Abstract
The epidermal growth factor receptor (EGFR) is a validated target in squamous cell carcinoma of the head and neck (HNSCC). However, despite high expression of EGFR in these cancers, EGFR inhibitor monotherapy has only had modest activity. Potential mechanisms of resistance to EGFR-targeted therapies involve EGFR and Ras mutations, epithelial-mesenchymal transition, and activation of alternative and downstream pathways. Strategies to optimize EGFR-targeted therapy in head and neck cancer involve not only the selection for patients most likely to benefit but also employing combination therapies to target the network of pathways involved in tumor growth, invasion, angiogenesis, and metastasis.
1. Background
Epidermal Growth Factor Receptors in Squamous Cell Carcinomas of the Head and Neck
Epidermal growth factor receptor (EGFR) is a ubiquitously expressed transmembrane glycoprotein in the ErbB/HER family of receptor tyrosine kinase. These receptors are composed of an extracellular ligand-binding domain, a hydrophobic transmembrane segment, and an intracellular tyrosine kinase domain. Binding of natural ligands (amphiregulin and transforming growth factor alpha (TGF-α) in head and neck cancer) to EGFR results in a conformational change in EGFR. This promotes homo- or heterodimerization with other ErbB/HER family of receptors with subsequent autophosphorylation and activation of the tyrosine kinase (1). This activation of EGFR leads to the initiation of intracellular signaling pathways which regulate the activation of cell proliferation, invasion, angiogenesis, and metastasis (1).
High expression of EGFR occurs in most epithelial malignancies including head and neck squamous cell carcinoma (HNSCC) (1). Elevated expression of EGFR in HNSCC correlates with poor prognosis (1). Two therapeutic strategies have been implemented in the inhibition of EGFR. The first utilizes monoclonal antibodies (mAb) to target the extracellular domain of EGFR and the second targets the intracellular EGFR domain with small molecule tyrosine kinase inhibitors (TKIs) (including gefitinib, erlotinib, and lapatinib). Despite near universal expression of EGFR in HNSCC, treatment with these anti-EGFR agents has only been modestly active in patients. Two FDA-approved monoclonal antibodies for targeting EGFR are cetuximab (a chimeric IgG1 mAb) and panitumumab (a fully human IgG2 mAb). Preclinical data from Bonner et al in 2000 showed that cetuximab and concurrent radiation resulted in a greater decrease in cell proliferation in a number of HNSCC cell lines (2). A multicenter phase III trial demonstrated an improvement in median overall survival in locoregionally advanced HNSCC patients treated with curative intent with definitive radiotherapy combined with weekly cetuximab versus the same radiotherapy regimen alone (3). There was an improvement in 3-year survival by 10% in patients receiving concurrent cetuximab and radiotherapy (3). However, the efficacy of cetuximab with radiotherapy compared with standard concomitant chemoradiotherapy remains under investigation. Preclinical data show that there is at least an additive effect of both classes of EGFR inhibitors when combined with cisplatin in the treatment of HNSCC (4).
Furthermore, cetuximab combined with platinum-fluorouracil chemotherapy improves survival compared with platinum-fluorouracil alone in patients with recurrent or metastatic HNSCC (5, 6). Adding cetuximab increased median overall survival from 7.4 months in the platinum chemotherapy-alone group to 10.1 months in the group receiving chemotherapy plus cetuximab (7). In a phase II trial of gefitinib in patients with recurrent or metastatic HNSCC, the overall response rate with gefitinib was 11% (8). In a similar population of recurrent and/or metastatic HNSCC patients, erlotinib was shown by Soulieres et al to have a response rate of 4% (9). A phase I study of chemoradiotherapy combined with lapatinib, a dual inhibitor of EGFR and HER2, for locally advanced HNSCC reported an overall response of 81% (10). BIBW2992, an irreversible dual inhibitor of EGFR and HER2 tyrosine kinase, which binds to Cys773 of EGFR and Cys805 of HER2, is currently being evaluated in clinical trials for HNSCC (11). A feature of BIBW2992 is its broad activity against multiple receptors in the ErbB family making it theoretically more effectively against tumor cells containing several ErbB family members and heterodimerizations. In preclinical studies it has been shown to inhibit cellular proliferation of lung cancer cell lines resistant to erlotinib, and cause tumor regression in xenografts and transgenic lung cancer models (11).
Mechanisms of Resistance to EGFR-Targeted Therapies
Even with high levels of EGFR expression within the tumor, clinical data demonstrate that many patients are refractory to EGFR inhibitor treatment underscoring that simple EGFR expression is not a reliable predictor of response to therapy. Primary resistance occurs in patients who either do not achieve stable disease or who progress within months after an initial clinical response while secondary or acquired resistance typically occurs after prolonged treatment. The majority of patients with HNSCC will be resistant to EGFR inhibitors and the mechanisms underlying this observation [Table 1] are beginning to be understood.
Table 1.
Mechanisms of Resistance to EGFR-Targeted Therapies
| EGFR Mutations |
|
| Ras Mutations |
|
| Epithelial-Mesenchymal Transition |
|
| Activation of Alternative/Downstream Pathways |
|
Among the first genetic alterations of the EGFR that have been identified, the type-III mutated variant (EGFRvIII) is characterized by an in-frame deletion from exons 2 through 7 in the extracellular domain which inhibits EGF and other EGFR ligands from binding and leads to constitutive activation of its tyrosine kinase domain (1). Structural changes in extracellular EGFR are hypothesized to affect the intracellular domain conformation and the ATP pocket leading to the constitutive activation by EGFRvIII and its resistance to EGFR-targeted therapy by monoclonal antibodies against the extracellular domain of EGFR (1). Irreversible EGFR inhibitors such as BIBW2992 have the added advantage in preclinical studies of being effective against EGFRvIII (12).
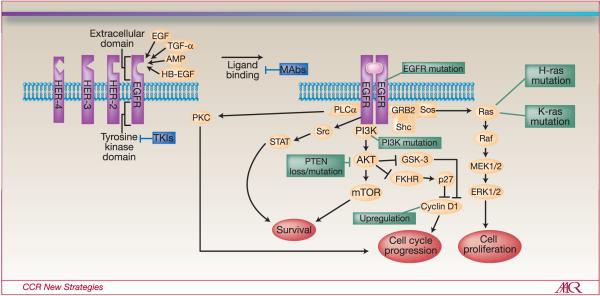
K-ras (v-KI-RAS2 Kirsten rat sarcoma viral oncogene homolog) mutations also predict resistance to EGFR inhibitors (Figure 1) (13). Retrospective analyses of clinical trials using TKIs have demonstrated a significant decrease in time to progression and survival in the presence of K-ras mutations in colorectal cancer (13). Data also suggest K-ras mutation status to be predictive of lack of response to mAbs cetuximab and panitumumab in metastatic colorectal cancer patients and is also associated with a worse prognosis (14, 15). However, K-ras mutations infrequently occur in HNSCC. Of the Ras proteins, however, H-ras mutations in HNSCC are likely more common than K-ras mutations and may play an important role in resistance to EGFR-targeted therapies (16, 17).
Figure 1.
EGFR Signaling Pathway and Several Mechanisms of Resistance to EGFR-Targeted Therapies
Abbreviations:
EGFR: epidermal growth factor receptor
K-Ras: v-KI-RAS2 Kirsten rat sarcoma viral oncogene homolog
H-Ras: v-Ha-ras Harvey rat sarcoma viral oncogene homolog
PTEN: phosphatidylinositol phosphatase
mTOR: mammalian target of rapamycin
mAbs: monoclonal antibodies
TKIs: tyrosine kinase inhibitors
In NSCLC, both high EGFR gene copy number as determined by fluorescent in situ hybridization (FISH) and EGFR tyrosine kinase mutations that lead to increased protein activity after ligand binding appear to be correlated with improved response to TKI (18). Even so, the same correlation rarely exists in HNSCC. In fact, EGFR TK mutations appear to be rare events in HNSCC underscoring essential differences between the two diseases. Moreover, recent findings by Lictra, et al demonstrated no association between overall survival in HNSCC and EGFR gene copy number as determined by FISH (19). The same negative findings were observed in a randomized study of gefitinib vs. methotrexate in recurrent/metastatic HNSCC where EGFR gene copy number was not predictive of a survival benefit in subjects treated with the EGFR TKI. Interestingly, response to TKIs in a subset of HNSCC may in fact be due to mutations in ErbB2 rather than EGFR but this preliminary finding has yet to be validated. (20).
Secondary or acquired resistance typically occurs in the setting of prolonged treatment. Several mechanisms contribute to the development of resistance including epithelial-mesenchymal transition, the development of secondary mutations in EGFR, activation of alternative pathways, and constitutive activation of downstream pathways. Epithelial to mesenchymal transition (EMT) is characterized by a change in the morphology with loss of polarity and cell-cell contacts by the epithelial cells with increased vimentin expression, and decreased E-cadherin, claudins 4 and 7 expression (21). Preclinical models of NSCLC cell lines and xenografts demonstrated a correlation between a mesenchymal phenotype and erlotinib resistance (21). In addition, clinical data in NSCLC patients receiving erlotinib and chemotherapy showed time to progression was better in patients with E-cadherin positive staining (22). Recently, cortactin, a cytoskeletal protein that regulates actin assembly, receptor-mediated endocytosis, and epithelial to mesenchymal phenotypic conversion of cells, has been associated with gefitinib resistance and invasive phenotype in HNSCC (23, 24). In addition, the E-cadherin repressor delta-crystallin enhancer binding factor 1 (deltaEF1) was recently identified as a regulator of mesenchymal phenotype and correlated with erlotinib resistance in HNSCC in vitro (24).
Inherent cell signaling is a significant factor in response to therapy. Upregulation of cyclin D1 in HNSCC cell lines is specifically associated with resistance to gefitinib by hyperphosphorylation of retinoblastoma protein (pRb) by cyclin D1-cyclin dependent kinase 4 (CDK4) (25). Furthermore, mutations or decreased expression of PTEN, a phosphatase regulator of PI3K/AKT signaling, is also associated with EGFR inhibitor resistance [Figure 1] (26). In cells dependent on EGFR, loss of PTEN was shown to uncouple the EGFR from its downstream signaling pathway and the presence of constitutive activation of AKT leads to cell survival independently of EGFR (26).
Cumulative evidence suggests that activation of signaling pathways downstream of EGFR may contribute to resistance to upstream inhibition of EGFR. Cancer cells have been shown to selectively activate alternative signaling pathways in the setting of single pathway inhibition. Stommel et al reported that in glioblastoma cell lines, xenografts and primary tumors, various receptor tyrosine kinases are simultaneously activated resulting in the sustained activation of signaling pathways in the face of receptor TKI monotherapy (27). In fact, blockade of specific pathways have been shown to initiate feedback mechanisms that trigger pro-survival signaling cascades in cancer. For example, inhibition of the PI3K/Akt pathway stimulates the MAPK/ERK signaling cascade in different cancer models (28).
Activation of cytoplasmic signaling pathways in the setting of EGFR blockade can occur through several mechanisms including: 1) concomitant activation of other receptor and non-receptor kinases including c-Met, IGF-1R, and Src family kinases, among others; 2) G-protein-coupled receptor (GPCR)-mediated activation of EGFR-independent pathways; and/or 3) induction of alternative oncogenic pathways by EGFR blockade. Signal transducer and activator of transcription-3 (STAT3) mediates proliferative, survival and invasion pathways in HNSCC induced by upstream activation of EGFR, Src and/or IL-6/gp130 (29, 30). We previously reported that increased activation of signal transducer and STAT3 was associated with increased resistance to EGFR tyrosine kinase inhibition in HNSCC (31). Further investigation demonstrated that siRNA-mediated knockdown of EGFR or treatment with cetuximab induced oncogenic signaling through activation of p70S6 kinase in HNSCC, in the setting of GPCR stimulation (unpublished observations).
Single nucleotide polymorphisms (SNPs) play a role in drug pharmacokinetics and pharmacodynamic processes. They are not only a factor in drug efficacy but also contribute to drug toxicity. The first intron of the EGFR gene has an important regulatory function and contains a heritable polymorphic microsatellite sequence of 9–23 CA repeats (32). The number of CA repeats is inversely proportional to EGFR expression on both mRNA and protein level in vitro (32). Two SNPs associated with increased expression of EGFR are in the promoter (−216G>T and −191C>A) (33). Preclinical data show a nonsynonymous SNP (1808G>A) in the extracellular domain of EGFR is associated with a lower affinity for ligand (EGF and TGFα) binding and an abated growth response (34). Amador et al. reported higher sensitivity to erlotinib in cell lines with less than or equal to 35 CA repeats compared with cell lines with greater than 35 repeats. There was also increased incidence of skin toxicity in gefitinib treated colorectal patients with less than or equal to 35 CA repeats (32). However, in a single arm study in patients with HNSCC, NSCLC, and ovarian cancer treated with erlotinib, even though there was a correlation between diarrhea and two EGFR promoter SNPs, the same correlation was not seen with skin toxicity (35).
EGFR-targeted monoclonal antibodies (mAbs), but not tyrosine kinase inhibitors, are FDA-approved for use in HNSCC. This apparent increased activity of antibody-mediated therapeutic strategies suggests that the immune system may contribute to clinical responses to EGFR targeting. Currently, the two FDA-approved mAbs targeting EGFR are cetuximab and panitumumab. Monoclonal antibodies recognize determinants expressed on the extracellular domain of EGFR and antagonize normal ligand-receptor interactions thereby disrupting downstream signaling. The mechanism(s) underlying the clinical response to EGFR-specific mAb-based immunotherapy are poorly understood. Evidence to date suggests that mAbs may induce activation of cellular immunity, including natural killer and T cells, thereby contributing to clinical response. Monoclonal antibodies have been shown to mediate antibody-dependent cellular cytotoxicity, complement-dependent lysis, and activation of tumor antigen-specific T cells. Cell-mediated cytotoxicity of target cells triggered by EGFR-specific mAbs appears to play a role in the clinical outcome of colorectal carcinoma patients (36). The variables influencing the extent of lysis of HNSCC cells by NK cells and EGFR-specific mAbs have been characterized, and have shown to include the level of EGFR expression, the amount of mAb, and the genotype of the Fcγ receptor (FcγR) which mediates the interactions of NK cells with the mAbs bound to target cells, i.e. FcγR IIIa (37). In addition, mAbs may mediate NK cell-dependent lysis of HNSCC cells. The lysis of HNSCC cells by NK cells and the EGFR-specific mAb cetuximab may trigger a series of events, which lead to the generation of cytotoxic T lymphocytes (CTL) recognizing tumor antigens expressed on the HNSCC cells. A variety of factors such as polymorphisms in Fcγ receptors expressed by immune cells, activity of T-regulatory cells, and tumor escape through downregulation of antigen-processing machinery in tumor cells, may modulate the immune activation mediated by therapeutic mAbs. Understanding the interplay of these factors is likely to improve the selection of the most appropriate candidates for mAb-based immunotherapy, prediction of clinical response, and our understanding of mechanisms of tumor escape from therapeutic mAbs.
2. On the Horizon
Predictive Markers for Response to EGFR-Targeted Therapies
In the current era of targeted therapies, the identification of predictive markers remains a challenge. Predicting outcome in EGFR-targeted therapies is complex, involves genetic and clinical characteristics, and the interplay of a network of pathways. The human papilloma virus (HVP), i.e. HPV type 16, has recently been identified to be associated with a subset of HNSCC, especially those arising from the lingual or palatine tonsils (38). Studies in oropharyngeal cancers have shown an association between lower HPV titers and high EGFR expression with worse overall survival (39). Several studies report a positive association between EGFR gene amplification and response to EGFR-directed antibody treatment in NSCLC and metastatic colorectal cancers (40, 41). Although recent reports suggest that EGFR gene copy number by FISH is not correlated with response in HNSCC, different methodologies and scoring methods are used which is compounded by the intra-tumor heterogeneity observed in EGFR gene copy in HNSCC. These factors account for the variation in EGFR gene amplification and copy number rates reported thus far in HNSCC and further investigation to characterize the role of EGFR gene amplification remains to be performed. The presence of EGFR gene amplification in a significant portion of HNSCC suggests that this still may be a potential predictive marker for response to EGFR-targeted therapies.
Other candidate predictive markers for EGFR-targeted therapies include K-ras/H-ras mutations, PI3k/Akt pathway mutations, and polymorphisms in EGFR, FcγRIIa and FcγRIIIa. K-ras mutations in NSCLC vary from 8–20% and approximately 30% in colon cancers (42). K-ras mutations are relatively rare (3–7%) in HNSCC; however, H-ras mutations occur at a higher rate (22% per one published study available) and may be a potential marker for decreased response to EGFR-targeted treatment (16, 17). Interestingly, H-ras mutations frequently occur in HNSCC patients from India and Southeast Asia and the oral carcinogenesis is likely related to betel quid use (43, 44). In metastatic colorectal cancer, mutations in the PI3K catalytic subunit (PIK3CA) have been reported to correlate with EGFR mAb resistance (45). Although PIK3CA mutations only occur in up to 8% of HNSCC, loss of the expression of the phosphatidylinositol phosphatase, PTEN, results in the loss of negative regulation on the Akt signaling pathway which leads to resultant activation of downstream survival mechanisms (26). Therefore, even though few data in HNSCC are available, the data from metastatic colorectal cancer suggest PTEN expression, PIK3CA mutation status, and Akt amplification may be potential predictive markers in HNSCC and warrant further investigation.
Finally, matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) has been shown to predict survival after treatment of lung cancer patients with EGFR-targeted therapy and recent data suggest MALDI-MS also has predictive value in HNSCC (46). A MALDI-MS profile was previously determined from over four hundred NSCLC patient serum or plasma samples to predict overall survival after EGFR TKI treatment (47). More than 300 samples from 5 HNSCC treatment groups (gefitinib, erlotinib and bevacizumab, cetuximab, surgery, and palliative chemotherapy) were then stratified into good or poor prognostic groups using the same MALDI-MS algorithm generated from NSCLC patients. Results demonstrated 98% success in classifying the HNSCC samples and predicting survival benefit in the EGFR-inhibitor treated groups (46).
The observation that cetuximab, but not EGFR TKI, prolong HNSCC survival when combined with standard therapeutic approaches suggests that the mechanism of EGFR targeting may be important (48). Based on improved anti-tumor effects in HNSCC preclinical models when EGFR expression was downregulated, a phase I antisense gene therapy trial was carried out (49). In this study, EGFR antisense therapy decreased EGFR protein expression in nearly all 17 patients treated where higher baseline levels of EGFR in the tumor were associated with an enhanced clinical response (49). Studies are underway to develop an antisense strategy to target EGFR that can be safely and effectively delivered systemically to HNSCC patients. In addition to antisense, RNA interference (RNAi) approaches are being developed to suppress EGFR expression as a potential clinical strategy (50).
Combination Therapy to Overcome Resistance to EGFR-Targeted Therapy
One way to overcome resistance to EGFR-targeted therapy is to use a combination of monotherapeutic agents with different mechanisms of action to target the network of pathways involved in HNSCC pathogenesis. Approaches to combination therapy can involve adding STAT or SRC inhibitors, c-MET or IGFR inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and other receptor TKIs, to anti-EGFR agents. Preclinical studies of HNSCC involving both EGFR and STAT3 inhibition have been effective in increasing apoptosis (51). EGFR and IGF-IR provide compensatory activation of similar downstream pathways when either is inhibited. AKT is controlled by both EGFR and IGF-IR. Buck et al demonstrated that inhibition of EGFR or IGFR activated the reciprocal receptor with a shift of EGFR inhibition of AKT from EGFR to IGF-IR (52). Guix et al reported the association between loss of IGF-binding protein as a mechanism of acquired EGFR TKI resistance and suggests the combination of EGFR and IGFIR inhibitors may be effective in abrogating this resistance(53). Similarly, gastric cancer and breast cancer cells treated with the combination of anti-EGFR and a c-MET inhibitor resulted in decreased cell proliferation warranting preclinical evaluation of this combination in HNSCC (54, 55).
mTOR inhibitors block activation of PI3K and AKT pathway signals involved in cellular proliferation and angiogenesis (56). Studies involving mTOR inhibitors and TKIs (erlotinib and gefitinib) have demonstrated an additive antitumor effect in HNSCC, colon and pancreatic cancer cell lines forming the basis for ongoing phase I trials of the mTOR inhibitor RAD001 in combination with cetuximab (56, 57). The vast majority of HNSCC express VEGF or VEGFR and therapy combining bevacizumab (a monoclonal antibody against VEGF-A) and erlotinib are promising with a response rate of 15% (58). Preclinical studies of human A431 squamous cell cancer xenografts show acquired resistance to mAb can develop via increased expression of VEGF further underscoring the multiple growth controlling pathways involved in tumorigenesis (59). Targeting other ErbB family receptors may also have synergistic effect in the treatment of HNSCC. Overexpression of ErbB2 is associated with gefitinib resistance and combination of pertuzumab (a monoclonal antibody against ErbB2) with gefitinib in HNSCC cell lines resistant to gefinitib monotherapy resulted in increased inhibition of cell growth (60). The role of ErbB3 and ErbB4 in HNSCC remains under investigation.
3. Summary
HNSCC is a heterogeneous disease and despite high expression of EGFR, resistance to EGFR-targeted therapies, especially as monotherapy, is common. Because EGFR signaling involves an interplay of other oncogenic pathways, improving response to EGFR-targeted therapies will not only involve using different genomic and proteomic biomarkers to select for improved patient response but also utilizing combination therapies to target the multiple pathways involved in neoplastic transformation. Currently, no biomarker has proven to predict response to EGFR-targeted therapy. Future studies will lead to improved optimization of the current challenges in anti-EGFR therapy.
REFERENCES
- 1.Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003 Jul;39(10):1348–54. doi: 10.1016/s0959-8049(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 2.Bonner JA, Raisch KP, Trummell HQ, Robert F, Meredith RF, Spencer SA, et al. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J Clin Oncol. 2000 Nov 1;18(21 Suppl):47S–53S. [PubMed] [Google Scholar]
- 3.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006 Feb 9;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 4.Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999 Nov;291(2):739–48. [PubMed] [Google Scholar]
- 5.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005 Dec 1;23(34):8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 6.Bourhis J, Rivera F, Mesia R, Awada A, Geoffrois L, Borel C, et al. Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006 Jun 20;24(18):2866–72. doi: 10.1200/JCO.2005.04.3547. [DOI] [PubMed] [Google Scholar]
- 7.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008 Sep 11;359(11):1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 8.Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003 May 15;21(10):1980–7. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 9.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004 Jan 1;22(1):77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 10.Harrington KJ, El-Hariry IA, Holford CS, Lusinchi A, Nutting CM, Rosine D, et al. Phase I study of lapatinib in combination with chemoradiation in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2009 Mar 1;27(7):1100–7. doi: 10.1200/JCO.2008.17.5349. [DOI] [PubMed] [Google Scholar]
- 11.Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer. 2008 Jan 15;98(1):80–5. doi: 10.1038/sj.bjc.6604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006 Sep 1;12(17):5064–73. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 13.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005 Sep 1;23(25):5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 14.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006 Apr 15;66(8):3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 15.Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007 Apr 23;96(8):1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC) Curr Protoc Hum Genet. 2008 Apr; doi: 10.1002/0471142905.hg1011s57. Chapter 10:Unit 10 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson JA, Irish JC, McLachlin CM, Ngan BY. H-ras oncogene mutation and human papillomavirus infection in oral carcinomas. Arch Otolaryngol Head Neck Surg. 1994 Jul;120(7):755–60. doi: 10.1001/archotol.1994.01880310059011. [DOI] [PubMed] [Google Scholar]
- 18.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007 Feb 10;25(5):587–95. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 19.Licitra LRF, Bokemeyer C, Remenar E, Kienzer H, Stoerkel S, Scheid S, Stroh C, Mesia C. Biomarker potential of EGFR gene copy number by FISH in the phase III EXTREME study: Platinum-based CT plus cetuximab in first-line R/M SCCHN. J Clin Oncol. 2009;27:15s. 2009 (suppl; abstr 6005) [Google Scholar]
- 20.Cohen EE, Lingen MW, Martin LE, Harris PL, Brannigan BW, Haserlat SM, et al. Response of some head and neck cancers to epidermal growth factor receptor tyrosine kinase inhibitors may be linked to mutation of ERBB2 rather than EGFR. Clin Cancer Res. 2005 Nov 15;11(22):8105–8. doi: 10.1158/1078-0432.CCR-05-0926. [DOI] [PubMed] [Google Scholar]
- 21.Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005 Oct 15;65(20):9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 22.Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005 Dec 15;11(24 Pt 1):8686–98. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 23.Timpson P, Wilson AS, Lehrbach GM, Sutherland RL, Musgrove EA, Daly RJ. Aberrant expression of cortactin in head and neck squamous cell carcinoma cells is associated with enhanced cell proliferation and resistance to the epidermal growth factor receptor inhibitor gefitinib. Cancer Res. 2007 Oct 1;67(19):9304–14. doi: 10.1158/0008-5472.CAN-07-0798. [DOI] [PubMed] [Google Scholar]
- 24.Haddad Y, Choi W, McConkey DJ. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clin Cancer Res. 2009 Jan 15;15(2):532–42. doi: 10.1158/1078-0432.CCR-08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalish LH, Kwong RA, Cole IE, Gallagher RM, Sutherland RL, Musgrove EA. Deregulated cyclin D1 expression is associated with decreased efficacy of the selective epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2004 Nov 15;10(22):7764–74. doi: 10.1158/1078-0432.CCR-04-0012. [DOI] [PubMed] [Google Scholar]
- 26.Sos ML, Koker M, Weir BA, Heynck S, Rabinovsky R, Zander T, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009 Apr 15;69(8):3256–61. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of Receptor Tyrosine Kinases Affects the Response of Tumor Cells to Targeted Therapies. Science. 2007 October 12;318(5848):287–90. doi: 10.1126/science.1142946. 2007. [DOI] [PubMed] [Google Scholar]
- 28.Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2007;27(20):2934–40. doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- 29.Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003 Jun 1;63(11):2948–56. [PubMed] [Google Scholar]
- 30.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003 Aug 22;278(34):31574–83. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 31.Kijima T, Niwa H, Steinman RA, Drenning SD, Gooding WE, Wentzel AL, et al. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002 Aug;13(8):355–62. [PubMed] [Google Scholar]
- 32.Amador ML, Oppenheimer D, Perea S, Maitra A, Cusatis G, Iacobuzio-Donahue C, et al. An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res. 2004 Dec 15;64(24):9139–43. doi: 10.1158/0008-5472.CAN-04-1036. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Innocenti F, Wu MH, Desai AA, Dolan ME, Cook EH, Jr., et al. A functional common polymorphism in a Sp1 recognition site of the epidermal growth factor receptor gene promoter. Cancer Res. 2005 Jan 1;65(1):46–53. [PubMed] [Google Scholar]
- 34.Moriai T, Kobrin MS, Hope C, Speck L, Korc M. A variant epidermal growth factor receptor exhibits altered type alpha transforming growth factor binding and transmembrane signaling. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10217–21. doi: 10.1073/pnas.91.21.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudin CM, Liu W, Desai A, Karrison T, Jiang X, Janisch L, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008 Mar 1;26(7):1119–27. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy EM, Sycz G, Arriaga JM, Barrio MM, von Euw EM, Morales SB, et al. Cetuximab-mediated cellular cytotoxicity is inhibited by HLA-E membrane expression in colon cancer cells. Innate Immun. 2009 Apr;15(2):91–100. doi: 10.1177/1753425908101404. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009 Nov;58(11):1853–64. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimers N, Kasper HU, Weissenborn SJ, Stutzer H, Preuss SF, Hoffmann TK, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007 Apr 15;120(8):1731–8. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 39.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008 Jul 1;26(19):3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappuzzo F, Finocchiaro G, Rossi E, Janne PA, Carnaghi C, Calandri C, et al. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol. 2008 Apr;19(4):717–23. doi: 10.1093/annonc/mdm492. [DOI] [PubMed] [Google Scholar]
- 41.Hirsch FR, Herbst RS, Olsen C, Chansky K, Crowley J, Kelly K, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008 Jul 10;26(20):3351–7. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Calvez F, Mukeria A, Hunt JD, Kelm O, Hung RJ, Taniere P, et al. TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: distinct patterns in never, former, and current smokers. Cancer Res. 2005 Jun 15;65(12):5076–83. doi: 10.1158/0008-5472.CAN-05-0551. [DOI] [PubMed] [Google Scholar]
- 43.Murugan AK, Hong NT, Cuc TT, Hung NC, Munirajan AK, Ikeda MA, et al. Detection of two novel mutations and relatively high incidence of H-RAS mutations in Vietnamese oral cancer. Oral Oncol. 2009 Oct;45(10):e161–6. doi: 10.1016/j.oraloncology.2009.05.638. [DOI] [PubMed] [Google Scholar]
- 44.Munirajan AK, Mohanprasad BK, Shanmugam G, Tsuchida N. Detection of a rare point mutation at codon 59 and relatively high incidence of H-ras mutation in Indian oral cancer. Int J Oncol. 1998 Nov;13(5):971–4. doi: 10.3892/ijo.13.5.971. [DOI] [PubMed] [Google Scholar]
- 45.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009 Mar 1;69(5):1851–7. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 46.Chung CH, Seeley EH, Grigorieva J, Yarbrough WG, Gilbert J, Murphy BA, et al. Mass spectrometry profile as a predictor of overall survival benefit after treatment with epidermal growth factor receptor inhibitors in head and neck squamous cell carcinoma. J Clin Oncol (Meeting Abstracts) 2009 May 20;27(15S):6000. 2009. [Google Scholar]
- 47.Taguchi F, Solomon B, Gregorc V, Roder H, Gray R, Kasahara K, et al. Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. J Natl Cancer Inst. 2007 Jun 6;99(11):838–46. doi: 10.1093/jnci/djk195. [DOI] [PubMed] [Google Scholar]
- 48.Argiris AGM, Gilbert J, Burtness B, Forastiere A. A Phase III randomized, placebo-controlled trial of docetaxel (D) with or without gefitinib (G) in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): A trail of the Eastern Cooperative Oncology Group (ECOG) J Clin Oncol. 2009;27:15s. doi: 10.1200/JCO.2012.45.4272. 2009 (suppl; abstr 6011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai SY, Koppikar P, Thomas SM, Childs EE, Egloff AM, Seethala RR, et al. Intratumoral epidermal growth factor receptor antisense DNA therapy in head and neck cancer: first human application and potential antitumor mechanisms. J Clin Oncol. 2009 Mar 10;27(8):1235–42. doi: 10.1200/JCO.2008.17.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang C, Pu P, Jiang H. Silencing epidermal growth factor receptor by RNA interference in glioma. Methods Mol Biol. 2009;542:335–49. doi: 10.1007/978-1-59745-561-9_18. [DOI] [PubMed] [Google Scholar]
- 51.Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4138–43. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008 Oct 15;68(20):8322–32. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 53.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008 Jul;118(7):2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bachleitner-Hofmann T, Sun MY, Chen CT, Tang L, Song L, Zeng Z, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther. 2008 Nov;7(11):3499–508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 55.Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008 May 1;68(9):3314–22. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azzariti A, Porcelli L, Gatti G, Nicolin A, Paradiso A. Synergic antiproliferative and antiangiogenic effects of EGFR and mTor inhibitors on pancreatic cancer cells. Biochem Pharmacol. 2008 Mar 1;75(5):1035–44. doi: 10.1016/j.bcp.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Jimeno A, Kulesza P, Wheelhouse J, Chan A, Zhang X, Kincaid E, et al. Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br J Cancer. 2007 Mar 26;96(6):952–9. doi: 10.1038/sj.bjc.6603656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seiwert TY, Cohen EE. Targeting angiogenesis in head and neck cancer. Semin Oncol. 2008 Jun;35(3):274–85. doi: 10.1053/j.seminoncol.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001 Jul 1;61(13):5090–101. [PubMed] [Google Scholar]
- 60.Erjala K, Sundvall M, Junttila TT, Zhang N, Savisalo M, Mali P, et al. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin Cancer Res. 2006 Jul 1;12(13):4103–11. doi: 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]