Abstract
Cell division requires the tight coordination of multiple cytoskeletal pathways. The best understood of these involves myosin-II-dependent constriction around the cell equator, but both Dictyostelium and mammalian cells also use a parallel, adhesion-dependent mechanism to generate furrows. We show that the actin nucleation factor SCAR/WAVE is strongly activated during Dictyostelium cytokinesis. This activation localises to large polar protrusions, driving separation of the daughter cells. This continues for 10 minutes after division before the daughter cells revert to normal random motility, indicating that this is a tightly regulated process. We demonstrate that SCAR activity is essential to drive myosin-II-independent cytokinesis, and stabilises the furrow, ensuring symmetrical division. SCAR is also responsible for the generation of MiDASes, mitosis-specific actin-rich adhesions. Loss of SCAR in both Dictyostelium and Drosophila leads to a similar mitotic phenotype, with severe mitotic blebbing, indicating conserved functionality. We also find that the microtubule end-binding protein EB1 is required to restrict SCAR localisation and direct migration. EB1-null cells also exhibit decreased adhesion during mitosis. Our data reveal a spindle-directed signalling pathway that regulates SCAR activity, migration and adhesion at mitosis.
Keywords: Dictyostelium, SCAR/WAVE, Cytokinesis
Introduction
Cell division is highly complex, involving the coordination of a number of cellular processes. At mitosis, in addition to the duplication, condensation and sorting of chromosomes, a cell must also undergo a dramatic series of morphological changes, unique to mitosis, which results in the production of two independent daughter cells (Zhang and Robinson, 2005). This is known as cytokinesis.
In eukaryotes, entry into mitosis coincides with the silencing of the actin cytoskeleton and retraction of cellular protrusions resulting in a round cell. These rounded cells then rapidly pinch in two, by the formation of an equatorial furrow. This is a complex process that is still not completely understood, but requires both myosin II contraction, and exocytosis (De Lozanne and Spudich, 1987; Lecuit and Wieschaus, 2000; Mabuchi and Okuno, 1977).
Although equatorial constriction appears to be the dominant mechanism for cell division in metazoa, other parallel mechanisms for splitting the cell in two also exist. The first indication of this was in studies using the social amoeba Dictyostelium discoideum, which were still able to divide with fairly normal speed and efficiency after genetic disruption of myosin heavy chain (mhcA) – provided they were attached to a surface (De Lozanne and Spudich, 1987; Neujahr et al., 1997). Cytokinesis is therefore so important that Dictyostelium cells possess at least two mechanisms for cell division, working in parallel to ensure success. These have been termed cytokinesis A (myosin-II dependent) and cytokinesis B (myosin-II independent, adhesion dependent) (Uyeda et al., 2000; Zang et al., 1997). Even if both these pathways fail, and a multinucleate cell is produced, Dictyostelium remains able to recover single cells by tearing the enlarged cell apart, in a cell-cycle-independent manner, known as traction-mediated cytofission or cytokinesis C (De Lozanne and Spudich, 1987; Nagasaki et al., 2002).
Dictyostelium is a unicellular organism but there is evidence that a pathway similar to cytokinesis B is conserved in metazoans. In a number of cultured mammalian cell lines, mitotic cells become only weakly attached to the substratum, although others remain adherent. For example, HeLa cells are unable to complete cytokinesis in the presence of myosin II inhibitors (Straight et al., 2003), but more adherent cell lines such as NRK (normal rat kidney) and HT1080 fibrosarcoma cells are still able to make a furrow in an adhesion-dependent manner (although cytokinesis ultimately fails) (Kanada et al., 2005). In addition, disruption of Rho, which lies upstream of myosin II, by either treatment with the Rho-kinase inhibitor Y27632, or microinjection with the Rho inhibitor C3 ribosyltransferase is only able to block the division of weakly adherent cells, whereas strongly attached cells are able to complete cytokinesis (O'Connell et al., 1999; Kanada et al., 2005). Like the work with Dictyostelium, these studies demonstrate a similar dependency on substrate adhesion, and therefore mammalian cells are likely to share a common mechanism for generating a furrow, independently of myosin II.
However, little is known about the mechanism of myosin-II-independent cytokinesis, and Dictyostelium remains the only organism where cytokinesis A and B can be clearly separated. We know it requires adhesion, and is partially blocked by the disruption of adhesion molecules such as talin, vinculin and paxillin (Hibi et al., 2004; Nagasaki et al., 2009) but the mechanical process and the signalling pathways responsible for its regulation remain unknown. One clue, however, is provided by the observation that the combined disruption of the actin-binding protein coronin and myosin II leads to a much more severe cytokinesis defect than each individual mutation, even when cells are attached to a surface (Nagasaki et al., 2002). Coronin strongly localises to the poles of mitotic cells (de Hostos et al., 1993) indicating that myosin-II-independent cytokinesis might be driven by actin-dependent processes at the cell extremities. This implies that actin plays a key, but undefined role at the poles as well as the furrow of dividing cells.
During Dictyostelium cytokinesis, a number of regulators of the cytoskeleton such as racE and phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] localise to the poles of dividing cells (Larochelle et al., 1996; Janetopoulos et al., 2005). Others have shown that in NRK cells, the localised application of cytochalasin D (an inhibitor of actin polymerisation) to the cell poles is catastrophic to cytokinesis, but has little effect at the furrow (O'Connell et al., 2001) and thus polar actin polymerisation appears to be crucial in mammalian cells too. It is therefore important to understand both the functional role and the nature of the pathways regulating the production of actin at the cortex of dividing cells.
Recent work has implicated the SCAR/WAVE complex, a potent activator of the ARP2/3 complex (Machesky and Insall, 1998), in regulating the cytoskeleton of mitotic cells. SCAR function is not essential for Dictyostelium cytokinesis (Blagg et al., 2003), but disruption of the Abi subunit of the complex leads to inappropriate SCAR activation (Pollitt and Insall, 2008), which can disrupt normal cell division. Consistent with this, others showed that cells overexpressing an N-terminal truncation of SCAR also rapidly become multinucleate (Caracino et al., 2007) indicating that SCAR activity must be tightly regulated for normal cytokinesis.
In this study we identify a defined period at the end of mitosis where Dictyostelium cells rapidly migrate apart. We show that during this time the SCAR/WAVE complex is strongly activated and constitutively localised to the polar cortex of dividing cells. This activation is essential in driving the migration of the daughter cells. We further demonstrate that this is crucial for successful myosin-II-independent division and show that the localisation of active SCAR is regulated by astral microtubules.
Results
Enhanced migration and activation of SCAR at cytokinesis
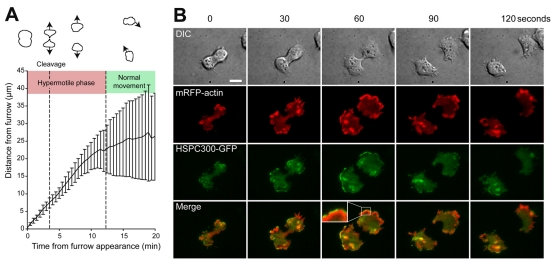
Mitosis involves a number of sequential rearrangements to the cytoskeleton. As previously described, the Dictyostelium mitotic cycle starts with an initial rounding up and loss of cellular polarity (Kitanishi-Yumura and Fukui, 1989). The first external sign of the internal polarity formed by the spindle is the formation of ruffles on opposing sides of the otherwise round cell. Subsequently, as the furrow starts to form and ingress, the cells simultaneously start to spread and the polar ruffles change into large, lamellipodia-type protrusions. At this stage, the daughter cells move away from the furrow (and each other) with high directionality (Fig. 1A; Fig. 7C).
Fig. 1.
Enhanced migration and SCAR localisation at cytokinesis. (A) Dividing wild-type (Ax3) cells were observed by video microscopy and the movement of each daughter cell away from the furrow was analysed. Values are the means of 10 divisions; error bars represent standard deviation. (B) Cytokinesis of Ax3 cells coexpressing HSPC300-GFP (green) and mRFPmars-actin (red) was observed by TIRF microscopy, showing a strong localisation and activation of SCAR at the end of mitosis (see Movie 2 in supplementary material for the full sequence). Scale bar: 10 μm.
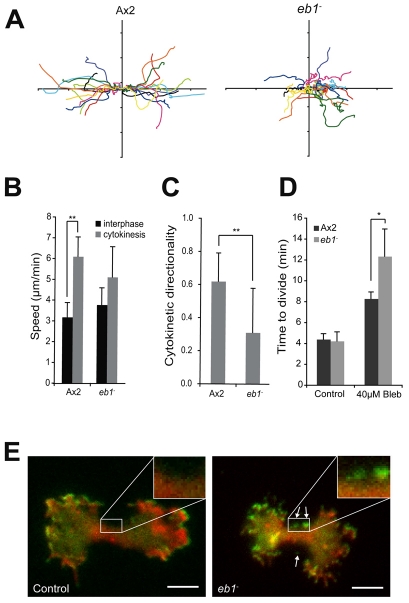
Fig. 7.
EB1 directs post-mitotic migration. (A) Paths of control (Ax2) and eb1-null cells at cytokinesis. Ten divisions are superimposed and the plots for each pairs of daughter cells are same colour. (B) Speed of migration of both dividing and interphase cells and (C) Cytokinetic directionality (defined as the distance moved perpendicular to the furrow divided by the total path length) of mutant cells. (D) Time to complete division, from the initiation of furrowing to abscission of control (Ax2) and eb1-null cells after treatment for 1 hour with 40 μM blebbistatin. *P<0.01; **P<0.05, Student's t-test. (E) TIRF images of cells coexpressing HSPC300-GFP (green) and mRFPmars-actin (red). Scale bar: 10 μm. Arrows indicate regions where SCAR is inappropriately localised in eb1-null cells (for the full sequence see Movie 10 in supplementary material).
Interestingly, when we examined this process in more detail, we noticed that the period in which cells maintained a spread, lamella morphology and moved in a directional manner lasted almost 12 minutes from the initiation of furrowing (as indicated by the sudden change in gradient in Fig. 1A). During this hypermotile phase, wild-type cells maintained their directionality away from the furrow and moved much faster (6.1 μm/minute) than randomly moving interphase cells (3.2 μm/minute; Fig. 7B). Importantly, this phase continued for almost 10 minutes after the cells had completed division (Fig. 1A), and therefore cannot be merely explained mechanically by the repression of pseudopod extension at the furrow by myosin II assembly. In addition, the end of this phase occurs as a sudden ‘switch’ back to normal interphase morphology and motility rather than a slow dissipation of polarity. We conclude that the mitotic activation of motility is a highly regulated process, specifically activated for a defined period as the cells begin to separate.
Previous work has shown that in Dictyostelium, misregulation of the SCAR complex by disruption of the Abi subunit leads to aberrant mitosis (Pollitt and Insall, 2008). The only in vivo assay for SCAR activity is to image the localisation of the complex, which coincides with areas of polymerised actin (Weiner et al., 2007). The dynamics of the SCAR/WAVE complex can be observed in live neutrophils by imaging GFP-tagged fusions of complex members with TIRF microscopy (Weiner et al., 2007). We expressed a number of different subunits of the SCAR complex fused to GFP in Dictyostelium. Using TIRF microscopy, HSCP300-GFP, GFP-NapA (not shown) and SCAR-GFP all had a highly dynamic localisation, and were clearly recruited to the very leading edge of cellular protrusions (Fig. 1B; supplementary material Fig. S1) (D.M.V. and R.H.I., unpublished). Of all the probes, HSPC300-GFP gave the highest contrast images and was used for all further experiments. The localisation of HSPC300-GFP was always closely followed by both a narrower band of ARP2/3 (indicated by mRFP-ARPC4; supplementary material Fig. S1B and Movie 1) and a broad band of mRFP-actin (Fig. 1B; supplementary material Movie 2). Therefore, although it is currently impossible to formally prove that SCAR complex localisation is equivalent to its activity, this appears to be the case. Expression of HSPC300-GFP is able to rescue both morphology and cytokinesis defects of hspc300-null cells (data not shown and supplementary material Fig. S3) and we conclude that this probe gives an authentic representation of SCAR localisation. This enabled us for the first time to image SCAR dynamics in live Dictyostelium, giving a similar but much clearer localisation than previous images of fixed cells using a SCAR-specific antibody (Pollitt et al., 2006) and with temporal resolution.
During interphase, Ax3 cells only have weak SCAR-complex localisation (Pollitt et al., 2006) and HSPC300-GFP only transiently localised to the small pseudopodia that vegetative Dictyostelium cells make (data not shown). However, during cytokinesis, the SCAR complex became strongly localised to the large lamellipodial protrusions of each daughter cell, just ahead of a broad band of actin and ARPC4 and was excluded from the furrow (Fig. 1B; supplementary material Movies 1 and 2). This strongly suggests that SCAR is highly activated at the poles, leading to local ARP2/3 activity and actin polymerisation. The localisation of both HSPC300-GFP and mRFP-actin is much stronger at cytokinesis than in interphase. Therefore SCAR activity is strongly activated at mitosis, and is specifically localised to the cell poles.
SCAR is responsible for generating MiDASes
In addition to its localisation at the mitotic cortex, we frequently observed that mRFP-actin localised to small patches beneath the nuclei of dividing cells (Fig. 2A). These actin patches have been described previously and termed MiDASes (mitosis-specific dynamic actin structures) (Itoh and Yumura, 2007). MiDASes are highly dynamic structures formed underneath the mitotic spindle and appear to be the major sites of cell attachment to the substratum at cytokinesis. This and the observation that MiDASes become much more stable in myosin-II-deficient cells led to the hypothesis that they play an important role in adhesion-dependent cytokinesis as points through which the cell can exert a mechanical force (Itoh and Yumura, 2007).
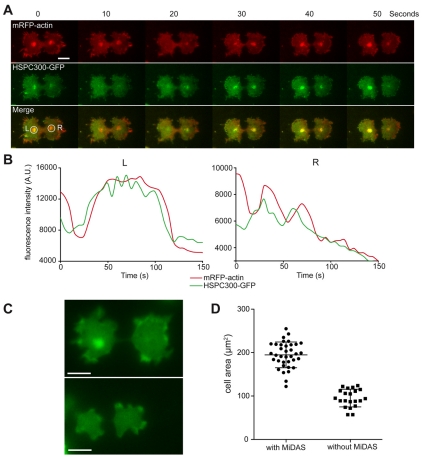
Fig. 2.
SCAR localisation precedes MiDAS formation. (A) HSPC300-GFP and mRFPmars-actin were imaged simultaneously by TIRF microscopy in dividing Ax3 cells (see Movie 2 in supplementary material). Scale bar: 10 μm. Both MiDAS and SCAR localisation appeared and disappeared over the course of the movie; the intensity of both the red and the green channels within the circled regions (labelled L and R) are plotted in B. Not all cells produced MIDASes, and when Ax3 cells expressing GFP-actin were observed at mitosis (in widefield), cells that produced MiDASes (at any point) had a significantly more spread morphology than those that did not. (C) Cells dividing either with (top) or without (bottom) MiDASes. Scale bar: 10 μm. The area of these cells just before abscission was measured and is plotted in D. Error bars indicate the standard deviation.
When we coexpressed mRFP-actin and HSPC300-GFP, we observed that MiDASes always colocalised with the SCAR complex as well as mRFP-ARPC4 (a subunit of the ARP2/3 complex) (Fig. 2A; supplementary material Fig. S1B). Furthermore, as MiDASes were transient – often appearing and disappearing several times over the course of the mitosis – we were able to investigate the causal relationship between the two. When we simultaneously imaged HSPC300-GFP and mRFP-actin we saw that the HSPC300-GFP localisation always preceded the actin by ~5 seconds (Fig. 2; supplementary material Movie 3). In addition, MiDASes were present in 67% of mitoses in control cells, but were never observed in any of the 36 scar-null mitoses that we observed (for example see Fig. 3B) so we conclude that MiDASes are generated by localised ARP2/3 activity driven by SCAR.
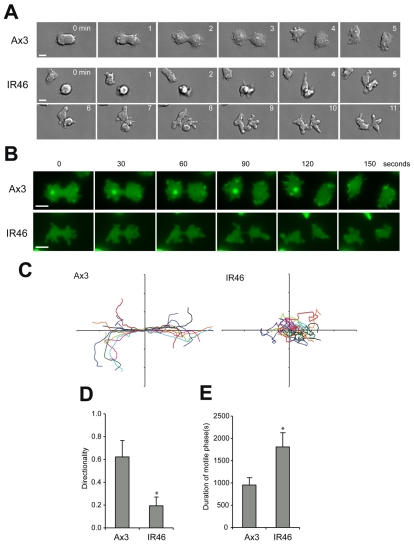
Fig. 3.
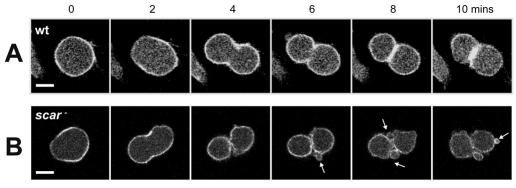
Abnormal mitosis of Dictyostelium cells lacking SCAR. (A) DIC images of control (Ax3) and scar-null (IR46) cells dividing on a glass surface. (B) Wide-field fluorescence images of dividing cells expressing GFP-actin. Scale bar: 10 μm. Note the lack of MiDASes and cortical actin in IR46 cells. (C) Plot indicating the paths of cells at cytokinesis. Movies of dividing cells were orientated such that the furrow was formed perpendicular to the x-axis and the centroids of the daughter cells were tracked manually from the first frame at which the furrow could be seen. Ten divisions of each cell line are superimposed; the paths of each pair of daughter cells are same colour. (D) Directionality of the cell paths plotted in C. (E) The duration of the motile phase, from the initiation of furrowing to a return to interphase morphology and behaviour (i.e. random migration and the cessation of blebbing). Values are the means ± standard deviation of 20 cells from 10 divisions. *P<0.005, Students t-test.
Dictyostelium cells have two parallel mechanisms for cytokinesis, cytokinesis A that is myosin-II dependent and cytokinesis B that is adhesion dependent. MiDASes are much more frequent and stable in cells lacking myosin-II (Itoh and Yumura, 2007), and therefore it is probable that they are upregulated as part of a switch to cytokinesis B. When we measured the area of dividing wild-type cells, we noticed that they split into two clear populations, and cells that produced MiDASes had almost twofold the area of cells without (Fig. 2C,D). Cells that had MiDASes also had more cortical actin, and therefore it appears that all SCAR-generated structures are coordinately upregulated. Furthermore, the fact that the two populations are distinct and not part of a continuum indicates that there is a switch that determines whether a cell divides using predominantly SCAR or myosin II as the primary driver of cytokinesis.
Cells lacking SCAR have abnormal mitosis
The mitotic activation of SCAR at the cortex and MiDASes implies an important role for SCAR in cell division. To investigate this further, we looked at mitosis in cells in which SCAR had been disrupted. Previous work had shown that the loss of SCAR has a surprisingly mild effects on the growth, motility and development of Dictyostelium (Bear et al., 1998). Indeed, although hyperactivation of SCAR by disruption of the Abi subunit leads to defects in cytokinesis, cells lacking SCAR remain almost entirely mononucleate in both adherent and suspension culture (Pollitt and Insall, 2008).
When we looked more closely by high-resolution DIC microscopy we observed that although scar-null cells were able to efficiently complete cytokinesis, they did so with a dramatically altered morphology. Unlike wild-type cells, which appeared strongly adhered and formed flat, lamella structures (Fig. 3A; supplementary material Movie 4) scar-null cells produced numerous large bulbous projections (Fig. 3A; supplementary material Movie 5). As these projections do not contain actin (Fig. 3B) we conclude that they are membrane blebs, caused by hydrostatic pressure. Similar to the activation of SCAR, these blebs only appeared as the furrow formed and lasted almost 30 minutes, long after division had completed, and twice as long as the hypermotile phase of wild-type cells (Fig. 3E). Similar to the hypermotile phase of wild-type cells, scar-null cells reverted to a normal vegetative morphology with a sudden, rapid change, indicating a specific change in upstream signalling.
In addition, although control cells remained adherent throughout mitosis (100% of over 50 divisions), scar-null cells frequently detached with at least one daughter cell released from the substrate, indicating reduced adhesion, consistent with the loss of MiDASes (see supplementary material Table S1). In many divisions, it also appeared that the furrow of scar-null cells was formed in the wrong orientation, pinching off the top of the cell, rather than forming perpendicular to the substratum. When we directly observed the mitotic spindle in three dimensions using GFP-tubulin, we observed that in scar-null cells, the spindle appeared less constrained and more mobile, frequently elongating at an angle to the substratum, whereas in wild-type cells, the spindle always remained parallel to the surface (see supplementary material Fig. S2).
From these experiments it is impossible to disseminate whether reduced adhesion is responsible for aberrant spindle extension, or caused by it, but it is an attractive hypothesis that interactions between the spindle and the actin cytoskeleton act as a scaffold to hold the spindle in place to orientate the plane of division (see supplementary material Fig. S2C). Nonetheless, the defects in adhesion and furrow orientation, combined with a loss of actin-rich lamellipodial protrusions, lead to a complete loss of directional migration, and scar-null cells moved randomly at cytokinesis (Fig. 3C,D). Therefore SCAR is essential for directed migration at mitosis.
In order to investigate the evolutionary conservation of the role SCAR in metazoa, we looked at cell division within columnar epithelial cells in the dorsal thorax of developing Drosophila melanogaster pupae. In this organism, we were able to develop a genetic system where a small, random population of scar-null cells grow and divide within a wild-type tissue. We observed cell division in real time by using Neuralized-Gal4 (Reddy and Rodrigues, 1999) to drive expression of UAS-GFP:Moe, which labels actin filaments, specifically within well-spaced sensory organ precursor cells. The MARCM system (Lee and Luo, 2001) was used to positively label scar mutant cells within this tissue and observe their behaviour over time. When scar-null cells divided, they also produced numerous blebs – strikingly similar to the phenotype of Dictyostelium SCAR mutants (Fig. 4; supplementary material Movies 6 and 7). Wild-type controls did not do this, indicating that SCAR is active at mitosis and suppresses blebbing. Therefore, although dividing cells are unable to migrate apart within a tissue, SCAR is important to maintain cell morphology and form proper F-actin structures.
Fig. 4.
Abnormal mitosis of Drosophila columnar epithelial cells lacking SCAR. Images of (A) control and (B) scar mutant sensory organ precursor cells undergoing cell division during pupal development. Cells are labelled with GFP-Moe. Scale bar: 5 μm. Optical Z-sections through the tissue were taken every 2 minutes on a confocal microscope and representative slices through a dividing cell are shown. Arrows indicate blebs produced when cells lacking SCAR divide. The full sequences can be viewed in Movies 6 and 7 in supplementary material.
SCAR drives myosin-II-independent cytokinesis
Loss of SCAR radically changes the morphology of cells at mitosis, yet both Dictyostelium and Drosophila mutants are still able to complete division. Previous work has shown that both Dictyostelium and some mammalian cell lines in tissue culture are able to form a cleavage furrow independently of myosin II, in an adhesion-dependent manner (Kanada et al., 2005) and Dictyostelium cells lacking myosin II are still able to divide efficiently, using this alternative pathway (Uyeda et al., 2000; Zang et al., 1997).
As SCAR is a key regulator of directed migration during cytokinesis, we asked whether SCAR is important for myosin-II-independent division. To test this, we grew both wild-type and scar-null Dictyostelium cells in the presence of the myosin-II-specific inhibitor blebbistatin (Straight et al., 2003). Whereas wild-type (Ax3) cells were able to grow and divide normally by adhesion-dependent cytokinesis, scar-null cells rapidly became multinucleate, such that after 3 days 85% of cells were multinucleate, with the majority having four or more nuclei (Fig. 5). Previously it has been observed that in Dictyostelium, different genetic backgrounds use SCAR to a greater or lesser extent during vegetative growth (Pollitt et al., 2006). When we repeated these experiments with scar-null cells in an Ax2 genetic background [a kind gift from Jan Faix, Hannover Medical School, Hannover, Germany (Steffen et al., 2006)] we saw the same effect, with a large increase in multinucleate cells upon blebbistatin treatment as well as a disorganised, blebby morphology as the cells divided (supplementary material Fig. S3 and data not shown).
Fig. 5.
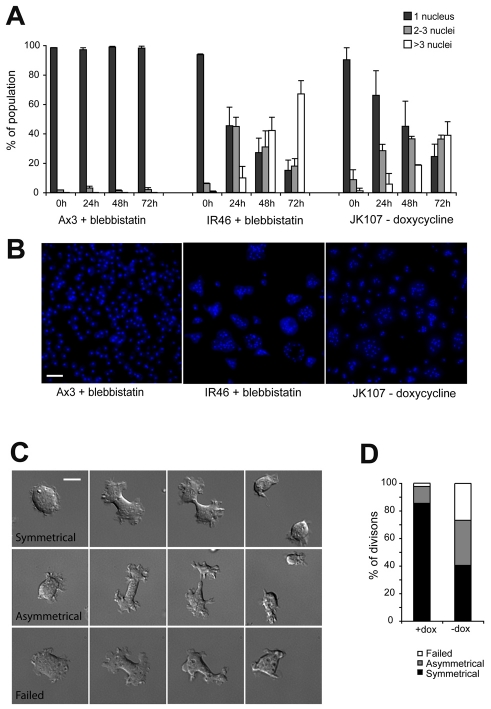
SCAR is essential for efficient myosin-II-independent cytokinesis. Control (Ax3) and scar-null cells were grown in glass dishes in the presence of 40 μM blebbistatin. JK107 cells grown in the presence of 10 μg/ml doxycycline were transferred to medium lacking it (i.e. scar expression was repressed). (A) Every 24 hours cells were fixed, stained with DAPI and the number of nuclei per cell were counted. At least 100 cells were counted for each condition and the values plotted are the means ± standard deviation of three independent experiments. (B) Representative images of DAPI stained, fixed cells after 3 days growth in the conditions indicated. Scale bar: 50 μm. (C) JK107 cells were grown in medium lacking doxycycline for 24 hours and cytokinesis was imaged by DIC microscopy. Scale bar: 10 μm. Mitosis frequently resulted in a failed (bottom), or asymmetric (middle) division, with one daughter cell significantly larger than the other. (D) Frequency of symmetrical, asymmetric and failed cytokinesis in JK107 cells grown in either the presence or absence of 10 μg/ml doxycycline.
We also tested mutants of other members of the SCAR complex; consistent with their loss of SCAR function phenotype (Ibarra et al., 2006; Pollitt and Insall, 2009), disruption of either hspc300 or napA led to multinucleate cells when grown in the presence of blebbistatin (supplementary material Fig. S3). This was rescued by expression of the HSPC300-GFP probe in hspc300-null cells. Loss of the PIR121 subunit leads to increased pseudopod activity (Blagg et al., 2003) and in this mutant, treatment with blebbistatin had no effect on the number of multinucleate cells. Abi-null cells have a specific defect of increased SCAR activity at cytokinesis (Pollitt and Insall, 2008) and have an intermediate phenotype, becoming only moderately more multinucleate upon blebbistatin treatment (supplementary material Fig. S3).
Although blebbistatin appears to be a highly specific inhibitor of myosin II, others have shown that in Dictyostelium, the inhibited myosin II molecules form aggregates, leading to additional effects on normally myosin-independent processes (Shu et al., 2005). To ensure that this is not responsible for the effects we see upon disruption of scar we developed a genetic system, in which myosin heavy chain (mhcA) was constitutively disrupted, but we were able to inducibly switch off SCAR expression using the Tet-On promoter (supplementary material Fig. S4). These cells (named JK107) divided efficiently and remained mononucleate in the presence of doxycycline (i.e. with SCAR expressed), however, they also rapidly became multinucleate when doxycycline was removed and scar expression was repressed (Fig. 5A,B). Therefore SCAR is a major effector for myosin-II-independent cytokinesis.
This inducible system allowed us to study in detail the division of mononucleate cells as they try to divide without myosin II or SCAR. When cells lacking myosin II but expressing SCAR divide they migrate apart, generating a symmetrical furrow and split into two cells of equal size (Fig. 5D). This process is completed in approximately the same time in wild-type, scar-null and mhcA-null cells (Fig. 6C). However, in the absence of both myosin II and SCAR, furrow progression is extremely slow and unstable, leading to an asymmetric (where one daughter cell is much larger than the other) or failed division in 60% of cases (Figs 5C,D). Frequently however, mhcA−/scar− double mutants did succeed in splitting into two cells, but this was very slow, taking four times longer to divide than when SCAR was expressed (Fig. 6C).
Fig. 6.
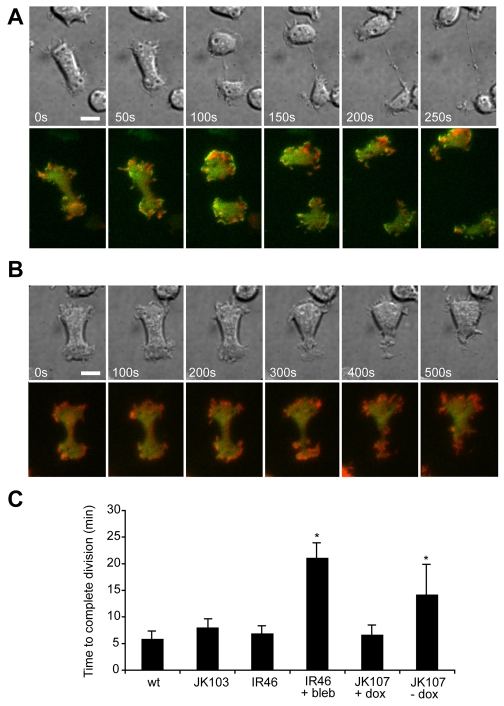
Cytokinesis of cells lacking scar and mhcA. JK107 cells coexpressing HSPC300-GFP and mRFPmars-actin were grown overnight in the presence (A) or absence (B) of 10 μg/ml doxycycline and imaged by TIRF microscopy. Scale bar: 10 μm. Note the frame interval in B is twice as long as in A. (C) Cells lacking both SCAR and myosin II take significantly longer to divide. Movies were recorded of dividing cells and the time from furrow formation to abscission was measured for control (Ax3), mhcA-null (JK103), scar-null (IR46) and inducible scar/mhcA-null cells (JK107). Values plotted are the mean ± standard deviation of at least five cells. *P<0.005.
When we looked at actin and SCAR dynamics in JK107 cells, we saw that when SCAR was induced, HSPC300-GFP localised to the initial polar ruffles, as well as large lamellae (supplementary material Movie 8). When SCAR expression was repressed, HSPC300-GFP became uniformly distributed, and the flat lamella structures were lost (Fig. 6; supplementary material Movie 9). Cells were still able to produce actin-rich ruffles, indicating that these can be generated independently of SCAR, but these ruffles were not sufficient to drive migration. Therefore dividing cells exhibit two overlapping actin processes at the cell poles, SCAR-independent polar ruffling, and a superimposed activation of SCAR at the cortex and under the spindle, driving cell spreading and migration (supplementary material Fig. S2C).
The mitotic activation of SCAR may therefore serve two purposes: first, by causing daughter cells to migrate apart, and increasing adhesion via MiDASes, SCAR provides a secondary mechanism for cytokinesis in the absence of myosin II, improving the robustness of mitosis; second, activation of SCAR appears to stabilise the mitotic furrow, ensuring it is correctly positioned for an equal division. This agrees with previous studies that demonstrated that it is the mechanical properties of both the cortex and the furrow that determine the morphology of the mitotic cell and ensure a symmetrical division (Girard et al., 2004).
It is interesting that disruption of Abi, which leads to hyperactive SCAR, or coronin, which also regulates polar actin, result in cytokinesis defects in suspension where migration is impossible (Pollitt and Insall, 2008; de Hostos et al., 1993). Therefore, whereas migration-assisted division may be limited to unicellular organisms or a specific subset of metazoan cells, SCAR-mediated stabilisation of the furrow may be more generally applicable to cell division when movement is restricted.
SCAR is also essential for traction-mediated cytofission
Dictyostelium cells are extremely robust at dividing, and in addition to myosin-II- and adhesion-dependent cytokinesis, a number of cytokinetic mutants remain able to maintain a mononucleate population by cytokinesis C (also known as traction-mediated cytofission) (De Lozanne and Spudich, 1987; Vithalani et al., 1996). In this process, multinucleate cells are able to tear themselves into individual cells in a cell-cycle-independent but adhesion-dependent manner.
Although cells lacking both SCAR and myosin II have severely defective cytokinesis, they do frequently succeed (70% end up as two cells; Fig. 5D). However after just six generations, over 85% of scar-null cells grown in blebbistatin have become multinucleate (Fig. 5A). We therefore investigated whether SCAR was also important for traction-mediated cytofission. To do this, we generated multinucleate cells by growing them in suspension with blebbistatin for 2 days, then washed out the drug and let them attach and divide on a surface. In this assay, both wild-type and scar-null cells were initially large and multinucleate, but whereas wild-type cells rapidly tore themselves into smaller fragments, cells lacking SCAR were completely unable to do this, and remained multinucleate throughout the assay unless they divided by a cell-cycle-dependent multinucleate mitosis, resulting in multiple daughter cells from a single mitosis (supplementary material Fig. S5). Interestingly, although vegetative scar-null cells are still able to move (Pollitt et al., 2006) multinucleate scar-null cells are stationary and did not significantly move over several hours. SCAR is thus more important for multinucleate cell motility and is essential for traction-mediated cytofission.
Localised SCAR activation and motility are regulated by astral microtubules
We have demonstrated that at mitosis, SCAR is strongly localised to the distal poles of dividing cells leading to an extended period of rapid, directional migration. What then is responsible for localising and activating SCAR? Unlike chemotaxing cells, which respond to an extracellular gradient of chemoattractant, the directional migration of a dividing cell must be determined by an intrinsic, intracellular stimulus. The mitotic spindle is an obvious candidate for the regulation of the direction of migration, and it is the astral microtubules that provide the mitotic cell with an intrinsic polarity (Carminati and Stearns, 1997; Pearson and Bloom, 2004; Shaw et al., 1997). Therefore, we looked to see if the astral microtubules were responsible for directing migration and SCAR activation at mitosis.
EB1 is one of a number of proteins that bind to the growing ends of microtubules and localises to the astral microtubules, centrosomes and kinetochores at mitosis (Berrueta et al., 1998; Rehberg and Graf, 2002). Disruption of Dictyostelium EB1 has previously been reported to delay prometaphase progression, but subsequent spindle elongation and division occurs as normal and eb1-null cells are able to efficiently complete cytokinesis (Rehberg and Graf, 2002). However, when we observed the motility of eb1-null cells (a kind gift from Ralf Gräf, University of Potsdam, Germany), we saw that whereas interphase cells were indistinguishable from wild type, at mitosis their directional migration was lost, and they moved randomly (Fig. 7A,C). Like scar-null cells, they also exhibited reduced adhesion, with at least one cell detaching in 73% of divisions (supplementary material Table S1).
When we examined the localisation of HSPC300-GFP in eb1-null cells, similar to control cells, SCAR complex localisation was enhanced at mitosis, and cells had an overall increase in speed compared with interphase cells, and the mitotic activation of SCAR appeared to be intact (Fig. 7B,E). However, whereas wild-type cells have a highly organised SCAR localisation, producing wide protrusions exclusively at the cell extremities, cells lacking EB1 were much less organised producing smaller, incoherent protrusions which frequently extended from the rear of the cell and the furrow (Fig. 7E; supplementary material Movie 10).
We have shown that EB1 is required for the proper localisation of SCAR at mitosis, and subsequent directed migration of the daughter cells. Consistent with this, when treated with blebbistatin, furrow ingression was severely retarded and eb1-null cells took 50% longer to divide than control cells (P<0.01, Student's t-test; Fig. 7D). Unlike scar-null cells, eb1-null cells did eventually succeed in dividing, and did not become multinucleate (data not shown), showing that although SCAR activity is uncoordinated in these cells, it is sufficient to drive an inefficient cytokinesis.
Previous work has also shown that MiDASes colocalise with the microtubule tips underneath the spindle, and that they are disrupted by treatment with microtubule depolymerising drugs (Itoh and Yumura, 2007). Consistent with this, MiDASes were never observed in eb1-null cells (n=15) and in 73% of mitoses one of the daughter cells lost contact with the substratum before division was complete. Therefore, we conclude that microtubule plus ends direct the localised activity of SCAR and regulate the adhesion and furrow orientation of dividing cells.
Whether the regulation of SCAR by EB1 is constitutive, present in interphase and chemotaxing cells, or is specific to mitosis, requires further investigation. However, Dictyostelium EB1 has been shown to localise to the tips of pseudopodia in migrating cells (Rehberg and Graf, 2002). In addition, depletion of EB1 in mouse melanoma cells inhibits the formation of lamellae and migration (Schober et al., 2009) implying a more general role of EB1 and microtubule plus ends in the regulation of SCAR.
Discussion
In this study, we have shown that at the end of mitosis, cells initiate a highly motile phase, which continues for a defined period of time, ending suddenly. The switch-like characteristics of this motile phase indicate that it is a tightly regulated process. Migration is activated simultaneously with furrow formation and it is probable that it shares a common master-regulator with myosin II contraction. Although the primary mitotic signal is unknown, we show that one of the major effectors of this pathway is the SCAR/WAVE complex, which drives both migration and adhesion through the localised activation of the ARP2/3 complex.
The activation of SCAR at mitosis appears to serve a number of functions. Firstly, we demonstrate that SCAR activity and resultant migration is crucial for cytokinesis B and the completion of cell division when myosin II is compromised. Secondly, the dramatic mitotic blebbing seen upon disruption of SCAR indicates a role in maintaining the cell cortex and shape in order to cope with increased hydrostatic pressure. Thirdly, the demonstration that SCAR directly regulates the formation of MiDASes implies a role in regulating cell adhesion at mitosis. This is consistent with the observation that SCAR-null cells frequently detach when they divide. In addition, others have recently demonstrated that dividing cells possess a robust mechanosensing system, whereby the cell redistributes myosin II and cortexillin I to the cortex in order to dynamically correct for any defects in cell shape that may occur (Ren et al., 2009). We show that cells lacking both SCAR and myosin II are unable to correct asymmetric divisions as they progress, implying that SCAR may be an effector of this mechanosensory pathway.
Are these roles for SCAR conserved in higher eukaryotes? Although cells within a tissue cannot migrate when they divide, there is good evidence for the presence of myosin-independent, adhesion-mediated mechanism in mammalian cells. Normal rat kidney (NRK) epithelial cells are highly adherent and when treated with blebbistatin, are still able to generate a furrow (albeit irregular) provided they have a surface to adhere to (Kanada et al., 2005). Others have shown that both NRK cells and 3T3 fibroblasts are able to divide after microinjection of the Rho-inhibitor C3 ribosyltransferase, which also blocks the activity of myosin II (O'Connell et al., 1999). This evidence clearly indicates that metazoan cells also possess a secondary, adhesion-dependent pathway that reinforces the predominant myosin-II-dependent furrow formation. Indeed, it has been suggested that migration-dependent division is the more ancient mechanism, with myosin II contraction superimposed later as an adaptation to multicellularity (Gerisch and Weber, 2000).
The activation of SCAR and cell migration is clearly beneficial for the division of independent, single cells, but its importance within a tissue, where migration is obstructed by the surrounding cells is less obvious. Our observation that loss of SCAR results in aberrant mitotic blebbing in both Dictyostelium and Drosophila tissue indicates conserved function. At a basic level, SCAR-generated cortical actin is important to contain the increased hydrostatic pressure generated by myosin contraction at the furrow. There is also some evidence to suggest an additional, more specific role for SCAR at cytokinesis. When the Abi subunit of the SCAR complex is disrupted, cells extend aberrant protrusions and become multinucleate (Pollitt and Insall, 2008); as this also occurs when the cells are grown in suspension where migration is impossible, the defect in cytokinesis must be more complex than simple disruption of directional movement. Others have also observed that excessive, or disregulated cortical actin disrupts cell division, and null mutants of coronin, as well as overexpression of a truncated form of SCAR also leading to cytokinesis defects (Caracino et al., 2007; de Hostos et al., 1993). There is also evidence that it is the balance between the forces and mechanical properties at the cortex and the furrow which determines the shape of the dividing cell (Girard et al., 2004). As SCAR is a major regulator of the cortical cytoskeleton, it is probable that its tight regulation will be important for cell shape and symmetry independently of any role in migration.
In addition, mutants that lack or have uncoordinated SCAR activity and migration share a common defect in spindle and furrow orientation: whereas wild-type cells always produce a furrow perpendicular to the substratum, scar and eb1-null cells do not, and frequently produce furrows towards the top of the mitotic cell such that one daughter cell is detached (supplementary material Table S1). As MiDASes are the major points of adhesion within mitotic cells (Itoh and Yumura, 2007), and are generated by SCAR, this may be simply due to a loss of adhesion, but it is clear that in Dictyostelium at least, SCAR is important for maintaining the orientation of the division plane. In addition, in scar-null cells, the spindle appears to be more motile and we postulate that by pushing out the cell cortex, SCAR is able to increase tension along astral microtubules and stabilise the spindle and furrow (supplementary material Fig. S2C).
Indeed, it has been shown that in Drosophila neuroblasts, the repression of asymmetric division relies upon the presence of adherence junctions and is disrupted by depletion of either APC or EB1 (Lu et al., 2001). Others have recently shown that formation of cell-cell junctions is inhibited by depletion of SCAR (Yamazaki et al., 2007). Therefore the localised activation of SCAR and stabilisation of cell-cell junctions provides a plausible mechanism for orientating the mitotic spindle and organising asymmetric cell division.
In conclusion, the actin cytoskeleton plays a number of distinct roles during cell division, and its proper regulation is crucial. In this study we have demonstrated that SCAR is essential for the proper morphology of both Dictyostelium and Drosophila cells during mitosis, and is the major regulator of both migration and adhesion. Further studies are required to investigate the potential implications of this within a whole organism context, or during embryogenesis but it seems probably that, in at least a subset of cell divisions, SCAR is crucial.
Materials and Methods
Dictyostelium strains and cell culture
The genotypes of all the strains of Dictyostelium used are listed in supplementary material Table S1. When mutant strains were generated in Ax2 (Watts and Ashworth, 1970) or Ax3 (Sussman and Sussman, 1967) backgrounds the appropriate control was used. Unless otherwise stated, all strains were cultured at 22°C adhered to plastic dishes and maintained at low density. Cells were grown in HL-5 medium (Formedium), which was replaced with low-fluorescent medium (Formedium) 2 hours prior to fluorescence microscopy. Cells were transformed by electroporation and extrachromosomal vectors were selected and maintained in 50 μg/ml hygromycin (Invitrogen). Cells inducibly expressing SCAR were maintained in 10 μg/ml doxycycline (Sigma). The pure (−) enantiomer of blebbistatin (Tocris) was used in all experiments.
Generation of mutant strains and expression plasmids
An inducible knockout of SCAR was generated using the previously published scar-null cell line IR46 (Blagg et al., 2003), and re-expressing SCAR under the control of the Tet-On promoter. The full coding sequence of scar was amplified by adding 5′ BamHI and 3′ SpeI sites by PCR and cloning into the BglII-SpeI sites of the Tet-On expression vector pDM310 (Veltman et al., 2009b). The Dictyostelium origin of replication was then removed by MluI-HindIII digest, blunting with Klenow and religation (giving pJSK321). This was transformed into IR46 cells by REMI using XhoI (Kuspa and Loomis, 1992) and selection with 10 μg/ml G418. Several independent transformants were isolated and the regulation of SCAR expression tested by western blotting using a rabbit anti-SCAR antibody (Blagg et al., 2003) of whole cell lysates after growth in various concentrations of doxycycline (supplementary material Fig. S4). The strain with the most similar expression to control cells at 10 μg/ml doxycycline was used for further experiments (named SIKO2).
The mhcA gene was disrupted using the following construct. Briefly, 5′ and 3′ fragments of mhcA were amplified by PCR from genomic DNA, adding BglII and SalI sites at the 5′ and 3′ internal ends, respectively. The PCR products were then mixed and used as template for a secondary PCR giving a 2.4 kb fragment of the mhcA gene with a 105 bp deletion in the middle replaced by BglII and SalI sites. This was cloned into TOPO blunt II (Invitrogen). The blasticidin-selectable marker was then inserted as a BamHI-SalI fragment from pLPBLP (Faix et al., 2004). Clones transformed with this construct were screened for disruption of the mhcA locus by PCR and further validated by inability to divide in suspension culture (data not shown). mhcA was disrupted in both Ax3 (giving JK103) and the inducible SCAR knockout strain described above (giving JK107).
The full-length actin gene from Dictyostelium was amplified from genomic DNA and cloned into pDONR221 using the Gateway system (Invitrogen). The actin gene was subsequently cloned into pDM448 (Veltman et al., 2009a) for fusion to GFP (resulting in pDM478) and into pDM449 for fusion to mRFPmars (resulting in pDM463). The full open reading frame for HSPC300 was amplified by PCR from cDNA and cloned into the expression vector pDM377 (yielding pDM459) and shuttle vector pDM412 (yielding pDM557) (Veltman et al., 2009a) placing it N-terminal to GFP. The HSPC300-GFP expression cassette was excised from pDM557 with NgoMIV and ligated into the NgoMIV site of pDM463 to obtain a single vector expressing both HSPC300-GFP and mRFPmars-actin (pJSK352). The full-length coding sequences for α-tubulin, NapA, SCAR and ARPC4 were also amplified from cDNA by PCR and cloned into the vectors pDM449, pDM448, 450 and 579, respectively, to yield expression vectors pJSK336 (GFP-tubulin), pDM479 (GFP-NapA), pDM523 (SCAR-GFP) and the shuttle vector pDM601 (mRFPmars2-ArpC4). The mRFPmars2-ArpC4 expression cassette was then excised from pDM601 by NgoMIV digest and ligated into the NgoMIV site of pDM459 to yield pDM604, a HSPC300-GFP–mRFPmars2-ArpC4 coexpression vector.
Microscopy and image analysis
Cell morphology and migration were imaged using differential interference contrast (DIC) microscopy. Cells were seeded in glass-bottomed dishes (MatTek) and left for 2 hours prior to imaging using an inverted microscope fitted with a 100×/1.40 NA oil objective (Nikon). For analysis of migration, images were acquired every 10 seconds. The paths of daughter cells at cytokinesis were generated by first orientating each movie such that the furrow ingressed along the vertical axis. The centroids of each daughter cell were manually tracked using the MtrackJ plugin for ImageJ (http://rsb.info.nih.gov/ij) from the start of furrow formation for 5 minutes. Cytokinetic directionality was calculated as the distance moved directly away from the furrow (i.e. along the x-axis) divided by the total path length.
Total internal reflection fluorescence (TIRF) experiments were performed on a Nikon Eclipse TE 2000-U microscope equipped with a 100×/1.45 NA Nikon TIRF oil immersion objective. The microscope was equipped with a custom condenser in which laser light was introduced into the illumination pathway directly from an optical fibre output oriented parallel to the optical axis of the microscope. The light source for evanescent wave illumination was a 473 nm diode laser or 561 laser (Omicron), independently coupled into the condenser separately to allow individual TIRF angle adjustment. A green-red dual filterblock (ET-GFP/mcherry; AHF Analysentechnik, Germany) was used for 473/561 coexcitation. A Multi-Spec dual emission splitter (Optical Insights, NM) with a 595 nm dichroic and two bandpass filters (510-565 for green and 605-655nm for red) was used to separate both emissions. Images were acquired every 5 seconds with a 50 msecond exposure using a Cascade 512F EMCCD camera (Photometrics UK).
Wide-field images of live and fixed cells were obtained using a 60×/1.42 NA oil immersion objective (Nikon) on an Olympus IX81 inverted microscope. Cell area was measured by manually outlining each daughter cell at the point of abscission. Cells were scored as having MiDASes if at least one MiDAS was observed at any point in mitosis. To image the mitotic spindle in three dimensions, a Z-series (0.5 μm spacing) was taken at each time point and either extended focus (XY) images, or Z-axis maximum intensity projections produced using Volocity imaging software (Improvision).
Drosophila genetics and live-cell imaging
Live-cell imaging was performed with Neuralized GAL4:UAS GFP-Moesin flies. GFP-Moe is the actin-binding domain of Drosophila Moesin (C-terminal 137 residues) fused to GFP (Edwards et al., 1997). Positively marked clones for scarΔ37 (Zallen et al., 2002) were induced with Ubx-Flp. For live-cell imaging, animals were prepared, at approximately 14 hours after puparium formation, by cutting a window in the pupal case, attached to a slide with double-sided sticky tape. A coverslip with a drop of injection oil was then placed over the notum, supported by coverslips at either end to allow imaging from both upright and inverted Leica SP2 or SP5 microscopes.
Counting nuclei
Cells were grown without subculture for the duration of the experiment on glass-bottomed dishes (MatTek). Cells were fixed by replacing the medium with −20°C methanol and left for 5 minutes. Cells were then washed twice in KK2 buffer (16.5 mM KH2PO4, 3.8 mM K2HPO4 pH 6.2) and placed in KK2 with 0.5 μg/ml Hoechst (Sigma) to stain DNA. Cells were imaged on a fluorescence microscope and the number of nuclei were counted in at least 100 cells for each experiment.
Supplementary Material
Acknowledgments
The authors are grateful to Ralph Gräf for EB1-null cells and Jan Faix for Ax2-derived SCAR mutants. We also thank Dave Gillespie for critical reading of the manuscript and the Medical Research Council for a Senior Fellowship (G117/537) to R.H.I. and project grant support (G0600249) to J.K. Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/13/2246/DC1
References
- Bear J. E., Rawls J. F., Saxe C. L., 3rd (1998). SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J. Cell Biol. 142, 1325-1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L., Kraeft S. K., Tirnauer J. S., Schuyler S. C., Chen L. B., Hill D. E., Pellman D., Bierer B. E. (1998). The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc. Natl. Acad. Sci. USA 95, 10596-10601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagg S. L., Stewart M., Sambles C., Insall R. H. (2003). PIR121 regulates pseudopod dynamics and SCAR activity in Dictyostelium. Curr. Biol. 13, 1480-1487 [DOI] [PubMed] [Google Scholar]
- Caracino D., Jones C., Compton M., Saxe C. L. R. (2007). The N-terminus of Dictyostelium Scar interacts with Abi and HSPC300 and is essential for proper regulation and function. Mol. Biol. Cell 18, 1609-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati J. L., Stearns T. (1997). Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 138, 629-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hostos E. L., Rehfuess C., Bradtke B., Waddell D. R., Albrecht R., Murphy J., Gerisch G. (1993). Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J. Cell Biol. 120, 163-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. (1987). Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236, 1086-1091 [DOI] [PubMed] [Google Scholar]
- Edwards K. A., Demsky M., Montague R. A., Weymouth N., Kiehart D. P. (1997). GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 191, 103-117 [DOI] [PubMed] [Google Scholar]
- Faix J., Kreppel L., Shaulsky G., Schleicher M., Kimmel A. R. (2004). A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 32, e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Weber I. (2000). Cytokinesis without myosin II. Curr. Opin. Cell Biol. 12, 126-132 [DOI] [PubMed] [Google Scholar]
- Girard K. D., Chaney C., Delannoy M., Kuo S. C., Robinson D. N. (2004). Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 23, 1536-1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Nagasaki A., Takahashi M., Yamagishi A., Uyeda T. Q. (2004). Dictyostelium discoideum talin A is crucial for myosin II-independent and adhesion-dependent cytokinesis. J. Muscle Res. Cell Motil. 25, 127-140 [DOI] [PubMed] [Google Scholar]
- Ibarra N., Blagg S. L., Vazquez F., Insall R. H. (2006). Nap1 regulates Dictyostelium cell motility and adhesion through SCAR-dependent and -independent pathways. Curr. Biol. 16, 717-722 [DOI] [PubMed] [Google Scholar]
- Itoh G., Yumura S. (2007). A novel mitosis-specific dynamic actin structure in Dictyostelium cells. J. Cell Sci. 120, 4302-4309 [DOI] [PubMed] [Google Scholar]
- Janetopoulos C., Borleis J., Vazquez F., Iijima M., Devreotes P. (2005). Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev. Cell 8, 467-477 [DOI] [PubMed] [Google Scholar]
- Kanada M., Nagasaki A., Uyeda T. Q. (2005). Adhesion-dependent and contractile ring-independent equatorial furrowing during cytokinesis in mammalian cells. Mol. Biol. Cell 16, 3865-3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanishi-Yumura T., Fukui Y. (1989). Actomyosin organization during cytokinesis: reversible translocation and differential redistribution in Dictyostelium. Cell Motil. Cytoskeleton 12, 78-89 [DOI] [PubMed] [Google Scholar]
- Kuspa A., Loomis W. F. (1992). Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc. Natl. Acad. Sci. USA 89, 8803-8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle D. A., Vithalani K. K., De Lozanne A. (1996). A novel member of the rho family of small GTP-binding proteins is specifically required for cytokinesis. J. Cell Biol. 133, 1321-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T., Wieschaus E. (2000). Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J. Cell Biol. 150, 849-860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251-254 [DOI] [PubMed] [Google Scholar]
- Lu B., Roegiers F., Jan L. Y., Jan Y. N. (2001). Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 409, 522-525 [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. (1977). The effect of myosin antibody on the division of starfish blastomeres. J. Cell Biol. 74, 251-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Insall R. H. (1998). Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347-1356 [DOI] [PubMed] [Google Scholar]
- Nagasaki A., de Hostos E. L., Uyeda T. Q. (2002). Genetic and morphological evidence for two parallel pathways of cell-cycle-coupled cytokinesis in Dictyostelium. J. Cell Sci. 115, 2241-2251 [DOI] [PubMed] [Google Scholar]
- Nagasaki A., Kanada M., Uyeda T. Q. (2009). Cell adhesion molecules regulate contractile ring-independent cytokinesis in Dictyostelium discoideum. Cell Res. 19, 236-246 [DOI] [PubMed] [Google Scholar]
- Neujahr R., Heizer C., Gerisch G. (1997). Myosin II-independent processes in mitotic cells of Dictyostelium discoideum: redistribution of the nuclei, re-arrangement of the actin system and formation of the cleavage furrow. J. Cell Sci. 110, 123-137 [DOI] [PubMed] [Google Scholar]
- O'Connell C. B., Wheatley S. P., Ahmed S., Wang Y. L. (1999). The small GTP-binding protein rho regulates cortical activities in cultured cells during division. J. Cell Biol. 144, 305-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell C. B., Warner A. K., Wang Y. (2001). Distinct roles of the equatorial and polar cortices in the cleavage of adherent cells. Curr. Biol. 11, 702-707 [DOI] [PubMed] [Google Scholar]
- Pearson C. G., Bloom K. (2004). Dynamic microtubules lead the way for spindle positioning. Nat. Rev. Mol. Cell Biol. 5, 481-492 [DOI] [PubMed] [Google Scholar]
- Pollitt A. Y., Insall R. H. (2008). Abi mutants in Dictyostelium reveal specific roles for the SCAR/WAVE complex in cytokinesis. Curr. Biol. 18, 203-210 [DOI] [PubMed] [Google Scholar]
- Pollitt A. Y., Insall R. H. (2009). Loss of Dictyostelium HSPC300 causes a scar-like phenotype and loss of SCAR protein. BMC Cell Biol. 10, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt A. Y., Blagg S. L., Ibarra N., Insall R. H. (2006). Cell motility and SCAR localisation in axenically growing Dictyostelium cells. Eur. J. Cell Biol. 85, 1091-1098 [DOI] [PubMed] [Google Scholar]
- Reddy G. V., Rodrigues V. (1999). Sibling cell fate in the Drosophila adult external sense organ lineage is specified by prospero function, which is regulated by Numb and Notch. Development 126, 2083-2092 [DOI] [PubMed] [Google Scholar]
- Rehberg M., Graf R. (2002). Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol. Biol. Cell 13, 2301-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Effler J. C., Norstrom M., Luo T., Firtel R. A., Iglesias P. A., Rock R. S., Robinson D. N. (2009). Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr. Biol. 19, 1421-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober J. M., Cain J. M., Komarova Y. A., Borisy G. G. (2009). Migration and actin protrusion in melanoma cells are regulated by EB1 protein. Cancer Lett. 284, 30-36 [DOI] [PubMed] [Google Scholar]
- Shaw S. L., Yeh E., Maddox P., Salmon E. D., Bloom K. (1997). Astral microtubule dynamics in yeast: a microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J. Cell Biol. 139, 985-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu S., Liu X., Korn E. D. (2005). Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc. Natl. Acad. Sci. USA 102, 1472-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A., Faix J., Resch G. P., Linkner J., Wehland J., Small J. V., Rottner K., Stradal T. E. (2006). Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol. Biol. Cell 17, 2581-2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743-1747 [DOI] [PubMed] [Google Scholar]
- Sussman R., Sussman M. (1967). Cultivation of Dictyostelium discoideum in axenic medium. Biochem. Biophys. Res. Commun. 29, 53-55 [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Kitayama C., Yumura S. (2000). Myosin II-independent cytokinesis in Dictyostelium: its mechanism and implications. Cell Struct. Funct. 25, 1-10 [DOI] [PubMed] [Google Scholar]
- Veltman D. M., Akar G., Bosgraaf L., Van Haastert P. J. (2009a). A new set of small, extrachromosomal expression vectors for Dictyostelium discoideum. Plasmid 61, 110-118 [DOI] [PubMed] [Google Scholar]
- Veltman D. M., Keizer-Gunnink I., Haastert P. J. (2009b). An extrachromosomal, inducible expression system for Dictyostelium discoideum. Plasmid 61, 119-125 [DOI] [PubMed] [Google Scholar]
- Vithalani K. K., Shoffner J. D., De Lozanne A. (1996). Isolation and characterization of a novel cytokinesis-deficient mutant in Dictyostelium discoideum. J. Cell Biochem. 62, 290-301 [DOI] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. (1970). Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J. 119, 171-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner O. D., Marganski W. A., Wu L. F., Altschuler S. J., Kirschner M. W. (2007). An actin-based wave generator organizes cell motility. PLoS Biol. 5, e221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D., Oikawa T., Takenawa T. (2007). Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J. Cell Sci. 120, 86-100 [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Cohen Y., Hudson A. M., Cooley L., Wischaus E., Schejter E. D. (2002). SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J. Cell Biol. 156, 689-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang J. H., Cavet G., Sabry J. H., Wagner P., Moores S. L., Spudich J. A. (1997). On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol. Biol. Cell 8, 2617-2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Robinson D. N. (2005). Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc. Natl. Acad. Sci. USA 102, 7186-7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.