Abstract
Endothelial properties are affected by mechanical stresses. Several studies have shown that an acute application of shear stress increases the permeability of endothelial monolayers in culture. We investigated whether more prolonged application of shear has the opposite effect. Porcine aortic endothelial cells were cultured on Transwell filters to assess monolayer permeability to albumin. The medium above the cells was swirled using an orbital shaker; resultant shears were computed to lie within the physiological range. Acute application of shear increased permeability, but chronic application reduced it. The effect of chronic but not acute shear was reversed by inhibiting nitric oxide (NO) synthesis. The effect of chronic shear was also reversed by inhibiting phosphatidylinositol 3-OH kinase (PI3K) and soluble guanylyl cyclase. None of these interventions affected permeability under static conditions, and inhibition of cyclooxygenase was without effect. Chronic shear decreased mitosis rates by a fraction comparable to the reduction in permeability, but this effect was not reversed by inhibiting NO synthesis. We conclude that chronic application of shear stress reduces endothelial permeability to macromolecules by a PI3K-NO-cGMP-dependent mechanism. Since atherosclerosis can be triggered by excessive entry of plasma macromolecules into the arterial wall, the phenomenon may help explain the atheroprotective effects of shear and NO.
Keywords: endothelium, mass transport, hemodynamics, nitric oxide, mitosis
the vascular endothelium presents a significant barrier to the exchange of water and solutes between blood and tissue. Transendothelial transport is therefore critical to the normal functioning of tissues, and imbalances in such transport have been implicated in a large number of pathological states. Endothelial cells (ECs) are exposed to several types of mechanical stress in vivo; they are mechanosensitive and alter their properties in response to these stresses (9). Much attention has focused on the influence of hemodynamic wall shear stress (the frictional force per unit area exerted parallel to the endothelial surface by the flow of blood) on transendothelial transport. This topic is of interest because shear stress varies substantially between species, between different types of vessels, and between regions within single vessels (8, 20, 51) and, in particular, because both shear stress (5, 26) and transendothelial transport (50) play key roles in the development of atherosclerosis, the principle underlying cause of cardiovascular morbidity and mortality.
Endothelial transport properties are difficult to study in vivo since subendothelial tissues are always present and influence the exchange of water and solutes. It can also be difficult to define surface areas, potential gradients for transport, and the mechanical forces experienced by the cells, and it is impossible to use expensive or toxic reagents to modify signaling pathways or putative transport routes. Starting more than 25 yr ago (4, 24), many researchers have attempted to solve these problems by studying transport across the endothelium cultured in vitro. A number of investigations have used such techniques to show that shear stress increases the transport of macromolecules, ions, and water across endothelial monolayers (6, 23, 38, 42). However, these studies applied shear stress acutely, whereas shear occurs chronically in vivo (although its magnitude may vary with time). In the present study, we investigated whether the application of shear stress for longer periods than used in most previous studies has a different effect on transendothelial transport, and we examined the mechanisms involved.
METHODS
Isolation and culture of ECs.
Descending thoracic aortas of Landrace Cross pigs, aged 4–6 mo and weighing ∼80 kg, were obtained immediately after slaughter (Fresh Tissue Supplies, East Sussex, UK) and stored for 24 h in HBSS containing penicillin (200 U/ml), streptomycin (200 μg/ml), amphotericin (5 μg/ml), and gentamycin (100 μg/ml). Pig aortic ECs (PAECs) were isolated from them by collagenase digestion using the methods of Bogle et al. (3). Briefly, vessels were trimmed of fat and connective tissue, and intercostal branches were ligated. The proximal end of the aortic segment was cannulated, and the vessel was flushed with PBS. The distal end of the vessel was then clamped, and the aorta was filled with 0.2 mg/ml type II collagenase (Sigma). After an incubation at 37°C for 10 min, the aorta was gently massaged to loosen ECs, and the collagenase solution was collected and centrifuged at 1,000 rpm for 10 min. Cells were resuspended in DMEM supplemented with FCS [10% (vol/vol)], newborn calf serum [10% (vol/vol)], l-glutamine (5 mM), and EC growth factor (5 μg/ml, Sigma). Cells were plated into flasks (Nunc) coated with 1% gelatin. Medium was replaced every 2–3 days. When confluent, cells were passaged by a brief exposure to trypsin-EDTA (0.1:0.02%).
At the second passage, cells were seeded in stages onto Transwell filters (polyester, 0.4-μm pores, 24-mm membrane diameter, Costar) that had been coated with fibronectin (50 μg/ml) and inserted into six-well culture plates. Cells were seeded at a low density (104 cells/cm2) and cultured for a further 7–9 days to allow endothelial junctions to become established (14). Culture medium was changed every 2–3 days. One filter in each plate was kept free of cells and used to correct for the permeability of the filter itself. The permeability of the filter plus any extracellular matrix secreted by the ECs after they had been cultured for 9 days was determined by removing the cells with 1 mM EGTA in Ca2+/Mg2+-free PBS for 30 min.
Determination of EC purity.
EC purity was assessed from the internalization of acetyl-LDL. DiI-labeled acetyl-LDL (Molecular Probes) was diluted in DMEM supplemented with 10% serum to a final concentration of 10 μg/ml, added to the upper compartment of the Transwell, and incubated at 37°C for 4 h. Wells were then washed with PBS for 10 min three times, and the DiI fluorescence was imaged with a confocal microscope (Leica SP5, with excitation and emission at 561 and 575–610 nm, respectively).
Contamination of endothelial monolayers with vascular smooth muscle cells was assessed by staining with anti-smooth muscle α-actin monoclonal antibodies (A5228, Sigma). Monolayers were fixed in paraformaldehyde (4%) for 15 min, permeabilized with Triton X-100 (0.1%) for 3 min, and blocked with 1% bovine plasma albumin (BPA) in PBS at room temperature for 30 min before being incubated for 2 h at room temperature with the antibody diluted 1:500 in PBS containing 1% BPA. Wells were washed with PBS three times for 10 min before an incubation for 1 h at room temperature with Cy3-labeled donkey anti-mouse antibody (Jackson) diluted 1:500 in PBS containing 1% BPA. Wells were washed extensively with PBS before being examined by confocal microscopy (excitation and emission at 514 and 577–615 nm, respectively).
Application of shear stress.
Six-well plates containing the Transwell filters were placed on the platform of an orbital shaker (POS-300, Grant Instruments) housed in the incubator. The orbit of the platform was circular with a radius of 5 mm and a rotation rate set to 150 rpm; this movement induced a swirling motion of the medium over the cells. The monolayers had a shear stress applied to them in this way for 6–8 days, starting 24 h after cells had been seeded (at which point they were 80–100% confluent) and continuing to the end of the 7- to 9-day culture period [chronic shear stress (CSS)], or for 1 h at the end of the 7- to 9-day culture period [acute shear stress (ASS)]. Static controls, consisting of monolayers placed in the incubator for 7–9 days but not on the shaker platform, were included in each experiment.
Computation of shear stress.
The movement of the medium was modelled by solving three-dimensional Navier-Stokes equations with commercial computational fluid dynamics (CFD) software (Fluent 6.2). A three-dimensional cylindrical rendering of the well plate was created in the preprocessor, GAMBIT, with dimensions and orbital parameters that mimicked the actual Transwell filters and experimental conditions: diameter, 24 mm; initial fluid height, 3.3 mm; orbital radius, 5 mm; and rotation rate, 150 rpm. A mesh with 307,320 hexahedral computational cells was applied to the volume. The modelling technique, convergence criteria, grid optimization, and time needed to reach steady state for the transient solution have been previously described (2). Shear stress at the base of the cylinder was derived from the computed fluid motion.
Preparation of the fluorescent tracer.
Sulforhodamine B acid chloride (Sigma) was dissolved in acetone and added to 20 times its own weight of fatty acid-free BSA (fraction V, Sigma) at 2% (wt/vol) in carbonate buffer (0.33 mol/l, pH 9) at 4°C (16). The conjugate was purified of free dye on a gel filtration column (Sephadex G-25), frozen dropwise in liquid nitrogen, freeze dried, and stored at −20°C. Before use, the conjugate was reconstituted in buffer and purified of any remaining free dye by an incubation for 1 h with neutralized activated charcoal (0.35 g/g protein) on a rotating platform. Charcoal was removed by centrifugation (twice at 3,000 rpm for 30 min) followed by filtration through a 0.2-μm filter.
The removal of free dye was assessed by ultrafiltration of the conjugate through a centrifugal filter with a 10-kDa molecular weight cutoff (Amicon, Ultra-4, Millipore) at 3,000 rpm for 30 min. The endotoxin content of the conjugate was determined using the limulus amebocyte lysate assay (E-TOXATE kit, Sigma).
Measurement of endothelial monolayer permeability.
Permeability was measured 7–9 days after cells had been seeded, at which time the monolayers were fully confluent and free from overgrowth. The serum content of the culture medium was reduced from 20% to 10% 24 h before the measurement of permeability, and the solution in both the upper and lower compartments was replaced with serum-supplemented DMEM containing 1% BPA 1 h before measurement. For the measurement itself, the solution in the upper compartment of the Transwell was replaced with serum-supplemented DMEM containing 1% BPA and trace quantities (1 mg/ml) of rhodamine-labeled albumin. Samples were taken from the bottom compartment of the Transwell after 1 h, and tracer fluorescence in them was measured using a fluorimeter (model 6285, Jenway) with excitation and emission wavelengths of 570 and 600 nm, respectively. The concentration of rhodamine albumin in each sample was determined from a standard curve.
The permeability (P) of the monolayer may be defined as the rate of diffusion or transcytosis of solute (Js; in this case, rhodamine-labeled albumin) across the unit area of the membrane (S) per unit concentration difference across the membrane (ΔC) as follows:
Js was determined from the concentration of rhodamine albumin in the lower compartment (CLC; in μg/ml), the volume of the lower compartment (VLC; in ml), and the time after the addition of the tracer to the upper compartment (t; in s) as follows:
Permeability was derived from Js using the area of the Transwell filter (A) and the initial concentration of rhodamine-labeled albumin in the upper compartment (CUC) as follows:
Transport rates were sufficiently low that it was unnecessary to correct for changes in ΔC during the course of the experiment. Results were corrected for the permeability of the cell-free filter by subtracting the resistance of the filter from the resistance of the monolayers plus filters in the same plate. (Resistances were calculated as reciprocals of permeability.)
Assessment of rates of EC mitosis.
PAECs were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 for 3 min. Cells were washed in PBS for 10 min twice and then stained with Harris' hematoxylin solution for 30 s. Hematoxylin was removed, and cells were washed in PBS for 10 min twice. Cells were visualized en face by phase-contrast microscopy. Mitotic figures were identified in randomly selected fields of view according to the criteria of Lin et al. (31).
Statistics.
At least three independent cell isolations were conducted for each experiment. Data are expressed as means ± SE. Statistical significance was assessed by Student's unpaired t-test with a criterion of P < 0.05.
RESULTS
Characterization of cells.
DiI-labeled acetyl-LDL was taken up by 99.8% of the cells on Transwells (n = 3), indicating essentially pure EC monolayers. Staining with anti-smooth muscle actin demonstrated that the contamination of monolayers with vascular smooth muscle cells was <1 in every 104 ECs.
Characterization of shear stress.
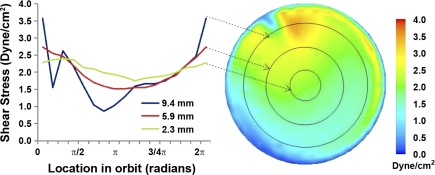
The CFD solution showed that a wave swirls around the Transwell as a result of the motion of the shaker platform. Fluid behavior was such that cells at all locations remain covered by medium throughout the entire orbit. Figure 1 shows a map of the shear stresses acting on the entire monolayer at one instant in time. The periodicity of the flow dictates that these steady-state contours remain the same as the map rotates synchronously with the orbiting motion of the well. The region of maximum shear stress, appearing as a small brown-orange area, coincides with the leading edge of the travelling wave. The drop in shear near the side wall of the well (outer annular blue region of the contour) arises from the no-slip boundary condition imposed in the solution.
Fig. 1.
Right: map of instantaneous shear stress magnitude acting on cells in the base of the Transwell. Left: shear stress magnitude for different radii throughout one orbit (2π radians). The arrows indicate the radial position for each curve.
Depending on location and time, cells experience shear stress magnitudes from 0.2 to 3.6 dyn/cm2. To illustrate the spatial and temporal variations, Fig. 1 shows oscillating resultant shear stress magnitudes at the bottom of the Transwell at radii of 2.3, 5.9, and 9.4 mm during one complete orbit. Near the center of the Transwell (at 2.3 mm), magnitudes fluctuate between 1.8 and 2.4 dyn/cm2. The amplitude of oscillation increases with increasing radius, reaching a peak in amplitude as well as magnitude near the periphery. Thus, at 5.9 mm, magnitudes fluctuate between 1.5 and 2.7 dyn/cm2, whereas at 9.4 mm they fluctuate between 0.9 and 3.6 dyn/cm2. The radial increase was reversed in the narrow band close to the side wall, where the response to the viscous layer on the side wall occured. The spatially and temporally averaged value of the entire contour is 1.82 dyn/cm2.
Characterization of the tracer.
Ultrafiltration of the tracer demonstrated that >99.9% of the dye was bound to albumin. The tracer was found to be essentially free of endotoxin (<0.015 EU/ml). Previous studies (17, 49) have shown that the tracer is stable in vitro with similar physical, chemical, and biological properties to the unlabeled protein.
Permeability of filters and the extracellular matrix.
The permeability of Transwell filters without cells averaged 6.1 ± 0.66 × 10−6 cm/s (n = 3). The extracellular matrix secreted by the monolayers over a period of 9 days did not add significantly to this value; after removal of the cells with EGTA, permeability was 5.2 ± 0.51 × 10−6 cm/s (n = 3, P = 0.35).
Effects of ASS and CSS on endothelial permeability.
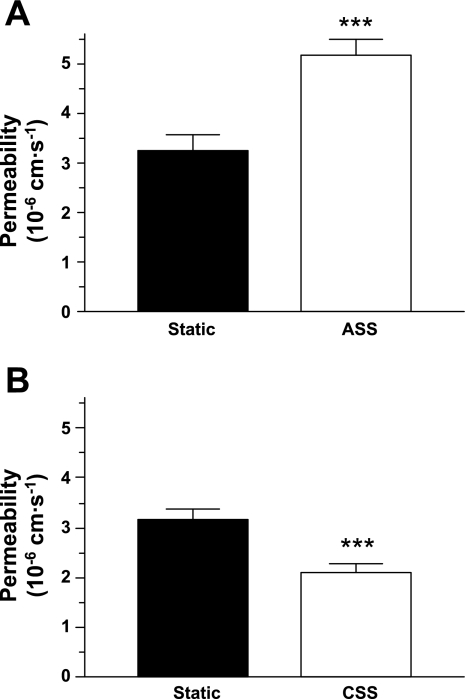
The permeability of endothelial monolayers was increased by ASS and decreased by CSS compared with unsheared controls (Fig. 2).
Fig. 2.
Permeability to rhodamine-labeled albumin of endothelial monolayers was increased by an acute exposure to shear stress (ASS; P = 0.003; A) and decreased by a chronic exposure to shear stress (CSS; P = 0.0004; B) compared with static conditions (n = 16–28). ***P < 0.005.
Role of nitric oxide.
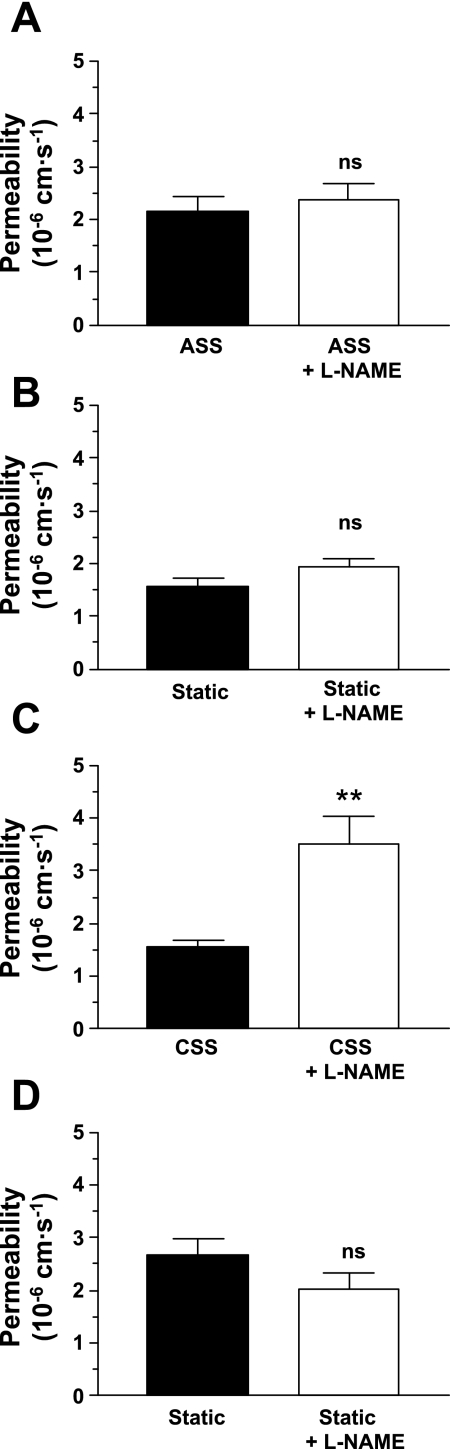
Inhibition of nitric oxide (NO) production by the addition of the nonmetabolized l-arginine analog Nω-nitro-l-arginine methyl ester (l-NAME; 500 μM) for 24 h before the addition of rhodamine-labeled albumin to the upper compartment did not significantly influence the permeability of monolayers in static culture or of monolayers exposed acutely to shear stress but increased the permeability of chronically sheared monolayers by greater than twofold, more than reversing the effect of CSS (Fig. 3).
Fig. 3.
Pretreatment with the endothelial nitric oxide (NO) synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 500 μM) for 24 h before the addition of the tracer did not significantly alter the permeability of monolayers exposed to ASS (P = 0.08; A) or of the corresponding static controls (P = 0.58; B). It significantly increased the permeability of monolayers exposed to CSS (P = 0.002; C), reversing the influence of CSS, but again did not affect the corresponding static controls (P = 0.15; D). As in Fig 2, ASS increased permeability and CSS decreased it relative to static controls (P = 0.04 and 0.002, one-tailed). n = 18–25. ns, Not significant. **P < 0.01.
Roles of phosphatidylinositol 3-OH kinase and soluble guanylyl cyclase.
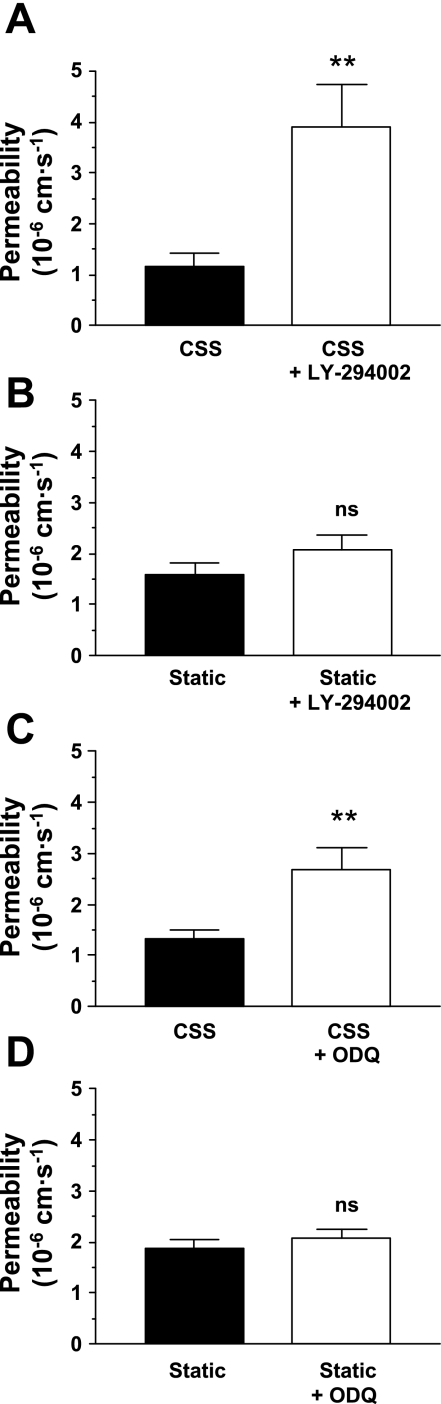
The involvement of phosphatidylinositol 3-OH kinase (PI3K) and soluble guanylyl cyclase (sGC) in the NO-mediated effect of CSS were investigated by the addition of LY-294002 (10 μM, Sigma), a PI3K inhibitor, or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 μM, Sigma), an inhibitor of NO binding to the heme site of sGC, 24 h before the addition of rhodamine-labeled albumin. Both inhibitors at least doubled the permeability of chronically sheared monolayers but were without significant effect on the permeability of unsheared cells (Fig. 4).
Fig. 4.
The phosphatidylinositol 3-OH-kinase inhibitor LY-294002 increased the permeability of endothelial monolayers exposed to CSS (P = 0.003; A) but had no effect on the permeability of unsheared (static) controls (P = 0.19; B). Similarly, the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) increased the permeability of endothelial monolayers exposed to CSS (P = 0.006; C) but had no effect on the permeability of unsheared (static) controls (P = 0.45; D). As in Fig 2, CSS decreased permeability relative to static controls (P = 0.009, one-tailed). n = 12–19. **P < 0.01.
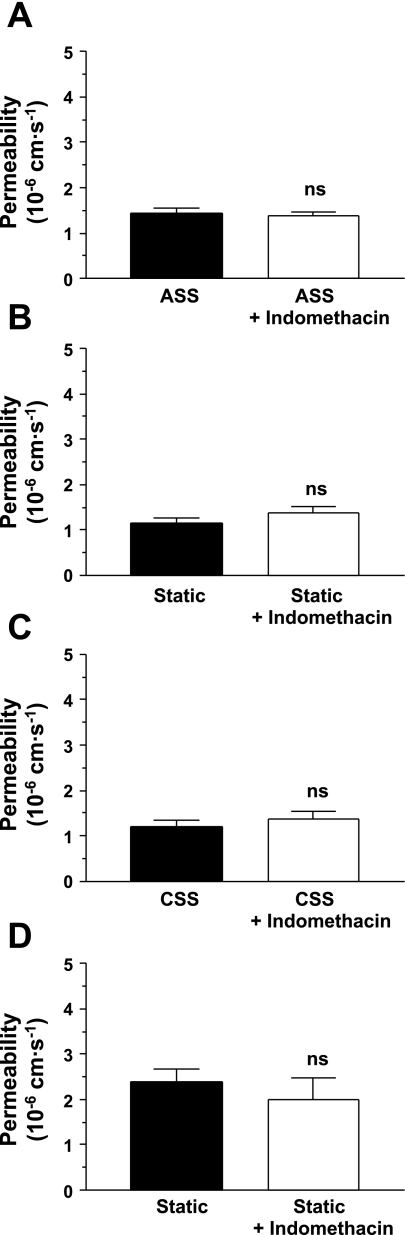
Roles of cyclooxygenase products.
The addition of the nonspecific cyclooxygenase (COX) inhibitor indomethacin (10 μM) for 24 h before the addition of rhodamine-labeled albumin did not have a significant effect on the permeability of monolayers cultured under static conditions or exposed acutely or chronically to shear stress (Fig. 5).
Fig. 5.
The cyclooxygenase inhibitor indomethacin did not alter the permeability of monolayers exposed to ASS (P = 0.60; A) or the corresponding unsheared (static) controls (P = 0.19; B). Similarly, it did not alter the permeability of monolayers exposed to CSS (P = 0.48; C) or the corresponding unsheared (static) controls (P = 0.46; D). As in Fig 2, ASS increased permeability and CSS decreased it, relative to static controls (P = 0.03 and 0.0001, one-tailed). n = 11–20.
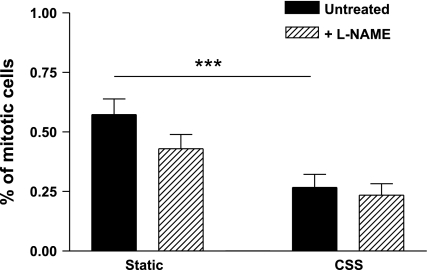
Effects of CSS on PAEC proliferation.
In chronically sheared ECs, the percentage of replicating cells was significantly reduced compared with static controls (Fig. 6). Pretreament with l-NAME (500 μM) for 24 h did not significantly affect the proliferation rate under either condition.
Fig. 6.
Rates of mitosis were significantly lower in wells exposed to CSS than in static controls (P = 0.0008, n = 9). Inhibition of NO synthesis by 24-h pretreatment with l-NAME had no effect in either group (P = 0.11 and 0.66). n = 9. ***P < 0.005.
DISCUSSION
The main finding of the present study was that although acute (1 h) application of shear stress increases the permeability of endothelial monolayers to albumin, chronic (1 wk) application of shear has the opposite effect. Many previous studies have demonstrated acute effects of shear on ECs, but investigations of chronic effects have been rare, reflecting the technical difficulty of applying defined fluid flows to large numbers of cells over many days under sterile conditions. These difficulties can be overcome by placing standard culture dishes on an orbital shaker in a cell culture incubator (13, 25, 29, 37, 46). This method gives high throughput, since culture plates may be stacked on top of one another, and cells can be cultured for at least as long as under static conditions.
Although the shear stress applied to the cells can be defined, doing so is more complex than in the case of parallel-plate flow chambers. An analytical expression has been derived (21) that estimates a constant scalar value of shear for the entire surface of the Transwell; technically, it is valid only for an infinitely wide plate with no side wall effects. Another approach is to measure the movement of particles in the fluid by optical methods (13). However, CFD methods are ideally suited to the problem since uncertainty concerning the geometry and other boundary conditions, which limits accuracy when applying such methods to in vivo flows, is negligible. (The variables are orbital radius, angular velocity, well diameter, and fluid depth; a separate solution is required for each combination of values.) The presence of a free surface adds complexity, but the problem is still solvable with commercial software (2).
The orbital movement of the platform, and hence of the dish, induces a swirling motion of the fluid within each well that is independent of the location of the well on the platform. A wave is generated that rotates around the well; the period of rotation of the wave is the same at all radial locations. The speed of the wave is therefore greater at the periphery of the well than at its center, but the height of the wave is also greater, which cancels some of the radial increase in shear stress on the bottom of the well that would otherwise occur. Postprocessing of the CFD solution shows that the time-averaged shear stress is relatively constant across the Transwell, but the difference between minimum and maximum shear is higher in a band near the perimeter. (There is also a viscous effect at the wall of the well, as also occurs in parallel-plate flow chambers, but the number of cells affected by this is negligible.) For the present study, this disadvantage as well as the restrictions the system imposes on the number of possible flow profiles are outweighed by the benefits of high throughput and prolonged exposure.
Cells were exposed to a time-averaged shear stress of ∼2 dyne/cm2. Recent experimental (20) and theoretical (51) studies and a literature review (8) have shown that aortic wall shear stress is not always at the commonly assumed value of 10–15 dyn/cm2 but strongly depends on body size. We are not aware of any measurements of shear stress in the pig, but values for the abdominal aorta of human subjects similar in weight to the pigs used here are in the range of 1–5 dyn/cm2 (7, 44). The frequency of the oscillation in shear was faster than the pig heart rate, 2.5 vs. 1.0–1.5 Hz (22), but in the aorta and other large arteries there are two periods of forward flow during each cardiac cycle (35). Therefore, the flow conditions imposed in vitro are broadly similar to those experienced by PAECs in vivo, although the details of the flow waveform are of course not entirely accurate.
Shear stress can rapidly induce the production of NO by causing the phosphorylation of endothelial NO synthase (eNOS or NOS III) through a PI3K-dependent pathway (14). In the longer term, shear also upregulates eNOS expression (39). Autocrine effects of NO can be mediated by its activation of sGC and consequent increase in endothelial cGMP (41). Similarly, shear induces the production of prostaglandins such as PGI2 by COX (18) and, in the longer term, upregulates COX-1 and COX-2 expression (45, 36). Both NO and prostaglandins alter endothelial permeability in vivo (27, 33). In the present study, the permeability-enhancing effects of ASS were not altered by inhibiting either eNOS or COX. [ASS also increases convective transport of water across cultured endothelium, and this effect is blocked by inhibiting eNOS (6); presumably, different transport pathways are involved.] The permeability-reducing effects of chronic exposure to shear were abrogated by inhibition of eNOS but not by inhibition of COX. They were similarly affected by inhibition of PI3K and by preventing NO binding to sGC. No effects of such inhibition were seen in static culture. The absence of an effect of l-NAME in static or ASS experiments suggests that either NO production was too low to influence permeability or, in the ASS case, that the increase in NO was of too short a duration or was overridden by other signaling pathways. We were not able to determine NO concentrations in our cultures, but it has previously been shown that PAECs cultured by similar methods to those we used produce ∼0.6 pmol·cm−2·min−1 in static culture and that this is increased severalfold by shear stress (1). There is conflicting evidence regarding the role of cGMP in modulating vascular permeability, but it appears to maintain the barrier function of the endothelium from large vessels (15, 43). It may do so directly, via activation of PKG, or indirectly, by inhibiting phosphodiesterases and consequently increasing intracellular cAMP (15).
In vivo, mitosis leads to foci of locally enhanced permeability that account for a significant proportion of total macromolecule entry into the arterial wall (10, 11, 30). In the present study, mitosis rates were 0.57 ± 0.07% in static culture, which is an order of magnitude higher than the 0.034% reported in vivo (30). This elevation may help explain the higher permeability observed in vitro, which occurs despite the absence of the pressure-driven convection that contributes to albumin transport in vivo. Mitosis rates were decreased by chronic application of shear stress to 0.27 ± 0.06%. A similar effect of laminar shear stress has previously been reported (19, 28, 32, 48). [Turbulent flow or very high shears increase proliferation (12, 34) but such flows are not relevant to the present study.] The percent reduction in mitosis we obtained was close to the percent reduction in permeability (53% and 34%, respectively). However, inhibition of eNOS did not reverse the antiproliferative effect of chronic shear. The effects of NO on endothelial proliferation have been controversial (19); Gooch et al. (19) similarly found that the antiproliferative effect of shear on the aortic endothelium was not reversed by inhibition of eNOS. Our result makes it unlikely that the effects of chronic shear on permeability are mediated by its effects on mitosis. There are many other possibilities, including effects of shear on vesicular transport, apoptosis, and expression of tight and junctional proteins, that remain to be investigated.
Finally, we speculate on the relevance of the results to atherogenesis. Excessive entry of macromolecules, particularly LDL, into the arterial wall is a plausible initiating event in the disease (50). The disease has also been associated with low time-averaged wall shear stress and with flow reversal during the cardiac cycle (5, 26). A link between these two is provided by the present observation that chronic exposure to unidirectional shear stress of a physiologically normal magnitude lowers endothelial permeability. [Acute application of shear, which increased permeability, may simulate proatherogenic shear stress patterns, to which ECs might never adapt (47)]. The role of NO in mediating the effects of chronic shear on permeability may help explain the atheroprotective effect of this molecule.
GRANTS
This work was funded by the Biotechnology and Biological Sciences Research Council.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Berkels R, Purol-Schnabel S, Roesen R. A new method to measure nitrate/nitrite with a NO-sensitive electrode. J Appl Physiol 90: 317–320, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Berson RE, Purcell MR, Sharp MK. Computationally determined shear on cells grown in orbiting culture dishes. Adv Exp Med Biol 614: 189–198, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bogle RG, Baydoun AR, Pearson JD, Mann GE. Regulation of l-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J Physiol 490: 229–241, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman PD, Ennis SR, Rarey KE, Betz AL, Goldstein GW. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann Neurol 14: 396–402, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci 177: 109–159, 1971 [DOI] [PubMed] [Google Scholar]
- 6.Chang YS, Yaccino JA, Lakshminarayanan S, Frangos JA, Tarbell JM. Shear-induced increase in hydraulic conductivity in endothelial cells is mediated by a nitric oxide-dependent mechanism. Arterioscler Thromb Vasc Biol 20: 35–42, 2000 [PubMed] [Google Scholar]
- 7.Cheng CP, Herfkens RJ, Taylor CA. Comparison of abdominal aortic hemodynamics between men and women at rest and during lower limb exercise. J Vasc Surg 37: 118–123, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NT, van Haperen R, Wentzel JJ, Gijsen F, van der Steen AF, de Crom R, Krams R. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195: 225–235, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chien S, Lin SJ, Weinbaum S, Lee MM, Jan KM. The role of arterial endothelial cell mitosis in macromolecular permeability. Adv Exp Med Biol 242: 59–73, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Chuang PT, Cheng HJ, Lin SJ, Jan KM, Lee MM, Chien S. Macromolecular transport across arterial and venous endothelium in rats. Studies with Evans blue-albumin and horseradish peroxidase. Arteriosclerosis 10: 188–197, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA 83: 2114–2117, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dardik A, Chen L, Frattini J, Asada H, Aziz F, Kudo FA, Sumpio BE. Differential effects of orbital and laminar shear stress on endothelial cells. J Vasc Surg 41: 869–880, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Draijer R, Atsma DE, van der Laarse A, van Hinsbergh VWM. cGMP and nitric oxide modulate thrombin-induced endothelial permeability: regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res 76: 199–208, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Fothergill JE. Fluorochromes and their conjugation with proteins. In: Fluorescent Protein Tracing (2nd ed.), edited by Nairn RC. Edinburgh: Livingstone, 1964, p. 4–33 [Google Scholar]
- 17.Fothergill JE. Properties of conjugated proteins. In: Fluorescent Protein Tracing (2nd ed.), edited by Nairn RC. Edinburgh: Livingstone, 1964, p. 34–59 [Google Scholar]
- 18.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science 227: 1477–1479, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Gooch KJ, Dangler CA, Frangos JA. Exogenous, basal, and flow-induced nitric oxide production and endothelial cell proliferation. J Cell Physiol 171: 252–258, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Greve JM, Les AS, Tang BT, Draney Blomme MT, Wilson NM, Dalman RL, Pelc NJ, Taylor CA. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am J Physiol Heart Circ Physiol 291: H1700–H1708, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hubbe MA. Adhesion and detachment of biological cells in vitro. Prog Surface Sci 11: 65–138, 1981 [Google Scholar]
- 22.Jackson PG, Cockcroft PD. Handbook of Pig Medicine Edinburgh: Saunders, 2007, p.7 [Google Scholar]
- 23.Jo H, Dull RO, Hollis TM, Tarbell JM. Endothelial albumin permeability is shear dependent, time dependent, and reversible. Am J Physiol Heart Circ Physiol 260: H1992–H1996, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Knox P. Filtration of plasma-proteins by human-endothelial cell-cultures or their extracellular-matrix (Abstract). J Physiol 340: 6–7, 1983 [Google Scholar]
- 25.Kraiss LW, Weyrich AS, Alto NM, Dixon DA, Ennis TM, Modur V, McIntyre TM, Prescott SM, Zimmerman GA. Fluid flow activates a regulator of translation, p70/p85 S6 kinase, in human endothelial cells. Am J Physiol Heart Circ Physiol 278: H1537–H1544, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5: 293–302, 1985 [DOI] [PubMed] [Google Scholar]
- 27.Kubes P. Nitric oxide affects microvascular permeability in the intact and inflamed vasculature. Microcirculation 2: 235–244, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Levesque MJ, Nerem RM, Sprague EA. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials 11: 702–707, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Ley K, Lundgren E, Berger E, Arfors KE. Shear-dependent inhibition of granulocyte adhesion to cultured endothelium by dextran sulfate. Blood 73: 1324–1330, 1989 [PubMed] [Google Scholar]
- 30.Lin SJ, Jan KM, Weinbaum S, Chien S. Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis 9: 230–236, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Lin SJ, Jan KM, Chien S. Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis 10: 703–709, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JY, Li YS, Chien S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci USA 97: 9385–9389, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik AB, Perlman MB, Cooper JA, Noonan T, Bizios R. Pulmonary microvascular effects of arachidonic acid metabolites and their role in lung vascular injury. Fed Proc 44: 36–42, 1985 [PubMed] [Google Scholar]
- 34.Metaxa E, Meng H, Kaluvala SR, Szymanski MP, Paluch RA, Kolega J. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am J Physiol Heart Circ Physiol 295: H736–H742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries (5th ed.). London: Hodder Arnold, 2005, p. 165 [Google Scholar]
- 36.Okahara K, Sun B, Kambayashi J. Cox 1 and 2: upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol 18: 1922–1926, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Pearce MJ, McIntyre TM, Prescott SM, Zimmerman GA, Whatley RE. Shear stress activates cytosolic phospholipase A2 (cPLA2) and MAP kinase in human endothelial cells. Biochem Biophys Res Commun 218: 500–504, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Phelps JE, DePaola N. Spatial variations in endothelial barrier function in disturbed flows in vitro. Am J Physiol Heart Circ Physiol 278: H469–H476, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Ranjan V, Xiao Z, Diamond SL. Constitutive nitric oxide synthase protein and mRNA levels are elevated in cultured human and bovine endothelial cells exposed to fluid shear stress. Am J Physiol Heart Circ Physiol 269: H550–H555, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Schaeffer RC, Jr, Gong F, Bitrick MS., Jr Restricted diffusion of macromolecules by endothelial monolayers and small-pore filters. Am J Physiol Lung Cell Mol Physiol 263: L27–L36, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Schröder H, Strobach H, Schrör K. Nitric oxide but not prostacyclin is an autocrine endothelial mediator. Biochem Pharmacol 43: 533–537, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Sill HW, Chang YS, Artman JR, Frangos JA, Hollis TM, Tarbell JM. Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am J Physiol Heart Circ Physiol 268: H535–H543, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Suttorp N, Hippenstie Sl Fuhrmann M, Krull M, Podzuweit T. Role of nitric oxide and phosphodiesterase isoenzyme II for reduction of endothelial hyperpermeability. Am J Physiol Cell Physiol 270: C778–C785, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Tang BT, Cheng CP, Draney MT, Wilson NM, Tsao PS, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: quantification using image-based computer modeling. Am J Physiol Heart Circ Physiol 291: H668–H676, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Cox-2: Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA 93: 10417–10422, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao PS, Lewis NP, Alpert S, Cooke JP. Exposure to shear stress alters endothelial adhesiveness: role of nitric oxide. Circulation 92: 3513–3519, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Wechezak AR, Viggers RF, Coan DE, Sauvage LR. Mitosis and cytokinesis in subconfluent endothelial cells exposed to increasing levels of shear stress. J Cell Physiol 159: 83–91, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Weinberg PD. Application of fluorescence densitometry to the study of net albumin uptake by the rabbit aortic wall up- and downstream of intercostal ostia. Atherosclerosis 74: 139–148, 1988 [DOI] [PubMed] [Google Scholar]
- 50.Weinberg PD. Rate-limiting steps in the development of atherosclerosis: the response-to-influx theory. J Vasc Res 41: 1–17, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Weinberg PD, Ethier CR. Twenty-fold difference in hemodynamic wall shear stress between murine and human aortas. J Biomech 40: 1594–1598, 2007 [DOI] [PubMed] [Google Scholar]