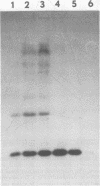
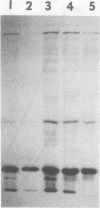
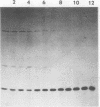
Abstract
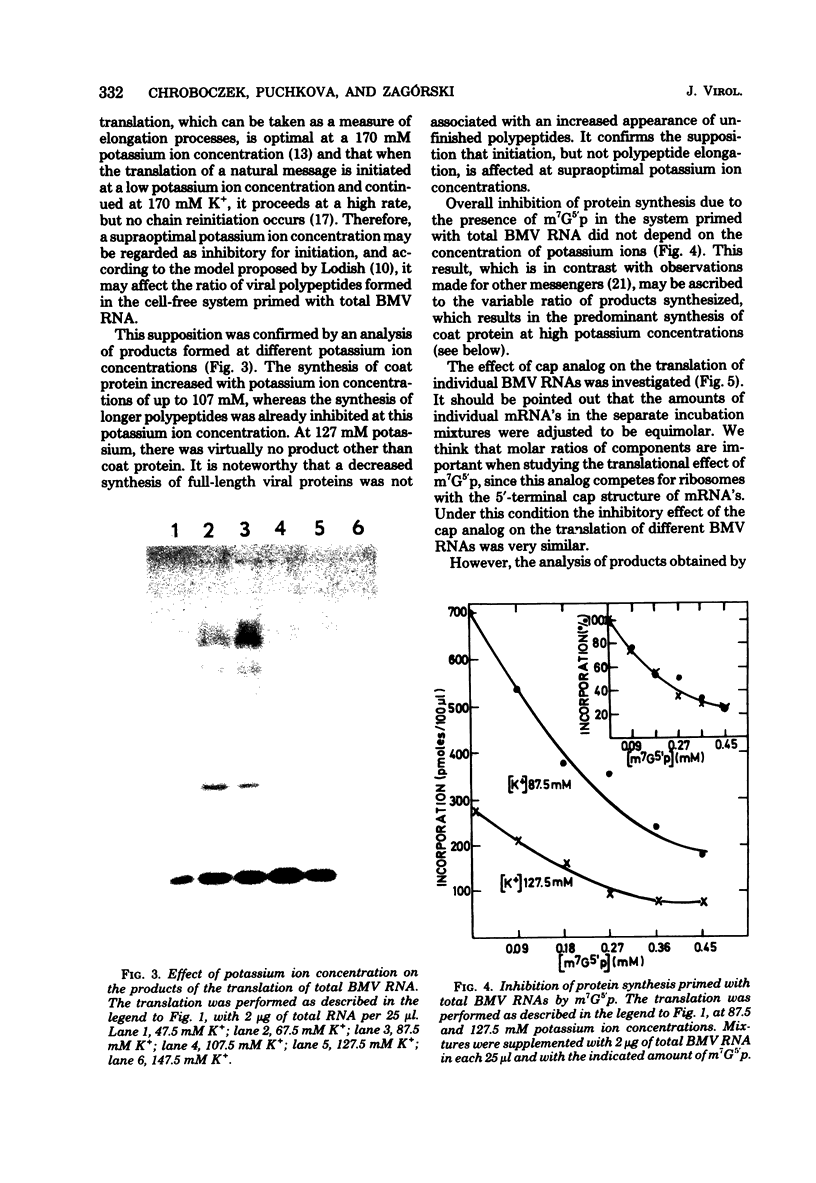
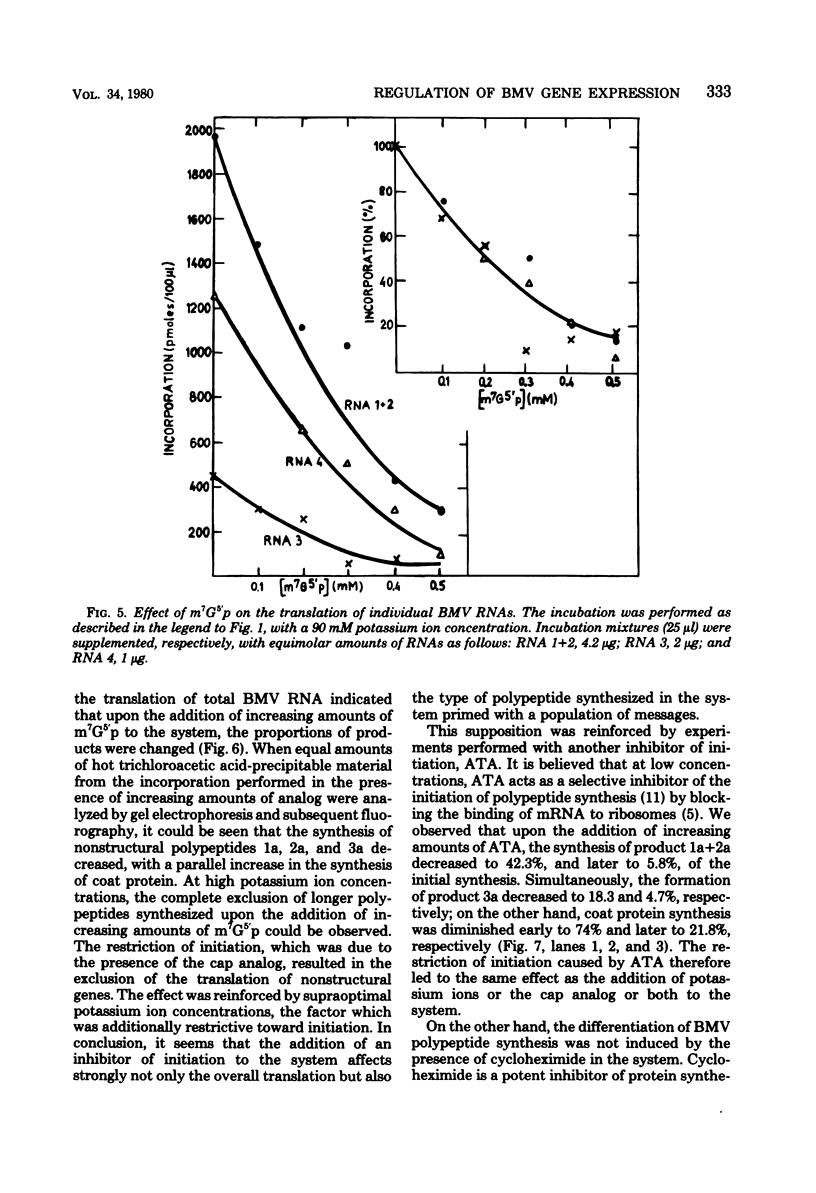
The translation of total and individual brome mosaic virus (BMV) RNAs was examined in a wheat germ cell-free system in the presence of various inhibitors. Inhibitors of the initiation of polypeptide synthesis, e.g., potassium ions, 7-methylguanosine 5′ -monophosphate, and aurintricarboxylic acid, were shown not only to inhibit overall BMV protein synthesis but also to change the ratio of BMV polypeptides synthesized. Under conditions restrictive for initiation, the translation of nonstructural BMV genes was suppressed, but coat protein synthesis proceeded at a high rate. A similar discrimination among BMV messengers was exerted by a regulatory protein kinase isolated from wheat germ. These results suggest that the regulation of the expression of BMV genes is based on a difference in the mechanism of formation of initiation complexes for individual BMV messages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Shih D. S., Zimmern D., Kaesberg P. Two-step binding of eukaryotic ribosomes to brome mosaic virus RNA3. Nature. 1979 Sep 27;281(5729):277–282. doi: 10.1038/281277a0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Fresno M., Vazquez D. Inhibitors of polypeptide elongation on yeast polysomes. J Antibiot (Tokyo) 1975 Jun;28(6):453–462. doi: 10.7164/antibiotics.28.453. [DOI] [PubMed] [Google Scholar]
- Benicourt C., Haenni A. L. Differential translation of turnip yellow mosaic virus mRNAs in vitro. Biochem Biophys Res Commun. 1978 Oct 30;84(4):831–839. doi: 10.1016/0006-291x(78)91659-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Grollman A. P. Effects of aurintricarboxylic acid on ribosomes and the biosynthesis of globin in rabbit reticulocytes. Mol Pharmacol. 1972 Mar;8(2):111–127. [PubMed] [Google Scholar]
- Kaesberg P. Structure and function of the RNAs of brome mosaic virus. Prog Nucleic Acid Res Mol Biol. 1976;19:465–471. doi: 10.1016/s0079-6603(08)60937-x. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Migration of 40 S ribosomal subunits on messenger RNA in the presence of edeine. J Biol Chem. 1978 Sep 25;253(18):6568–6577. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane L. C. The bromoviruses. Adv Virus Res. 1974;19:151–220. doi: 10.1016/s0065-3527(08)60660-0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- Marcus A., Bewley J. D., Weeks D. P. Aurintricarboxylic acid and initiation factors of wheat embryo. Science. 1970 Mar 27;167(3926):1735–1736. doi: 10.1126/science.167.3926.1735. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of the RNAs of brome mosaic virus: the monocistronic nature of RNA1 and RNA2. J Mol Biol. 1976 May 5;103(1):77–88. doi: 10.1016/0022-2836(76)90053-x. [DOI] [PubMed] [Google Scholar]
- Stubbs J. D., Kaesberg P. Amino acid incorporation in an Escherichia coli cell-free system directed by bromegrass mosaic virus ribonucleic acid. Virology. 1967 Nov;33(3):385–397. doi: 10.1016/0042-6822(67)90114-6. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Baglioni C. Influence of potassium salt concentration and temperature on inhibition of mRNA translation by 7-methylguanosine5'-monophosphate. J Biol Chem. 1978 Jan 10;253(1):178–183. [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Maroney P. A., Baglioni C. Inhibition of protein synthesis by Cl-. J Biol Chem. 1977 Jun 10;252(11):4007–4010. [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Nuss D. L., Baglioni C. 5'-Terminal 7-methylguanosine and mRNA function: influence of potassium concentration on translation in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3254–3258. doi: 10.1073/pnas.74.8.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]