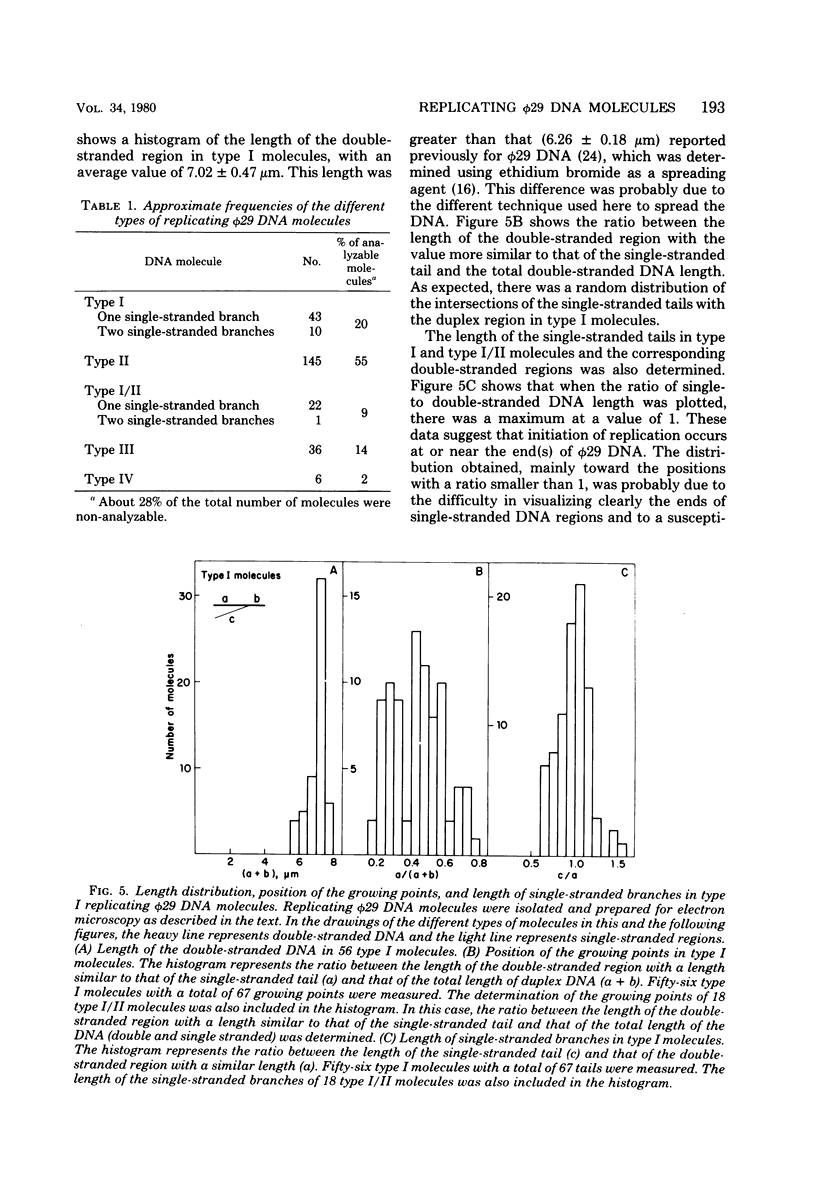
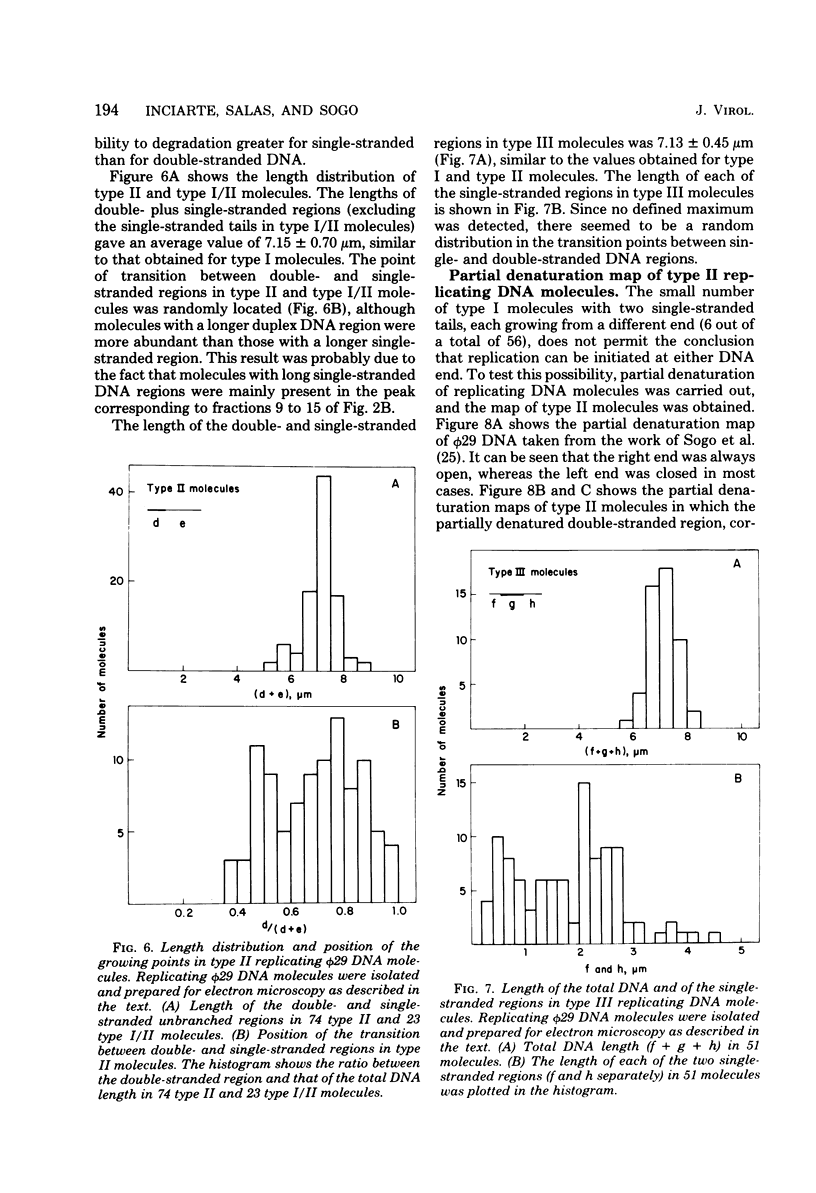
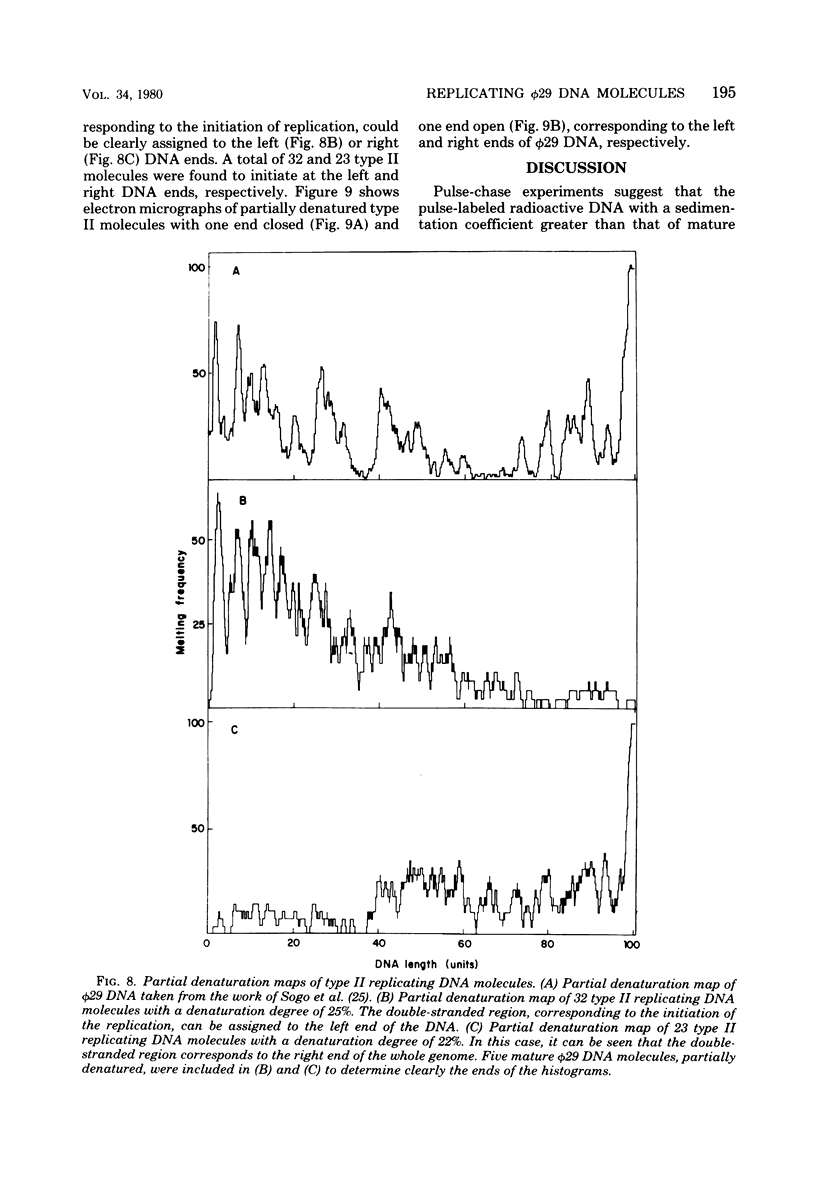
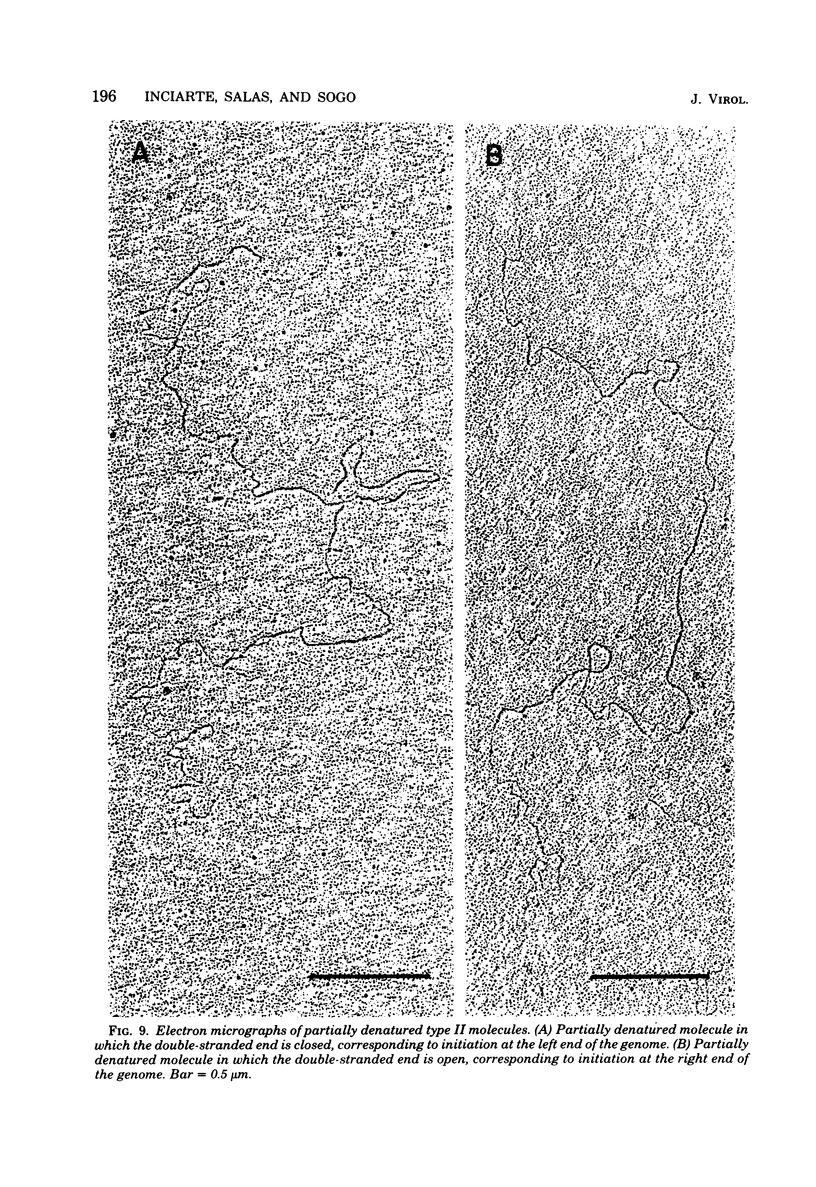
Abstract
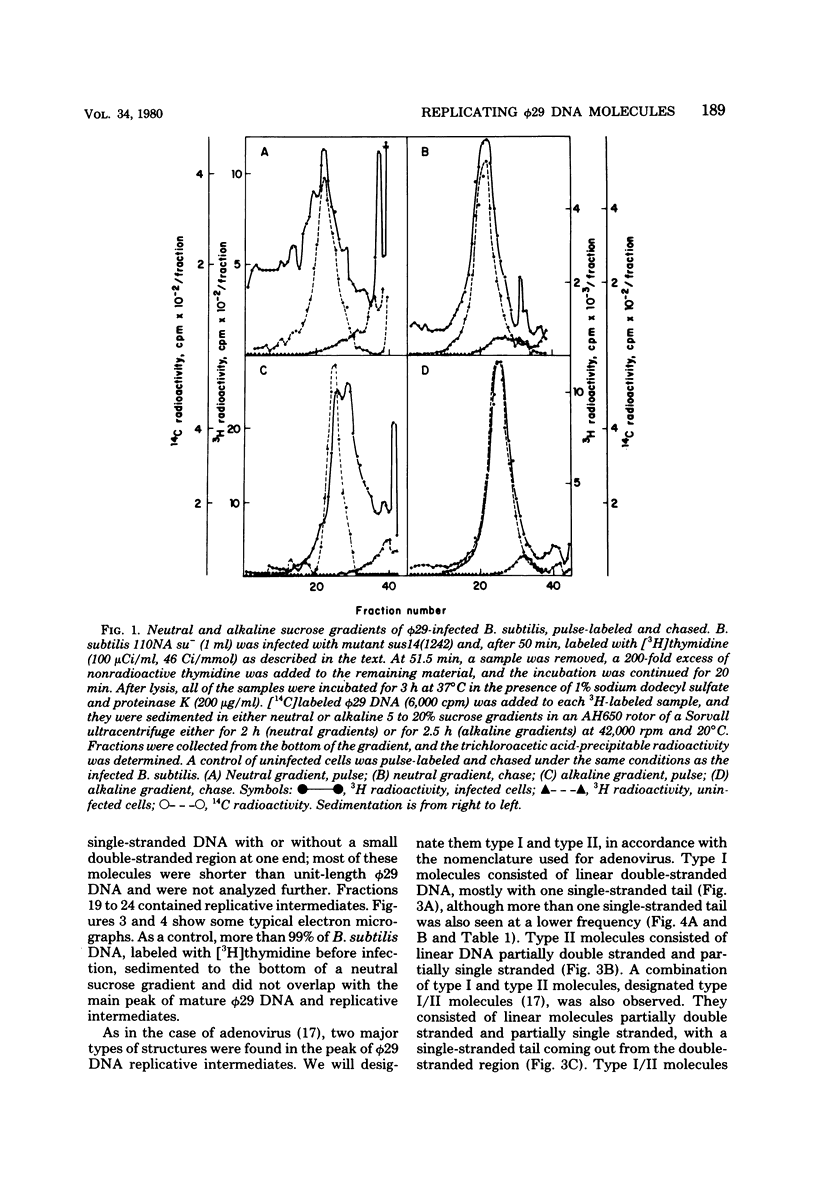
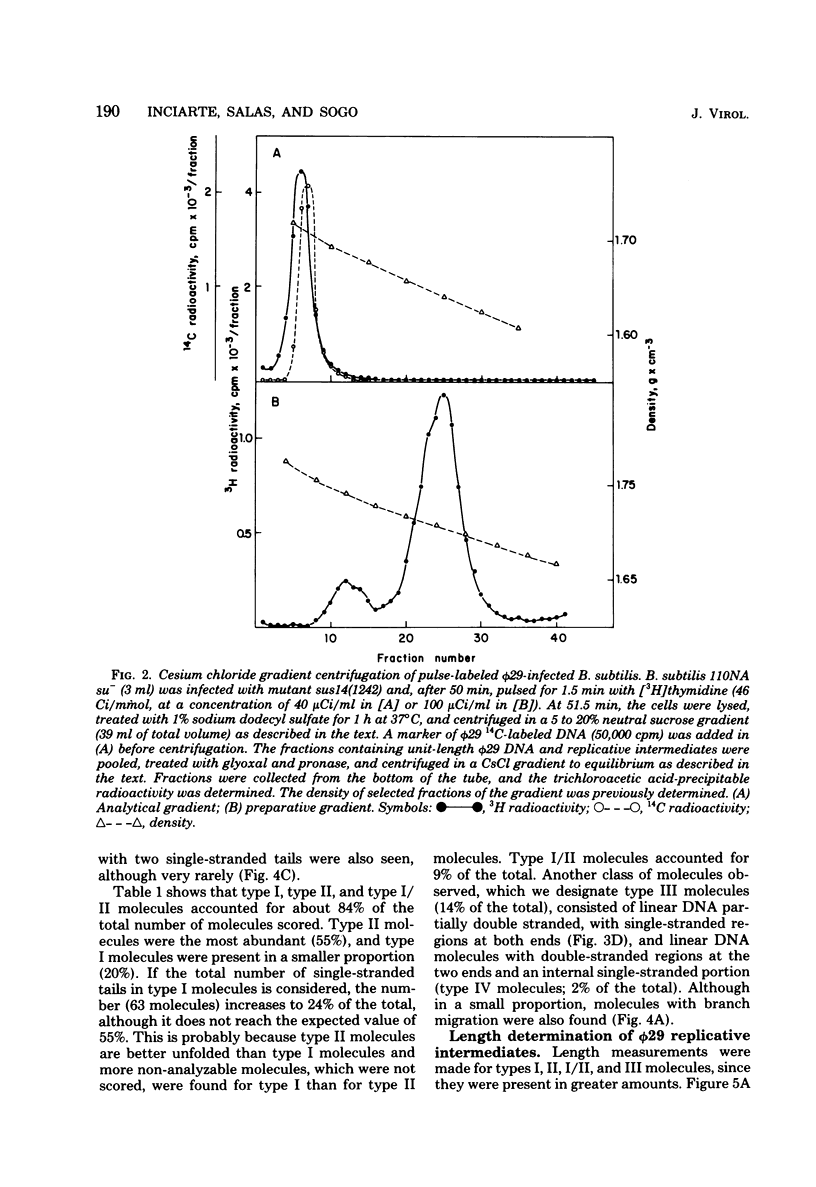
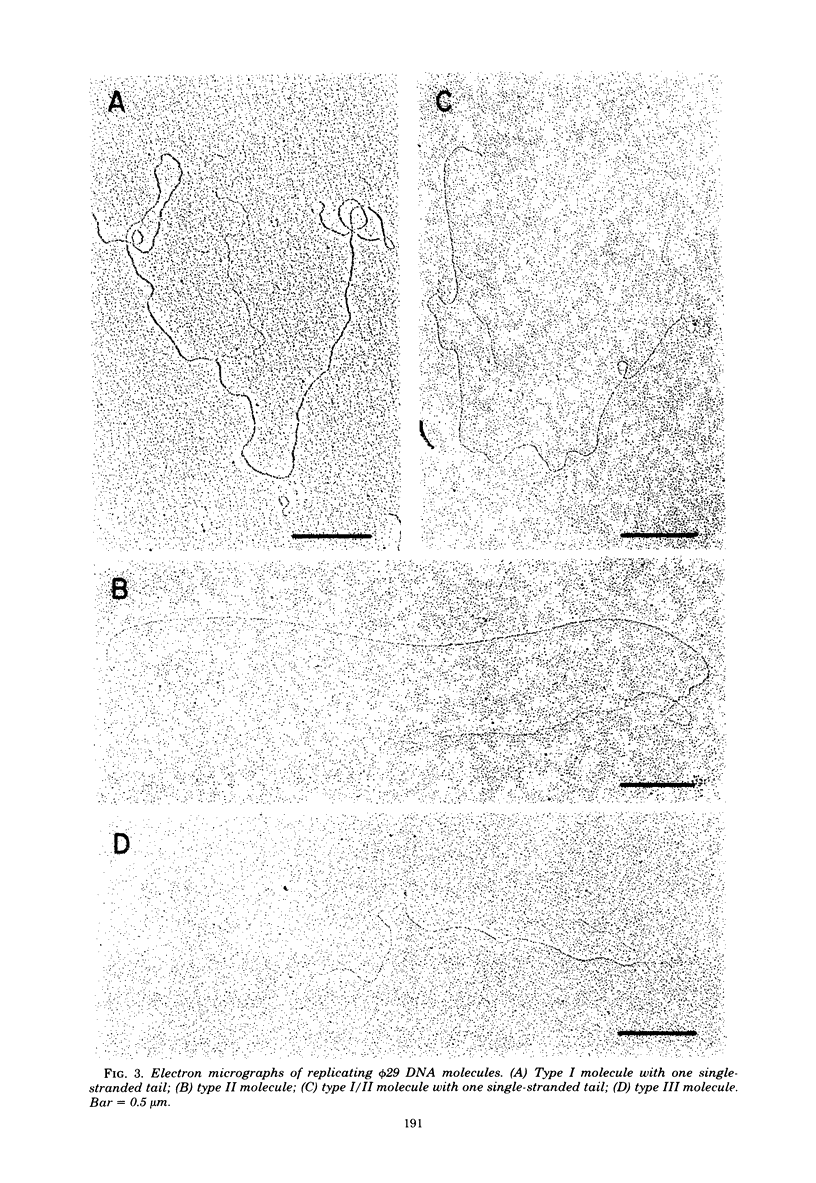
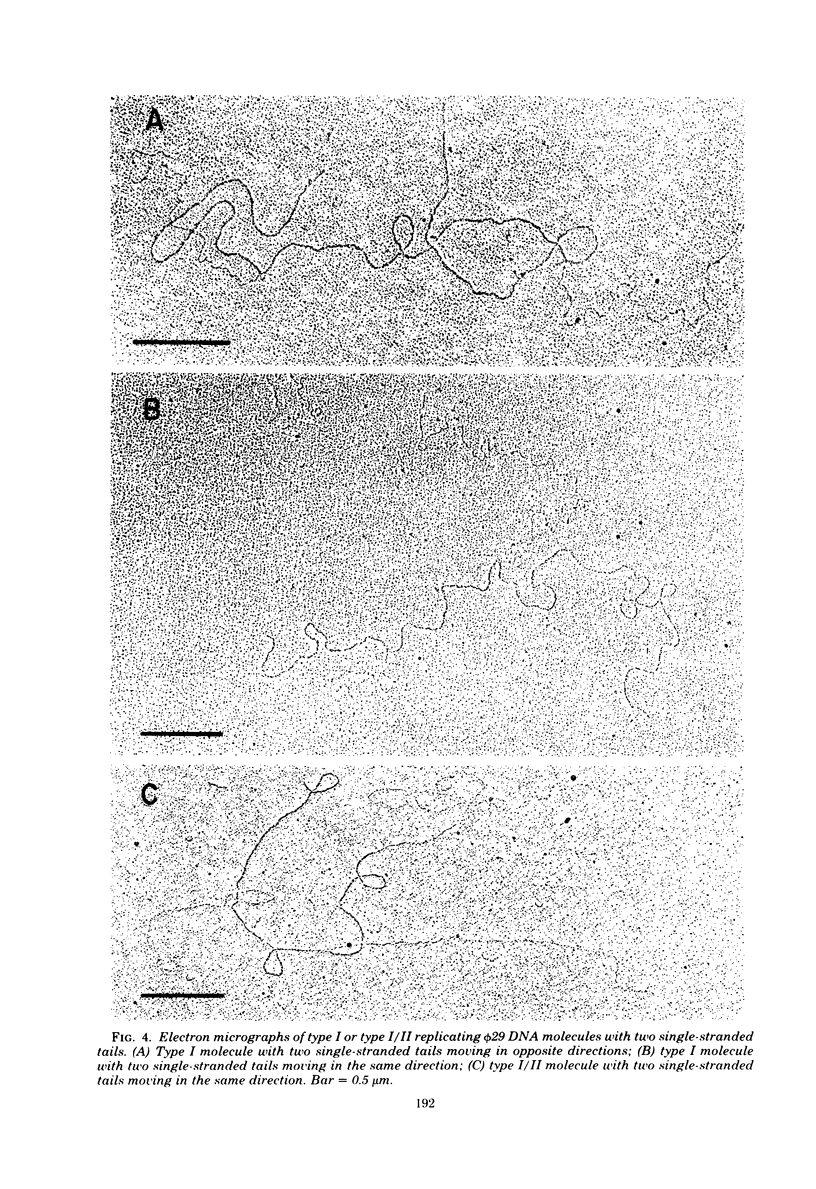
We isolated phi 29 DNA replicative intermediates from extracts of phage-infected Bacillus subtilis, pulsed-labeled with [3H]thymidine, by velocity sedimentation in neutral sucrose followed by CsCl equilibrium density gradient centrifugation. During a chase, the DNA with a higher sedimentation coefficient in neutral sucrose and a lower sedimentation rate in alkaline sucrose than that of viral phi 29 DNA was converted into mature DNA. The material with a density higher than that of mature phi 29 DNA consisted of replicative intermediates, as analyzed with an electron microscope. We found two major types of molecules. One consisted of unit-length duplex DNA with one single-stranded branch at a random position. The length of the single-stranded branches was similar to that of one of the double-stranded regions. The other type of molecules was unit-length DNA with one double-stranded region and one single-stranded region extending a variable distance from one end. Partial denaturation of the latter molecules showed that replication was initiated with a similar frequency from either DNA end. These findings suggest that phi 29 DNA replication occurs by a mechanism of strand displacement and that replication starts non-simultaneously from either DNA end, as in the case of adenovirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Shimojo H. Initiation and termination sites of adenovirus 12 DNA replication. Virology. 1977 May 15;78(2):415–424. doi: 10.1016/0042-6822(77)90118-0. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Roberts R. J. The nucleotide sequences at the termini of adenovirus-2 DNA. J Mol Biol. 1979 Mar 15;128(4):577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Moreno F., Jiménez F., Mellado R. P., Viñuela E., Salas M. Bacillus subtilis phage phi29. Characterization of gene products and functions. Eur J Biochem. 1976 Jul 1;66(2):229–241. doi: 10.1111/j.1432-1033.1976.tb10512.x. [DOI] [PubMed] [Google Scholar]
- Carrascosa J. L., Viñuela E., Salas M. Proteins induced in Bacillus subtilis infected with bacteriophage phi 29. Virology. 1973 Nov;56(1):291–299. [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen E. W., Reilly B. E., Tosi M. E., Anderson D. L. Analysis of gene function of bacteriophage phi 29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J Virol. 1976 Aug;19(2):501–517. doi: 10.1128/jvi.19.2.501-517.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hawley L. A., Reilly B. E., Hagen E. W., Anderson D. L. Viral protein synthesis in bacteriophage phi 29-infected Bacillus subtilis. J Virol. 1973 Nov;12(5):1149–1159. doi: 10.1128/jvi.12.5.1149-1159.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. Bacteriophage phi29 terminal protein: its association with the 5' termini of the phi29 genome. J Virol. 1978 Dec;28(3):895–904. doi: 10.1128/jvi.28.3.895-904.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra W., Van Wielink P. S., Sussenbach J. S. The visualization of a circular DNA-protein complex from adenovirions. Virology. 1977 Jan;76(1):444–447. doi: 10.1016/0042-6822(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Koller T., Sogo J. M., Bujard H. An electron microscopic method for studying nucleic acid-protein complexes. Visualization of RNA polymerase bound to the DNA of bacteriophages T7 and T3. Biopolymers. 1974 May;13(5):995–1009. doi: 10.1002/bip.1974.360130514. [DOI] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Moreno F. Suppressor-sensitive mutants and genetic map of Bacillus subtilis bacteriophage phi 29. Virology. 1974 Nov;62(1):1–16. doi: 10.1016/0042-6822(74)90298-0. [DOI] [PubMed] [Google Scholar]
- Ortin J., Viñuela E., Salas M., Vasquez C. DNA-protein complex in circular DNA from phage phi-29. Nat New Biol. 1971 Dec 29;234(52):275–277. doi: 10.1038/newbio234275a0. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bellett J. D. A circular DNA-protein complex adenoviruses and its possible role in DNA replication. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):523–531. doi: 10.1101/sqb.1974.039.01.064. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Inciarte M. R., Corral J., Viñuela E., Salas M. RNA polymerase binding sites and transcription map of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1979 Feb 5;127(4):411–436. doi: 10.1016/0022-2836(79)90230-4. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Rodeño P., Koller T., Viñuela E., Salas M. Comparison of the A-T rich regions and the Bacillus subtilis RNA polymerase binding sites in phage phi 29 DNA. Nucleic Acids Res. 1979 Sep 11;7(1):107–120. doi: 10.1093/nar/7.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D. W., Bieber D. Concentration of DNA solutions by extraction with 2-butanol. Biochim Biophys Acta. 1975 Jan 6;378(1):18–21. doi: 10.1016/0005-2787(75)90132-x. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. Studies on the mechanism of replication of adenovirus DNA. V. The location of termini of replication. Virology. 1977 Mar;77(1):149–157. doi: 10.1016/0042-6822(77)90414-7. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. The mechanism of replication of adenovirus DNA. VI. Localization of the origins of the displacement synthesis. Virology. 1978 Feb;84(2):509–517. doi: 10.1016/0042-6822(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Talavera A., Salas M., Viñuela E. Temperature-sensitive mutants affected in DNA synthesis in phage phi29 of Bacillus subtilis. Eur J Biochem. 1972 Dec 4;31(2):367–371. doi: 10.1111/j.1432-1033.1972.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky S., Kawamura F., Ito J. Thermolabile transfecting DNA from temperature-sensitive mutant of phage phi29. Nature. 1976 Jan 1;259(5538):60–63. doi: 10.1038/259060a0. [DOI] [PubMed] [Google Scholar]
- Yehle C. O. Genome-linked protein associated with the 5' termini of bacteriophage phi29 DNA. J Virol. 1978 Sep;27(3):776–783. doi: 10.1128/jvi.27.3.776-783.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]





