handling characterized by alternating large and small amplitudes of Ca2+ transients. Alternans constitutes an important phenomenon that is considered to be a sign of cardiac dysfunction (46). Although this type of event has been characterized for both isolated cells and intact preparations of various species (7, 29), there is no evidence that alternans are present in mouse ventricles working at physiological temperatures and heart rates.
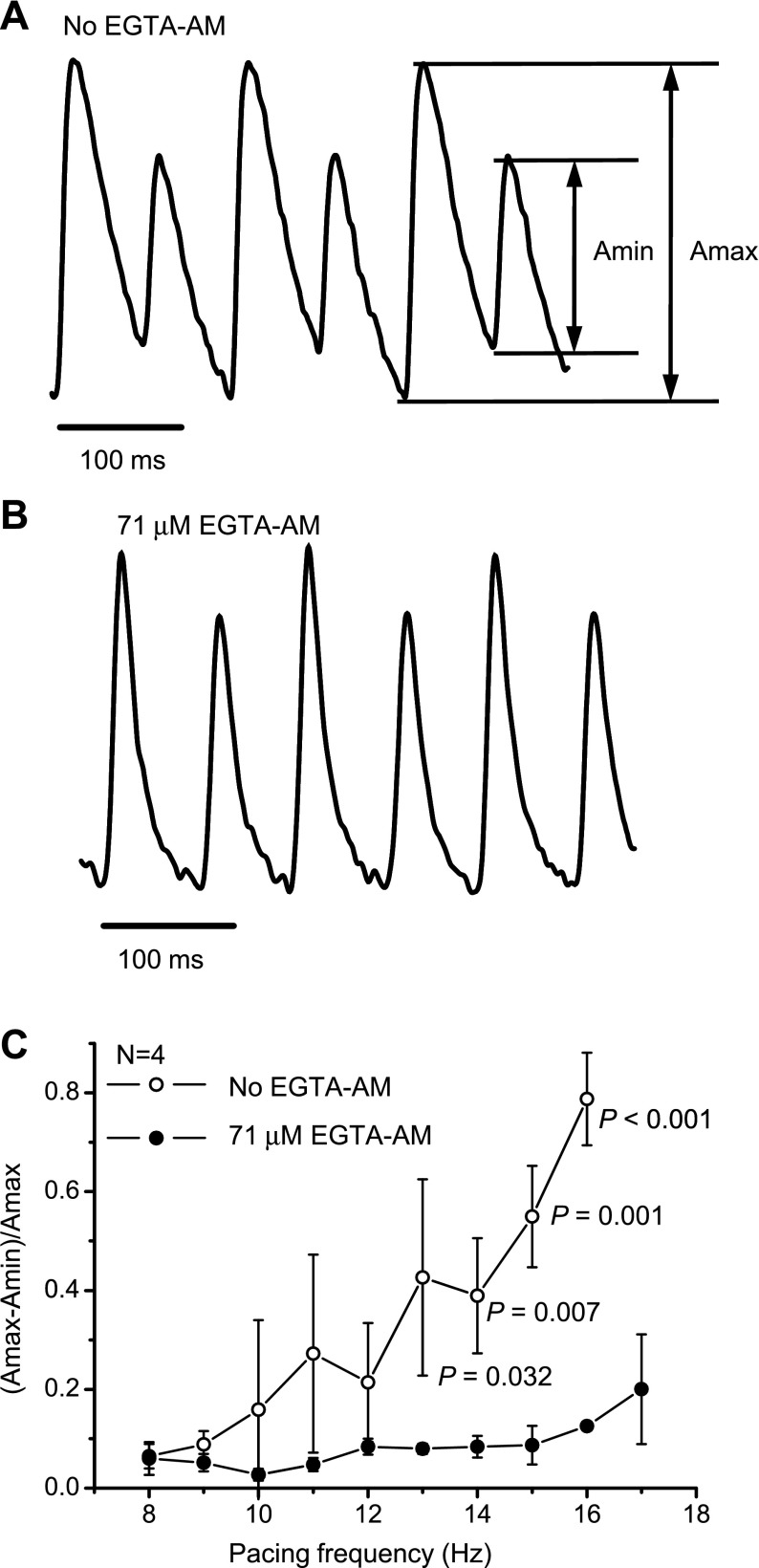
We proposed that the acceleration of the restitution of Ca2+ release in the presence of EGTA AM will affect the frequency dependence of Ca2+ alternans. Examples of Ca2+ alternans are shown in Fig. 12, A and B. Alternans was induced by pacing with a high stimulation frequency, i.e., 14 Hz. Treatment with 71 μM EGTA AM resulted in a diminished amplitude of Ca2+ alternans. The frequency dependence of Ca2+ alternans is shown in Fig. 12C. This plot demonstrates that, for tachycardic heart rates (higher than 13 Hz, 780 beats/min), the presence of EGTA AM dramatically reduced the amplitude of Ca2+ alternans, most likely due to the acceleration of the restitution of the Ca2+ transients. This suggests that the smaller the degree of SR Ca2+ depletion during each beat, the smaller the amplitude of Ca2+ alternans will be.
Fig. 12.
Effect of EGTA AM on Ca2+ alternans. Cytosolic Ca2+ transients were recorded with rhod-2 AM in the absence (A) and presence (B) of 71 μM EGTA AM at 37°C at a heart rate of 14 Hz. C: frequency dependence of Ca2+ alternans. The amplitude of the alternans was calculated as follows: (Amax − Amin)/Amax, where Amax and Amin are the maximum and minimum amplitudes of Ca2+ release, respectively. N is the number of independent experiments for each variant (total number of animals: 8). P values are the results of one-way ANOVA (no EGTA AM vs. 71 μM EGTA AM at the same pacing frequency).
DISCUSSION
Changes in the Luminal SR Ca2+ Content Can Be Detected Using Mag-fluo-4 AM
In this report, we show, for the first time, the regulation of luminal SR Ca2+ dynamics in an intact beating heart. Luminal SR Ca2+ dynamics were assessed with the low-affinity dye mag-fluo-4. After perfusion of the heart with mag-fluo-4 AM, a temperature cycle procedure was used to promote the washout of the dye from the cytosol to differentially stain the lumen of the SR. The results are consistent with data obtained by Shannon et al. (45) with fluo 5N, a low-affinity dye comparable to mag-fluo-4, implying that dyes of this type can be effectively wash out from the cytosol at 37°C. In that study, confocal measurements were used to show that the remaining fluorescence signals originated from the SR. Our experimental results in the presence of caffeine and a high concentration of ryanodine (Fig. 1 and Supplemental Fig. 2) are in agreement with that previous report and confirm the luminal origin of the fluorescence signal measured when the hearts were loaded with a fluorescein-based dye (mag-fluo-4 AM) at room temperature (21°C) and then warmed up to 37°C.
The addition of ryanodine in the Tyrode solution led not only to the inhibition of field-induced changes in the mag-fluo-4 signal but also to a decline in the diastolic level of Ca2+ within the SR (Fig. 1C and Supplemental Fig. 2, B and C). This can be interpreted as a depletion of the Ca2+ store due to ryanodine-induced locking of the Ca2+ release channel in a long-open dwell time subconducting state (1, 14). In accordance with this suggestion, the diastolic level of free Ca2+ in the cytosol, measured with rhod-2, increased when ryanodine was applied (Fig. 1F and Supplemental Fig. 3B). Caffeine-induced changes in the diastolic level also had different directions in the case of mag-fluo-4 and rhod-2 fluorescence (Fig. 1, B and E). All of the above-discussed results allowed us to conclude that the PLFF technique (33) in combination with the fluorescent dye mag-fluo-4 AM can be applied to successfully track luminal SR Ca2+ dynamics in intact beating mouse hearts. Furthermore, the similar temperature dependency of both cytosolic and intra-SR Ca2+ dynamics (Fig. 2) indicates that we obtained an equivalent (and specular) view of the same process from the cytoplasm and lumen of the SR.
Ca2+ Buffering by EGTA Modifies Cytosolic and Luminal SR Ca2+ Dynamics
As shown in Fig. 3, the presence of the Ca2+ buffer EGTA produced a reduction in the amplitude of diastolic and systolic levels of myoplasmic free Ca2+ at the intact heart level. This reduction was similar to the one reported by Diaz et al. (11) for isolated ventricular cardiac myocytes. The decrease in the amplitude was associated with profound changes in the kinetics (Fig. 3D) and restitution of Ca2+ transients measured using the fluorescent dyes located in the cytosol (Fig. 11). Therefore, our data on intact whole hearts are in accordance with those obtained for isolated rat myocytes using photolysis of “caged” EGTA (NP-EGTA) to modify the caffeine response (11).
The kinetics of relaxation of cytosolic Ca2+ transients were also dramatically modified by the presence of the Ca2+ buffer. The initial relaxation phase was significantly accelerated in the presence of EGTA AM. Similar effects have been observed in skeletal muscle (35, 39) and cardiac myocytes (11). These authors concluded that the increase in the rate of relaxation was due to the chelating kinetics of the exogenous buffer. Surprisingly, the presence of EGTA AM revealed a slow component in the relaxation of cytosolic Ca2+ transients (Fig. 3D). This could be explained in part by a slow unbinding of Ca2+ from EGTA or it could be associated with the competition between EGTA and the Ca2+-binding sites on the proteins related to Ca2+ removal processes, for instance, the Na+/Ca2+ exchanger, SERCA, etc. Thus, the differences between the effects of the amplitude of Ca2+ release on cytosolic Ca2+ transients observed by Bers and Berlin (5) and our data obtained with rhod-2 (Fig. 3A) can be explained by the direct influence of EGTA as a Ca2+ buffer competing with the proteins involved in chelating Ca2+ and transporting Ca2+ out of the cytosol.
The data shown in Fig. 4 are in accordance with the possibility that Ca2+ transport mediated by the SERCA pump is impaired in the presence of EGTA AM. Both the diastolic free Ca2+ concentration and amplitude of Ca2+ transients (depletion) inside the SR were significantly reduced. Interestingly, in contrast to what happened in the cytosol, the kinetics of the relaxation of Ca2+ transients in the SR lumen were slower in the presence of EGTA AM (Fig. 7). These results support two hypotheses. First, the buffering effect of EGTA in the SR lumen is minimal because the high free Ca2+ concentration in this compartment (between 100 μM and 2 mM) saturates a high-affinity buffer like EGTA (Kd = 50 nM). Second, the presence of EGTA in the cytosolic compartment of ventricular cells slows down Ca2+ transport into the SR. This reduction of the rate of uptake viewed from inside the SR indicates that the SR is reloading Ca2+ more slowly because the fraction of Ca2+ released from the SR is smaller [intraluminal control of the SERCA pump turnover (53)] and because the free Ca2+ concentration in the cytosol is lower in the presence of EGTA. This latter condition will decrease the probability of the cytosolic Ca2+-binding sites of the SERCA pump to be occupied and can reduce the final turnover of the pump (5). The slowness of replenishment of the SR with Ca2+ is expected to be translated into a reduction of the free Ca2+ inside the SR, which will lead to a reduction of the gain of the Ca2+-release process. Finally, the comparison of the relaxation kinetics of cytosolic and intra-SR transients (Fig. 7) suggests that the speed up induced by EGTA AM in the kinetics of the cytosolic Ca2+ transients is mostly an effect of competition between rhod-2 and EGTA AM and not the effect of EGTA AM on the Ca2+-release process. These results are in complete agreement with predictions obtained by numerically integrating the diffusion-reaction model presented in this report (Fig. 6 and Supplemental Material).
Effect of Ca2+ Buffering on the Restitution of Ca2+ Transients and AP in an Intact Beating Heart
This report describes in detail the restitution of the Ca2+-release process in the epicardial layer of an intact beating murine heart. The refractoriness of the Ca2+-release process is a central phenomenon in whole heart cardiac dynamics. Stroke volume and cardiac efficiency are direct functions of myocardial contractility. The changes in cardiac contractility in response to the increase in heart rate will depend on how fast the Ca2+-release process can be restituted. Additionally, the kinetics of restitution of SR Ca2+ release provide direct experimental evidence on how the heart will respond during an anomalous spontaneous extrasystolic episode.
The time courses of the restitution of cytosolic and luminal SR Ca2+ transients were not significantly different (Fig. 8). However, our experiments revealed that the addition of EGTA AM accelerated the restitution process of the SR more than cytosolic Ca2+ transients (Fig. 11). Additionally, it was demonstrated that the restitutions of the AP as well as the Ca2+ influx via voltage-dependent Ca2+ channels are faster than the restitution of Ca2+ transients generated by the release of Ca2+ from the SR (Figs. 9 and 10). These findings indicate that Ca2+ release from the SR is a rate-limiting process regulating cardiac contractility.
Interestingly, the addition of EGTA AM not only accelerated the restitution process in the SR more than the restitution of cytosolic Ca2+ transients (Figs. 10 and 11) but also led to an acceleration of AP restitution (Fig. 11E). Nevertheless, the AP was still restituted much faster than Ca2+ release from the SR.
There are several reasons why increasing the Ca2+ buffering capacity of the myoplasm with an exogenous buffer can modify myoplasmic Ca2+ dynamics. Those include 1) the ability of an exogenous buffer to bind Ca2+ permeating through L-type Ca2+ channels in the plasma membrane that may prevent these ions from activating the RyR2, 2) the attenuation of the feedthrough mechanism of Ca2+ release from the SR through the same or neighboring RyR2s, and 3) the acceleration of the diffusion of Ca2+ from the dyadic space. Although all these processes can alter luminal SR Ca2+ dynamics because they modify Ca2+ release from this compartment, the possibility that EGTA AM can act through any of these three proposed mechanisms is unlikely due to its very slow association rate constant [5 × 106 M−1·s−1 (13)].
Another possibility is that EGTA AM affects the restitution of Ca2+ transients by modifying the gain of the Ca2+-induced Ca2+-release process. This might happen due to a faster relaxation of myoplasmic Ca2+ transients (reducing Ca2+-induced Ca2+-dependent inactivation of RyR2) and/or due to a lesser degree of beat-to-beat SR Ca2+ depletion. Our results suggest that the most likely mechanism by which an addition of this intracellular exogenous buffer modifies the restitution of intracellular Ca2+ transients is by decreasing the gain of the Ca2+-release process via reducing the free diastolic Ca2+ concentration inside the SR and, consequently, diminishing the relative fraction of beat-to-beat Ca2+ depletion.
Effect of Ca2+ Buffering on the Frequency Dependence of Ca2+ Release
Tachycardia is an increase in the heart rate that can induce several physiopathological responses. One example is T-wave alternans. T-wave alternans is observed as alternating beat-to-beat changes in the T wave of the ECG and constitutes an important arrhythmogenic mechanism that can lead to sudden cardiac death (37). T-wave alternans is likely to increase with tachycardia and is thought to be associated with abnormalities in intracellular Ca2+ handling and/or cellular metabolism (7, 18, 27). Despite years of studies and debates, the mechanistic links among T-wave alternans, tachycardia, intracellular Ca2+ handling, and cellular metabolism are still unclear.
Our data showed that treatment with EGTA AM resulted in the acceleration of the restitution of Ca2+ release (Fig. 11) and attenuation of Ca2+ alternans (Fig. 12). Wan and coworkers (52) were able to demonstrate that endocardial cells isolated from guinea pig ventricles are more prone to show alternans than epicardial cells due to their reduced capacity to uptake Ca2+ into the SR. Here, we show that the relaxation kinetics of intra-SR Ca2+ transients were slower in mouse hearts treated with EGTA AM than in control hearts (Fig. 7). In addition, the intra-SR diastolic Ca2+ concentration declined in the presence of the buffer (Fig. 4). If the development of Ca2+ alternans depends only on the efficiency of Ca2+ uptake, the alternans would be more pronounced after treatment with EGTA AM. However, the effect of EGTA was the opposite (Fig. 12). This implies that another factor, namely, the difference between the diastolic and systolic level of intra-SR Ca2+ (the degree of Ca2+ depletion), may play an important role in the restitution of Ca2+ release and, consequently, in the generation of cardiac alternans. At the molecular level, this could be explained by the dynamic regulation of RyR2 activity through the Ca2+-binding protein CSQ2 (23).
Conclusions
The changes in intracellular Ca2+ dynamics induced by EGTA AM indicate that an exogenous buffer significantly modifies Ca2+ release from the SR. The acceleration of the restitution of Ca2+ transients in the presence of EGTA supports our hypothesis that the amplitude of Ca2+ release (the degree of intra-SR Ca2+ depletion during each heart beat) is a major factor regulating the refractoriness of Ca2+ transients and that the refractoriness defines the frequency dependency of Ca2+ alternans.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-084487.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Guillermo J. Perez, Dr. Julio A. Copello, Dr. Alicia Mattiazzi, Dr. Patricio Velez, and Heather R. Orrell for helpful comments and suggestions on the manuscript.
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Anderson K, Lai FA, Liu QY, Rousseau E, Erickson HP, Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem 264: 1329–1335, 1989. [PubMed] [Google Scholar]
- 2. Antzelevitch C. The Brugada syndrome: diagnostic criteria and cellular mechanisms. Eur Heart J 22: 356–363, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 7: 964–972, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 293: H2024–H2038, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bers DM, Berlin JR. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol Cell Physiol 268: C271–C277, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Bers DM, Guo T. Ca2+ signaling in cardiac ventricular myocytes. Ann NY Acad Sci 1047: 86–98, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Blatter LA, Kockskamper J, Sheehan KA, Zima AV, Huser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol 546: 19–31, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cannell MB, Allen DG. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J 45: 913–925, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caputo C, Bolaños P, Escobar AL. Fast calcium removal during single twitches in amphibian skeletal muscle fibers. J Muscle Res Cell Motil 20: 555–567, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Colquhoun D, Hawkes AG. On the stochastic properties of single ion channels. Proc R Soc Lond B 211: 205–235, 1981. [DOI] [PubMed] [Google Scholar]
- 11. Diaz ME, Trafford AW, Eisner DA. The effect of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophys J 20: 1915–1925, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisner DA, Diaz ME, Li Y, O'Neill SC, Trafford AW. Stability and instability of regulation of intracellular calcium. Exp Physiol 90: 3–12, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Escobar AL, Vélez P, Kim AM, Cifuentes F, Fill M, Vergara JL. Kinetic properties of DM-nitrophen and calcium indicators: rapid transient response to flash photolysis. Pflügers Arch 434: 615–631, 1997. [DOI] [PubMed] [Google Scholar]
- 14. Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Fill M, Zahradníková A, Villalba-Galea CA, Zahradník I, Escobar AL, Györke S. Ryanodine receptor adaptation. J Gen Physiol 116: 873–882, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J 65: 865–881, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hollingworth S, Gee KR, Baylor SM. Low-affinity Ca2+ indicators compared in measurements of skeletal muscle Ca2+ transients. Biophys J 97: 1864–1872, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol 524: 415–422, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo D, Young LH, Patel C, Jiao Z, Wu Y, Liu T, Kowey PR, Yan GX. Calcium-activated chloride current contributes to alternations in left ventricular hypertrophy rabbit. Am J Physiol Heart Circ Physiol 295: H97–H104, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Györke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca2+-induced Ca2+ release in heart. Science 260: 807–809, 1993. [DOI] [PubMed] [Google Scholar]
- 21. Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J 75: 2801–2810, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Györke S, Györke I, Lukyanenko V, Terentyev D, Viatchenko-Karpinski S, Wiesner TF. Regulation of sarcoplasmic reticulum calcium release by lumenal calcium in cardiac muscle. Front Biosci 7: 1454–1463, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Györke I, Hester N, Jones LR, Györke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to lumenal calcium. Biophys J 86: 2121–2128, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Györke S, Terentyev D. Modulation of ryanodine receptor by lumenal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res 77: 245–255, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Györke S, Vélez P, Suárez-Isla B, Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+. Biophys J 66: 1879–1886, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knollman BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodeling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol 525: 483–498, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kockskamper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol 545: 65–79, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai FA, Anderson K, Rousseau E, Liu QY, Meissner G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun 151: 441–449, 1988. [DOI] [PubMed] [Google Scholar]
- 29. Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res 92: 668–675, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Laver DR. Regulation of ryanodine receptors from skeletal and cardiac muscle during rest and excitation. Clin Exp Pharmacol Physiol 33: 1107–1113, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Li GR, Du XL, Siow YL, Karmin O, Tse HF, Lau CP. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc Res 58: 89–98, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res 62: 116–126, 1988. [DOI] [PubMed] [Google Scholar]
- 33. Mejía-Alvarez R, Manno C, Villalba-Galea CA, del Valle Fernández L, Costa R, Fill M, Gharbi T, Escobar AL. Pulsed local-field fluorescence microscopy: a new approach for measuring cellular signals in the beating heart. Pflügers Arch 445: 747–758, 2003. [DOI] [PubMed] [Google Scholar]
- 34. Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol 12: 88–118, 1965. [DOI] [PubMed] [Google Scholar]
- 35. Novo D, DiFranco M, Vergara JL. Comparison between the predictions of diffusion-reaction models and localized Ca2+ transients in amphibian skeletal muscle fibers. Biophys J 5: 1080–1097, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pessah IN, Waterhouse AL, Casida JE. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun 128: 449–456, 1985. [DOI] [PubMed] [Google Scholar]
- 37. Pham Q, Quan KJ, Rosenbaum DS. T-wave alternans: marker, mechanism, and methodology for predicting sudden cardiac death. J Electrocardiol 36: 75–81, 2003. [DOI] [PubMed] [Google Scholar]
- 38. Picht E, de Santiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum Ca content fluctuations. Circ Res 99: 740–748, 2006. [DOI] [PubMed] [Google Scholar]
- 39. Pizarro G, Rios E. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev 71: 849–908, 1991. [DOI] [PubMed] [Google Scholar]
- 40. Pizarro G, Csernoch L, Uribe I, Rodriguez M, Rios E. The relationship between Qy and Ca release from the sarcoplasmic reticulum in skeletal muscle. J Gen Physiol 97: 913–947, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puglisi JL, Bers DM. LabHEART: an interactive computer model of rabbit ventricular myocyte ion channels and Ca transport. Am J Physiol Cell Physiol 281: C2049–C2060, 2001. [DOI] [PubMed] [Google Scholar]
- 42. Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Lumenal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol 131: 325–334, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosales RA, Fill M, Escobar AL. Calcium regulation of single ryanodine receptor channel gating analyzed using HMM/MCMC statistical methods. J Gen Physiol 121: 533–553, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sato Y, Ferguson DG, Sako H, Dorn GW, II, Kadambi VJ, Yatani A, Hoit BD, Walsh RA, Kranias EG. Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J Biol Chem 273: 28470–28477, 1998. [DOI] [PubMed] [Google Scholar]
- 45. Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res 93: 40–45, 2003. [DOI] [PubMed] [Google Scholar]
- 46. Shimizu W, Antzelevitch C. Cellular and ionic basis for T-wave alternans under long-QT conditions. Circulation 99: 1499–1507, 1999. [DOI] [PubMed] [Google Scholar]
- 47. Stern MD. Buffering calcium in the vicinity of a channel pore. Cell Calcium 13: 183–192, 1992. [DOI] [PubMed] [Google Scholar]
- 48. Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Györke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res 91: 414–420, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Tikhonov AN, Samrskii AA. Equations of Mathematical Physics. New York: McMillan, 1963. [Google Scholar]
- 50. Tinker A, Sutko JL, Ruest L, Deslongchamps P, Welch W, Airey JA, Gerzon K, Bidasee KR, Besch HR, Jr, Williams AJ. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys J 70: 2110–2119, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Villalba-Galea CA, Suarez-Isla BA, Fill M, Escobar AL. Kinetic model for ryanodine receptor adaptation (Abstract). Biophys J 74: A58, 1998. [Google Scholar]
- 52. Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol 39: 419–428, 2005. [DOI] [PubMed] [Google Scholar]
- 53. Yu X, Inesi G. Variable stoichiometric efficiency of Ca2+ and Sr2+ transport by the sarcoplasmic reticulum ATPase. J Biol Chem 270: 4361–4367, 1995. [DOI] [PubMed] [Google Scholar]
- 54. Zoghbi ME, Bolaños P, Villalba-Galea C, Marcano A, Hernández E, Fill M, Escobar AL. Spatial Ca2+ distribution in contracting skeletal and cardiac muscle cells. Biophys J 78: 164–173, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.