Summary
Many studies have addressed the effect of dietary glycemic index on obesity and diabetes, but little is known about its effect on lifespan itself. We found that adding a small amount of glucose to the medium (0.1-2%) shortened the lifespan of C. elegans. Glucose shortened lifespan by inhibiting the activities of lifespan-extending transcription factors that are also inhibited by insulin signaling: the FOXO family member DAF-16 and the heat shock factor HSF-1. This effect involved the down-regulation of an aquaporin glycerol channel, aqp-1. We show that changes in glycerol metabolism are likely to underlie the lifespan-shortening effect of glucose, and that aqp-1 may act cell non-autonomously as a feedback regulator in the insulin/IGF-1 signaling pathway. Insulin down-regulates similar glycerol channels in mammals, suggesting that this glucose-responsive pathway might be conserved evolutionarily. Together these findings raise the possibility that a low-sugar diet might have beneficial effects on lifespan in higher organisms.
Introduction
Diets of industrialized countries tend to have high glycemic index (GI) values. The high-GI diets, which are enriched in processed carbohydrates or sugars, are easily metabolized to glucose and raise blood glucose level very quickly. High-GI diets have been linked to obesity, type 2 diabetes and cardiovascular diseases (Aston, 2006; Venn and Green, 2007). How high-GI diets may accelerate the onset and progression of these diseases is not clear. Possible mechanisms include direct metabolic effects, changes in body weight and alterations in hormonal regulatory systems. The hormone insulin may mediate at least some of the effects of the high-GI diets on human health. Blood insulin levels rise rapidly after the consumption of high-GI foods and then fall quickly (Aston, 2006; Venn and Green, 2007). This dramatic fluctuation in insulin levels may lead to insulin resistance and eventually to type 2 diabetes, although further research on the molecular mechanisms of insulin fluctuations is required.
The insulin signaling pathway is evolutionarily well conserved from C. elegans to mammals (Barbieri et al., 2003; Katic and Kahn, 2005). In mammals, insulin and its close homolog IGF-1 bind to tyrosine-kinase receptors and result in the inhibition of the FOXO transcription factor, an important transcriptional regulator of many cellular processes such as metabolism, stress response and apoptosis (Barbieri et al., 2003; Katic and Kahn, 2005; Salih and Brunet, 2008).
The insulin/IGF-1 signaling pathway has been shown to regulate the lifespan of many organisms (Barbieri et al., 2003; Katic and Kahn, 2005; Kenyon, 2005). Reducing the activity of this pathway; for example, by mutation of the C. elegans daf-2 insulin/IGF-1 receptor gene (Kimura et al., 1997), slows the aging process and doubles lifespan (Kenyon et al., 1993). This extended lifespan requires the activity of the FOXO transcription factor DAF-16 and the heat-shock transcription factor HSF-1 (Henderson and Johnson, 2001; Hsu et al., 2003; Kenyon et al., 1993; Lee et al., 2001; Lin et al., 1997; Lin et al., 2001; Morley and Morimoto, 2004; Ogg et al., 1997). In addition, DAF-16 and HSF-1 contribute to the longevity of wild-type animals cultured on bacteria under standard laboratory conditions, as reducing either daf-16 or hsf-1 gene activity accelerates the rate of tissue aging and shortens lifespan (Garigan et al., 2002; Herndon et al., 2002; Kenyon et al., 1993; Lin et al., 2001). Although the connection between the insulin/IGF-1 signaling pathway and aging in C. elegans has been well established, our current knowledge on the effect of glucose on the C. elegans insulin/IGF-1 signaling pathway and on lifespan is very limited.
In this study, we tested whether glucose feeding affected the lifespan of C. elegans. As recently shown by Schulz et al. in an independent study (Schulz et al., 2007), we found that a glucose-enriched diet significantly shortened the lifespan of C. elegans. We then found that glucose decreased lifespan by inhibiting the DAF-16 and HSF-1 transcription factors. In addition, we found that the downstream aquaporin gene aqp-1, which encodes a glycerol channel, was responsible for the lifespan-shortening effect of a glucose-containing diet. In mammals, insulin represses the expression of genes encoding similar aquaporin glycerol channels (Kishida et al., 2001; Kuriyama et al., 2002). Genetic depletion of one of these aquaporin channels in mice causes obesity and insulin resistance, pointing at the importance of these glycerol channels in mammalian glucose metabolism (Hara-Chikuma et al., 2005; Hibuse et al., 2005). The role of glycerol channels in glucose metabolism seems to be conserved in C. elegans since we found that C. elegans aqp-1 was also a glucose-regulated downstream target of DAF-16 and HSF-1. Furthermore, we showed that aqp-1, in turn, influenced DAF-16 activity. This positive-feedback loop may act cell non-autonomously to adjust the level of DAF-16/FOXO activity among the tissues. It is possible that, like many other pathways that affect aging (Barbieri et al., 2003; Guarente, 2007; Katic and Kahn, 2005; Kenyon, 2005; Piper and Bartke, 2008; Stepanyan et al., 2006), the glucose-responsive pathway might be conserved from C. elegans to mammals. If so, then low-sugar diets might have beneficial effects on mammalian aging. Surprisingly, dietary glucose could completely suppress the long lifespan of daf-2(-) insulin/IGF-1 receptor mutants in C. elegans, suggesting that individuals with an impaired insulin receptor might benefit disproportionally from a low-sugar diet.
Results
Dietary glucose shortens the lifespan of C. elegans
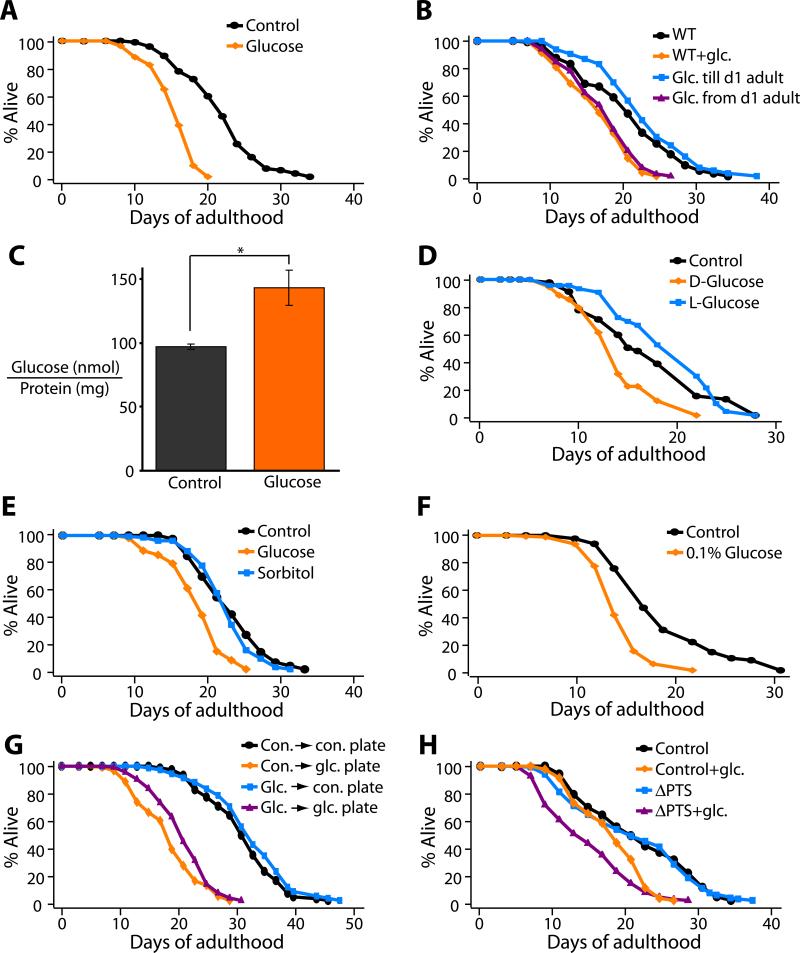
To ask whether glucose might influence the lifespan of C. elegans, which are normally fed a diet of E. coli OP50 bacteria, we added 2% glucose to culture plates containing normal NG medium and a lawn of bacteria. We found that glucose addition decreased lifespan by approximately 20% (Figure 1A). This lifespan shortening required glucose treatment during adulthood, as feeding only during development had no effect on adult lifespan (Figure 1B).
Figure 1. Glucose feeding shortens the adult lifespan of C. elegans.
(A) A diet containing 2% D-glucose (glucose) decreased the lifespan of wild-type (N2) animals significantly. (B) Glucose feeding initiated on day-1 of adulthood (Glc. from d1 adult) shortened the lifespan of wild-type animals but glucose feeding during entire larval developmental period (Glc. until d1 adult) did not. (C) Glucose feeding increased the glucose level inside the animals. Error bars represent s.e.m (*P<0.05, two-tailed Student's t-Test). (D and E) In contrast to D-glucose, diet containing 2% L-glucose (D) or sorbitol (E) did not shorten lifespan. See Table S1 for additional trials and statistical analysis for these and all other lifespan data. (F) 0.1% glucose was sufficient to affect lifespan. See Figure S2 for other glucose concentrations. (G) Worms grown on glucose-containing plates (glc. plate) with dead bacteria were short lived whether the bacteria had been previously cultured with glucose (glc. → glc. plate) or not (con. → glc. plate). In contrast, growing bacteria in 2% glucose media prior to killing the bacteria and moving to a control plate without glucose (glc. → con. plate) was not sufficient to affect lifespan. (H) Worms fed with glucose-uptake defective ΔPTS OP50 double mutant bacteria (ΔPTS) (See Figure S3) grown on 2% glucose plates were short-lived. Finally, since progeny number is negatively correlated with lifespan in many cases, we measured the effect of glucose feeding on total progeny number. However, glucose feeding decreased the total progeny number (see Figure S1).
We confirmed that the animals ingested glucose, as glucose-treated worms contained ~50% more glucose than control animals (Figure 1C). To test whether an indirect effect of glucose addition might influence lifespan, we asked whether increased osmolarity or glucose-fed bacteria could shorten the lifespan of C. elegans. Neither of these treatments appeared to have any effect. First, 2% L-glucose or D-sorbitol did not affect lifespan (Figure 1, D and E), whereas as little as 0.1% (5.6 mM) D-glucose was sufficient to decrease lifespan significantly (Figure 1F). Second, when we cultured worms on normal NG medium (no added glucose) plated with bacteria pre-fed 2% glucose, the animals were not short lived (Figure 1G), arguing against the possibility that a metabolite of glucose produced by the bacteria was responsible for the shorter lifespan. Consistent with this, animals were short-lived when cultured on 2%-glucose plates containing killed bacteria (Figure 1G). Likewise, 2%-glucose plates seeded with ΔPTS mutant bacteria, which are defective in glucose-uptake (Deutscher et al., 2006), decreased the lifespan of the animals (Figure 1H). Taken together, these data suggest that glucose consumption directly decreases the lifespan of C. elegans. While this study was in progress, Schulz et al. reported independently that glucose shortens C. elegans’ lifespan, and described analogous control experiments (Schulz et al., 2007).
Glucose shortens lifespan by down-regulating the activities of the DAF-16/FOXO and HSF-1 transcription factor
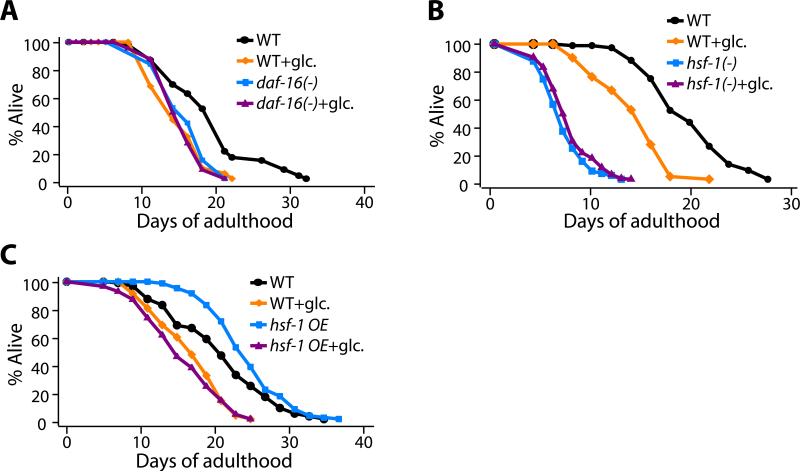
Because glucose stimulates insulin secretion in mammals, we wondered whether glucose might shorten the lifespan of C. elegans by influencing components of the insulin/IGF-1 signaling pathway. Insulin/IGF-1 signaling inhibits the transcriptional activity of DAF-16/FOXO (Salih and Brunet, 2008). When insulin/IGF-1 signaling is reduced, lifespan is doubled, and this lifespan extension requires daf-16 (Kenyon et al., 1993). Conversely, when daf-16 is deleted in otherwise normal animals, the rate of tissue aging is accelerated and lifespan is shortened by ~20% (Garigan et al., 2002; Kenyon et al., 1993). We found that glucose did not further shorten the lifespan of daf-16(-) animals (Figure 2A and Figure S4A).
Figure 2. The lifespan-shortening effect of glucose requires the DAF-16 and HSF-1 transcription factors.
(A, B) The short lifespan of the null allele daf-16(mu86) (A) [daf-16(-); in this and other figures, (-) refers to a reduction or loss-of-function mutation and the specific allele is given in the corresponding figure legend.] or hsf-1(sy441, RNAi) (B) animals was not further decreased by glucose feeding. In contrast, wild-type animals (WT) lived significantly shorter on a diet containing 2% glucose. (C) The long lifespan caused by overexpression of hsf-1 (hsf-1 OE) was suppressed by adding 2% glucose to the diet. See Figures S3-S5 for the lifespan data of additional mutants we tested: daf-16(RNAi), hsf-1(sy441), sir-2.1(ok434), aak-2(ok524), aak-1(RNAi), aak-1(RNAi); aak-2(ok524), nhr-49(gk405), daf-12(rh61rh411) animals.
The long lifespan produced by inhibiting insulin signaling in C. elegans also requires the heat shock transcription factor hsf-1 (Hsu et al., 2003), and, as with daf-16, reducing hsf-1 activity accelerates aging and shortens lifespan (Garigan et al., 2002). We found that glucose did not further shorten the lifespan of hsf-1(-) mutants (Figure 2B and Figure S4B). The effect of glucose on daf-16(-) and hsf-1(-) animals was specific, because glucose did shorten the lifespan of animals containing mutations in many other genes that affect lifespan, including: sir-2.1(-), aak-2(-), nhr-49(-) and daf-12(-) (Figure S5 and S6, described below). In addition, glucose treatment also completely suppressed the long lifespan caused by hsf-1 overexpression, which is daf-16 dependent (Hsu et al., 2003) (Figure 2C). This genetic specificity implicates daf-16 and hsf-1 in the longevity response to dietary glucose, and suggests that glucose is not simply toxic to worms in a general, non-specific way.
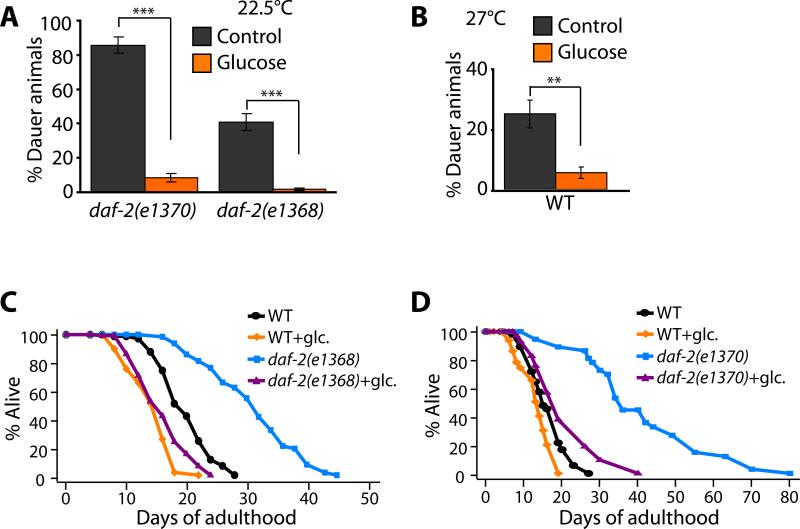
During development, the insulin/IGF-1 pathway regulates a hibernation-like state called dauer diapause (Hu, 2007). The dauer is an alternative third-larval stage that worms enter in response to food limitation and crowding. Whereas animals carrying weak daf-2 mutations become long-lived adults, animals carrying stronger mutations become dauers constitutively, as do animals carrying weak daf-2 mutations but grown at high temperature (Hu, 2007). Like adult lifespan extension, dauer formation requires both daf-16. We found that 2% glucose feeding reduced the fraction of the animals that became dauers at 22.5°C in strains carrying reduction-of-function mutations in daf-2 (Figure 3A). This was true for two different reduction-of-function alleles of daf-2, daf-2(e1368) and daf-2(e1370). In addition, glucose feeding suppressed dauer formation in wild-type animals grown at a higher temperature, 27°C (Figure 3B). These data are consistent with the hypothesis that glucose treatment up-regulates the insulin/IGF-1 signaling pathway, which in turn counteracts the reduced activity of the insulin/IGF-1 receptor in these animals.
Figure 3. Glucose may influence insulin/IGF-1 signaling to regulate dauer formation and lifespan.
(A-B) Glucose feeding significantly decreased dauer formation caused by daf-2(e1370) or daf-2(e1368) mutation at 22.5°C (A), or by growth at 27°C (B) (Error bars are s.e.m. ***P<0.001, **P<0.01, two-tailed Student's t-Test). (C-D) The extreme longevity caused by some daf-2/insulin-IGF-1 receptor mutations was completely suppressed by glucose feeding. Glucose feeding suppressed the longevity caused by daf-2(e1368) (C) and daf-2(e1370) (D) mutations.
Many DAF-16-regulated genes have been identified in microarray and other studies (Halaschek-Wiener et al., 2005; Lee et al., 2003; McElwee et al., 2003; McElwee et al., 2004; Murphy et al., 2003; Oh et al., 2006; Ookuma et al., 2003). To ask whether glucose affects the expression of known DAF-16-regulated genes, we carried out microarray analysis. We found that treating wild-type animals with 2% glucose produced a pattern of gene expression that overlapped significantly with that produced by genetic inhibition of daf-16 activity in daf-2 insulin/IGF-1-receptor mutants (hypergeometric probabilities, P <10-10; Table 1). We also observed changes in expression of several insulin-like genes, including the known DAF-16-target gene ins-7 (Figure S7A), which affects lifespan (Murphy et al., 2003; Murphy et al., 2007). [Consistent with this, we observed increased ins-7::gfp expression in transgenic animals upon addition of glucose; Figure S7B-D] In addition, we found that the DNA motif CTTATCA (Budovskaya et al., 2008) that we previously showed to be overrepresented in the upstream sequence of the DAF-16-regulated genes (Murphy et al., 2003), was significantly overrepresented in the promoter regions (2 kb) of the glucose-responsive genes as well (P < 10-5, Table 1). These findings support the hypothesis that glucose shortens lifespan by preventing DAF-16 from regulating the expression of specific target genes.
Table 1.
Genes differentially regulated by glucose treatment
| A. Genes upregulated by glucose treatment | |||
|---|---|---|---|
| Transcript/Gene | Brief description | DAF-16 binding sites# (bp) GTAAAC/TA | GATA transcription factor binding site (bp) CTTATCA |
| K09H11.7 | Haloacid dehalogenase-like hydrolase | −4863 | |
| dC53A3.2 | Haloacid dehalogenase-like hydrolase | −4908, −4873, −4189 | −4550, −1559 |
| C18H9.6 | Unknown | −4875 | −430 |
| F44E7.2 | Haloacid dehalogenase-like hydrolase | −2485, −1801, −1766 | −2124, −636 |
| K02G10.7 (aqp-8)* | Major intrinsic protein (MIP), aquaporin | −2919, −2080 | −230 |
| Y40B10A.6 | o-methyltransferase domain | 2021, −390 | |
| F21C10.9 | Unknown | −2937, −1952, −1637, −373 | |
| K11D12.4 | Choline or carnitine o-acyltransferase | −3785, −826, −380 | −4348, −2735 |
| dF21F8.4 | Putative aspartyl (acid) protease | −3035 | 4371, −4035, −3162, −2287, −1531, −1484 |
| C17C3.1d | Lipid storage | −4547, −2753, −2033 | |
| Y69A2AR.4 (smf-3)* | Solute carrier family 11 | −3187, −207, −194 | |
| F10D2.11 | UDP-glucoronosyl/glucosyl transferase | −843, −366 | 4076, −3374, −2136, −12 |
| Y53F4B.6 | Growth, size, and locomotor behavior | ||
| Y40B10A.2 | o-methyltransferase domain | −2335 | −1378 |
| F15H10.1 (col-12) | Collagen | −4123, 3192, −1405 | −3147 |
| C29E6.5 (nhr-43)* | Nuclear hormone receptor | −4528, −3392 | −1002, −943, −231 |
| F31F4.5 | Pseudogene | ||
| F09F3.9 | Choline or carnitine o-acyltransferase | −1395, −967, −42 | −988 |
| Y80D3A.7 (ptr-22) | Patched superfamily | −4270, −2815 | −3331 |
| F44G3.2 | Plant ATP-guanido phosphotransferase | −2651, −1490 | −4345 |
| F15B9.1 (far-3) | Fatty acid- and retinol-binding protein 3 | −1712, −421, −409 | −4749, −979 |
| C54D10.1 (cdr-2)* | Cadmium-responsive gene 2 | −4690, −2245, −2095, −2038, −1540, −1024 | |
| dC05E11.4 (amt-1) | Ammonium transporter family | −4905, −3889, −3671, −3440, −3246, −2591, −1606, 1327, −1240, −1118 | −1789, −1461 |
| B. Genes downregulated by glucose treatment | |||
|---|---|---|---|
| Transcript/Gene | Brief description | DAF-16 binding sites# (bp) GTAAAC/TA | GATA transcription factor binding site (bp) CTTATCA |
| dZK6.7a | Lipid storage, chromosome segregation, pathogen response | −2695, −859 | −4312, −3979, −80 |
| C14C6.5 | ShTK and CC domains | ||
| C50F7.5 | Unknown | −3658, −135 | −1107, −1084, −240, −209 |
| T20B12.4 | Unknown | ||
| dF48D6.4a | Unknown | −4869, −4476, −4316, −1427 | −3835, −403 |
| dF32A5.5b (aqp-1)* | Aquaporin | −172 | −2364, −2285, −112 |
| dF48D6.4c | Unknown | −4869, −4476, −4316, −1427 | −3835, −403 |
| dZK6.7b | Lipid storage, chromosome segregation, pathogen response | −2695, −859 | −4312, −3979, −80 |
| dY4C6B.6 | Glucosyl-ceramidases | −3890, −3363, −2443, −1909 | −155, −70 |
| dF48D6.4b | Unknown | −4869, −4476, −4316, −1427 | −3835, −403 |
| dF15E11.14 | Unknown | −2707 | −394 |
| dF27C8.4 | Downstream target of a VHL-1 pathway | −4923, −2524, −992 | −2810, −1883, −1007, −75 |
| Y37A1B.12 (tor-1) | May bind ATP | −113, −59 | −4039 |
| T08A9.9 | Similar to bactericidal amoebapores | −4817, −3857, −348 | −1039, −132 |
| F59B2.11 | Unknown | −4056 | |
| C25B8.3a (cpr-6)* | Cysteine protease domain | −4500, −2119 | −1972 |
| C23H5.8a* | Unknown | −4036 | −72 |
See Figure S8 for fold changes of these genes and Table S3 for fold changes of all the genes in microarray analysis.
Genes that were up- (or down-) regulated both by glucose treatment and by inactivation of daf-16.
The change in mRNA expression level upon glucose treatment was confirmed by qRT-PCR (Figure S9). The lifespan of deletion mutants for these genes was measured (Figure 4A and Figure S10)
We found that 37 of the 40 glucose-responsive transcripts (93%) contain one or more DAF-16 binding elements in their 5-kb upstream regulatory regions. This enrichment is statistically significant (P<0.05, hypergeometric probability) compared to that (78%) of random C. elegans genes (Kenyon and Murphy, 2006).
Glucose completely prevented daf-2 mutations from extending lifespan
Because the activity of the insulin/IGF-1 pathway is required for growth to adulthood (Hu, 2007), the daf-2 mutations that extend lifespan only partially inhibit gene activity. Thus it was hard to predict what effect glucose might have on the lifespans of daf-2 mutants. We found that glucose almost completely suppressed the lifespan extension of daf-2(e1370) (tyrosine-kinase) and daf-2(e1368) (ligand-binding domain) mutants (Kimura et al., 1997) back to wild type levels (Figure 3C and D). This dramatic effect, which was seen in multiple trials, suggests that glucose can suppress the lifespan-extending effect of even very high levels of DAF-16 activity. This finding suggested that glucose would also suppress the extended lifespan of ins-7(RNAi) animals, and we found that this was the case (Figure S7E).
Glucose may shorten lifespan by altering aqp-1 expression
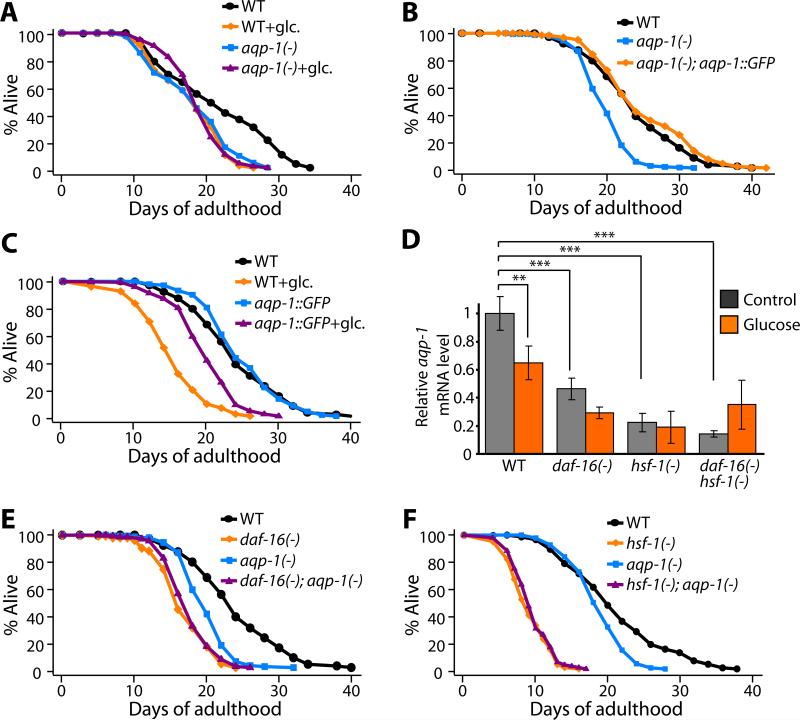
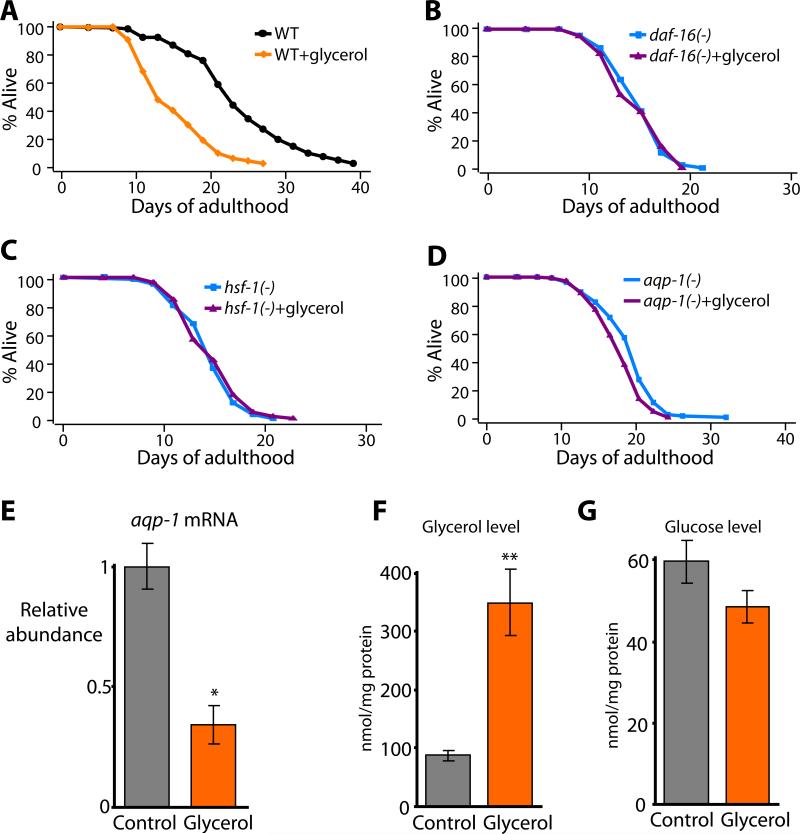
To better understand how glucose affects lifespan, we tested the functions of genes whose expression changed in response to glucose. To do this, we outcrossed available mutants to our wild-type (N2) strain, and measured lifespan (Figure 4A and Figure S10). Of 7 mutants that we tested, one, an aqp-1(-) deletion mutant, behaved remarkably like daf-16(-) and hsf-1(-) mutants: aqp-1 mutants were short-lived, and their short lifespan was not further decreased by glucose (Figure 4A). This finding suggested that glucose shortens lifespan by down-regulating the expression of aqp-1. Consistent with this interpretation, we found that overexpression of a functional aqp-1::GFP fusion (Huang et al., 2007), which rescued the short lifespan of the aqp-1 deletion mutant (Figure 4B), partially prevented glucose from shortening lifespan (Figure 4C). This observation is significant because it argues that reduction of aqp-1 activity is required for glucose to shorten lifespan. Together these findings indicate that glucose shortens lifespan by inhibiting aqp-1 activity.
Figure 4. Down-regulation of aqp-1, a glucose-responsive DAF-16/HSF-1 target gene, is responsible for the effect of glucose on lifespan.
(A) The short lifespan caused by aqp-1(-) [aqp-1(tm339)] mutation was not further shortened by a diet containing 2% glucose, whereas the lifespan of wild-type animals (WT) was shortened by a 2% glucose diet. (B) A transgene expressing aqp-1::GFP completely rescued the short lifespan of aqp-1(-) animals. (C) aqp-1::GFP transgenic animals were partially resistant to the lifespan shortening effect of glucose diet, indicating that reduction of aqp-1 levels is required for glucose to shorten lifespan. (D) Glucose feeding or mutation of daf-16 or hsf-1 lowered aqp-1 mRNA levels. Panel shows quantitative RT-PCR analysis of aqp-1 mRNA level in wild-type, daf-16(-) [daf-16(mu86)], hsf-1(-) [hsf-1(sy441, RNAi)] or daf-16(-) hsf-1(-) animals with (orange) or without (grey) glucose treatment. Error bars represent s.e.m. (***P<0.001, **P<0.01, two-way ANOVA followed by post-hoc Bonferroni tests). (E and F) In contrast to its lifespan-decreasing effect in wild-type animals, the aqp-1(-) mutation did not affect the short lifespan of daf-16(-) (E) or hsf-1(-) (F) animals.
Because of the similarities among the lifespan phenotypes of daf-16, hsf-1 and aqp-1 mutants, we hypothesized that aqp-1 is a particularly important transcriptional target of both DAF-16 and HSF-1 in animals fed glucose. We performed quantitative RT-PCR to compare the levels of aqp-1 transcripts in wild-type, daf-16(-), hsf-1(-) and daf-16(-) hsf-1(-) animals with and without glucose. We found that glucose feeding down-regulated aqp-1 in wild-type animals, consistent with our microarray data (Figure 4D and Table 1). In addition, aqp-1 was repressed in daf-16(-), hsf-1(-) and daf-16(-) hsf-1(-) animals compared to wild type, suggesting that normal expression of aqp-1 requires these transcription factors (Figure 4D). In contrast to the effects of dietary glucose on wild-type animals, glucose feeding did not significantly affect aqp-1 expression in these mutants (Figure 4D). In addition, we found that aqp-1(-) mutation did not further decrease the short lifespan caused by daf-16(-), hsf-1(-), or daf-16(-) hsf-1(-) mutations (Figure 4E-F and Figure S11). Together these data strongly suggest that glucose, aqp-1, hsf-1 and daf-16 all act in the same pathway, in which glucose shortens lifespan by down-regulating the activities of DAF-16 and HSF-1, which in turn down-regulates aqp-1 expression.
Glycerol may play an important role in lifespan shortening
aqp-1 encodes a glycerol channel (Huang et al., 2007), which is noteworthy because mammalian glycerol channels have been implicated in glucose homeostasis (Hara-Chikuma and Verkman, 2006). Because the AQP-1 channel may alter glycerol metabolism, we wondered what effect glycerol itself might have on lifespan. To investigate this, we measured the lifespan of animals grown on normal culture plates supplemented with 1% glycerol (a molarity equivalent to 2% glucose) and a lawn of E. coli bacteria. We found that glycerol feeding decreased the lifespan of wild-type animals by 23-36% (Figure 5A), which was similar to the effect of glucose feeding. We then tested whether glycerol treatment affected the lifespan of daf-16(-), hsf-1(-) or aqp-1(-) mutants. Similar to glucose treatment, we found that adding glycerol to the diet of these mutants had little or no effect on their already short lifespan (Figure 5B-D). In addition, we found that glycerol feeding repressed aqp-1 expression (Figure 5E).
Figure 5. The glycerol channel AQP-1 mediates the lifespan shortening effect of glycerol feeding.
(A) Diet containing 1% glycerol (glycerol) decreased the lifespan of wild-type animals significantly. (B-D) The short lifespan of daf-16(mu86) (B), hsf-1(sy441) (C) or aqp-1(tm339) (D) animals was not further decreased by glycerol feeding. The lifespan of hsf-1(sy441) mutants was decreased by glycerol feeding in one out of two trials (See Supplemental Table S1). However, the % decrease in lifespan (-11%) caused by glycerol feeding was much smaller than that of wild-type animals (-36%) in that trial. (E) Glycerol feeding (Glycerol) significantly decreased the mRNA level of aqp-1. (F and G) Glycerol treatment (Glycerol) increased the glycerol level inside the animals (F) but not the glucose level (G). Error bars represent s.e.m. *P<0.05, **P<0.005, two-tailed Student's t-test. In an effort to confirm the effect of glycerol on lifespan using genetic models, we measured the lifespan of mutant animals that have abnormal glycerol levels but the data were inconclusive at this point. See Figure S17 and the figure legend for detail.
The similarity between the effects of glucose and glycerol on the lifespan of wild-type, daf-16(-), hsf-1(-) and aqp-1(-) animals suggested that glucose and glycerol may act in the same way (or in the same pathway) to shorten lifespan. One possibility was that glucose and glycerol have similar effects on lifespan because they are metabolically interchangeable in C. elegans. We examined this possibility by measuring glucose and glycerol in the glucose-fed or glycerol-fed animals. We found that glucose feeding increased both glucose and glycerol in the animals (Figure S12A and B). However, glycerol-fed animals contained increased levels of glycerol but not glucose (Figure 5F and G). These data suggest that dietary glucose is metabolized to glycerol and that this glycerol may in turn decrease the lifespan of the animal.
The glycerol channel AQP-1 acts as a feedback regulator in the insulin/IGF-1 signaling pathway
The aqp-1 transgene is expressed in the pharynx and intestine (which behaves as the entire endoderm of the animal, including its adipose tissue) (Huang et al., 2007). We examined levels of glucose and glycerol in aqp-1 mutants with or without dietary glucose, but we did not find any significant difference between whole wild-type vs. aqp-1(-) animals (Figure S12A and B). Thus presumably aqp-1 influences the distribution of glycerol within the animal (something that we cannot measure with current technology).
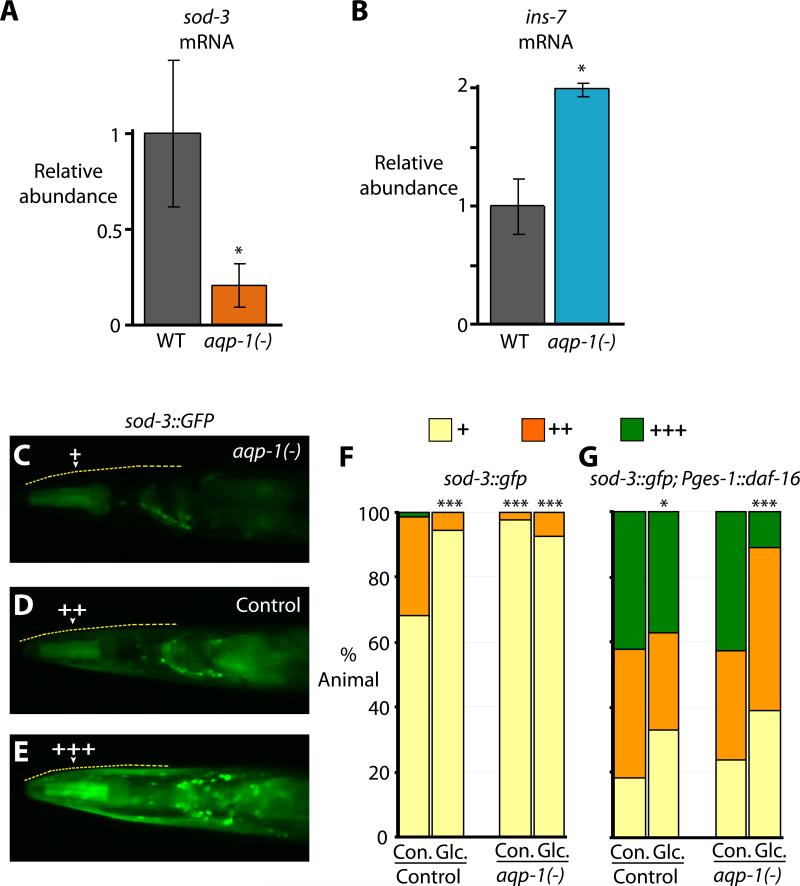
The fact that loss of aqp-1 caused a lifespan phenotype that was similar to that caused by loss of daf-16 itself was striking, and stimulated us to test the possibility that AQP-1 might be acting in a regulatory pathway. Specifically, we hypothesized that AQP-1 action might affect the transcription of DAF-16- and/or HSF-1-regulated genes. To test this hypothesis, we measured mRNA levels of 80 DAF-16 and/or HSF-1 target genes using quantitative RT-PCR analysis. We found that a significant fraction of the genes that are down-regulated by mutations in daf-16 and/or hsf-1 (8 out of 44) were also significantly down-regulated by the aqp-1 mutation (Figure 6A and S13). One daf-16 and hsf-1 target (out of 36 tested) was up-regulated by the aqp-1 mutation (Figure 6B and S14). This was the insulin-like peptide ins-7, which was also regulated by glucose, as mentioned above, and is known to influence lifespan (Murphy et al., 2007; Murphy et al., 2003).
Figure 6. The AQP-1/glycerol channel acts as a feedback regulator in the insulin/IGF-1 signaling pathway.
(A) aqp-1 mutation significantly decreased the mRNA level of a direct DAF-16 target gene, sod-3. (B) Expression of the insulin-like peptide gene ins-7 was induced by aqp-1 mutation. Error bars represent s.e.m. *P<0.05, two-tailed Student's t-test. These changes in sod-3 and ins-7 expression by aqp-1 mutation were not additive to daf-16(-) or hsf-1(-) mutations (Figure S16). (C) aqp-1 mutant animals that express sod-3::GFP generally contain GFP in the pharynx but not in the surrounding head muscles. (D, E) Some animals that express sod-3::GFP in a wild-type or in a Pges-1::GFP::daf-16 transgenic animal background contain GFP throughout the head, and often contain higher levels of GFP in the pharynx and intestine as well. The upper edge of the animal's head is indicated by the dashed yellow line. Arrowheads indicate head muscles. (F, G) Quantification of head-muscle sod-3::GFP expression for panel D-E without (F) or with (G) Pges-1::GFP::daf-16 [Pges-1::daf-16] (n≥75, *P<0.05, ***P<0.001, χ2 test (Tullet et al., 2008).
The finding that aqp-1 regulates ins-7 suggested that aqp-1 might influence DAF-16 target gene expression in a cell non-autonomous fashion. To test this idea, we crossed the aqp-1 mutation into a strain carrying a GFP reporter fusion to the promoter of a direct DAF-16 target gene, sod-3 (Honda and Honda, 1999). Interestingly, we found that the level of sod-3::GFP in the head muscle was decreased by aqp-1 mutation or glucose feeding (Figure 6C-F). Based on the transgene experiments (Huang et al., 2007) aqp-1 seems to be expressed only in the intestine and pharynx, and not in the muscle, although it remains possible that the aqp-1 transgene does not exactly reproduce the expression pattern of endogenous aqp-1. Thus, these data imply that aqp-1 mutation affects the transcriptional activity of DAF-16 in other tissues in a non-autonomous manner.
In otherwise wild-type animals, increasing DAF-16/FOXO levels in the intestine increases DAF-16/FOXO activity in other tissues in a process that appears to involve feedback regulation of ins-7 (Libina et al., 2003; Murphy et al., 2007). Since aqp-1 mutations increased ins-7 expression, we wondered whether the aqp-1 mutation would also influence this FOXO-to-FOXO signaling among tissues. To test this idea, we increased the level of DAF-16 in the intestine of otherwise wild-type animals using an intestine-specific daf-16 transgene (Pges-1::GFP::daf-16) (Libina et al., 2003), and assayed the level of sod-3::GFP in other tissues. In previous experiments, we showed that intestinal DAF-16 over-expression leads to a dramatic increase in head-muscle sod-3::GFP expression (Libina et al., 2003), as long as the head muscles are daf-16(+). We found that in the presence of glucose, the aqp-1 mutation reduced this up-regulation of sod-3::GFP expression in head-muscles (Figure 6G). Together these findings also imply a cell non-autonomous role for aqp-1 in the homeostasis of this hormonal signaling system.
Discussion
Glucose decreases the lifespan of C. elegans by inhibiting the DAF-16 and HSF-1 transcription factors
A major finding of this study is that glucose is a potent lifespan-shortening agent in C. elegans that appears to act by down-regulating the activities of the lifespan-extending proteins DAF-16/FOXO and HSF-1. Several lines of evidence lead to this conclusion. First, glucose shortened the lifespan of wild-type animals, but it did not further decrease the lifespan of the already short-lived daf-16(-) or hsf-1(-) mutants. Second, like daf-16(-) or hsf-1(-) mutations, glucose feeding could suppress the long lifespan of daf-2/insulin/IGF-1 receptor mutants and that of animals overexpressing hsf-1. Third, glucose treatment suppressed dauer formation, which is an alternative developmental stage that requires DAF-16 activity. Finally, glucose feeding generated a pattern of gene expression that overlapped significantly with that produced by daf-16(-) mutations. Since both DAF-16 and HSF-1 act downstream of, and are inhibited by, insulin/IGF-1 signaling in C. elegans, we propose that glucose may act by stimulating insulin/IGF-1 signaling. Consistent with this hypothesis, glucose stimulates the expression of several insulin genes.
Glucose dramatically shortens the long lifespan of daf-2 mutants
We were surprised to find that the long lifespan of animals carrying certain reduction-of-function mutations in the daf-2 insulin/IGF-1-receptor gene were drastically reduced by glucose feeding. It is possible that glucose suppresses the high level of DAF-16 activity in these mutants by the same mechanism that it uses to suppress normal DAF-16 activity in the wild-type animals. However in daf-2(-) mutants, loss of aqp-1 has a much more modest (~20%) effect on lifespan than does the addition of glucose (Murphy et al., 2003). The physiology of daf-2 mutants differs from that of wild type in many ways, and some aspect of this altered physiology must allow glucose to shorten lifespan independently of aqp-1. The mechanism likely involves daf-16 down regulation, as glucose did not further shorten the lifespan of daf-16(-); daf-2(-) double mutants (Figure S19). The ability of glucose to suppress the lifespan extensions of these daf-2 mutants is thought-provoking, because it raises the possibility that the more modest (15-30%) effects that inhibition of insulin/IGF-1 signaling has on the lifespan of mammals (Russell and Kahn, 2007), might be increased substantially by reducing the glycemic indexes of their diets.
Glucose does not appear to shorten lifespan by decreasing respiration
In their recent study, Schulz et al. found that glucose decreases oxygen consumption in wild-type C. elegans (Schulz et al., 2007), and we confirmed this finding in our laboratory (Figure S12C). However, we did not find a significant difference between the rates of oxygen consumption in aqp-1(-) animals and wild type (Figure S12C). Because the down-regulation of aqp-1 appears to be necessary and at least partially sufficient for glucose to shorten lifespan, these findings argue against the idea that glucose shortens lifespan by inhibiting respiration.
In addition, Schulz et al. proposed a model suggesting that glucose may shorten lifespan by inactivating the AMP-dependent kinase (AMPK) (Schulz et al., 2007). This model was mainly based on their findings that the AMPK subunit AAK-2 is inactivated by glucose treatment and that 2-deoxy-glucose (DOG), which acts oppositely to glucose and increases lifespan, does not lengthen the lifespan of aak-2 null mutants (Schulz et al., 2007). This model predicts that glucose would not further decrease the lifespan of aak-2 null mutants. However, we found that glucose feeding did further decrease the short lifespan caused by loss of aak-2, to the same extent as wild type (Figure S5). Thus our data imply that glucose does not shorten lifespan by inactivating AMPK. Taken together, these findings argue that whereas DOG may extend lifespan by activating AMP-dependent kinase and increasing respiration, the effect of glucose on lifespan is mediated by another pathway.
Interestingly, Schulz et al. also proposed that DOG extends lifespan by increasing the level of reactive oxygen species (ROS) (Schulz et al., 2007). However, Schotterer et al. recently showed that short-lived, glucose-treated worms also display increased ROS (Schlotterer et al., 2009). How this apparent paradox can be resolved is not yet clear.
Similar effects of glucose and glycerol on lifespan
We found that glucose and glycerol have very similar effects on lifespan. Both glucose and glycerol significantly decreased the lifespan of wild-type animals but did not further shorten the lifespan of daf-16(-), hsf-1(-) or aqp-1(-) mutants. This was unexpected. When present as the sole carbon source, glucose and glycerol have opposite effects on intermediary metabolism in yeast (Brisson et al., 2001). For example, growth on glucose triggers catabolite repression, stimulates glycolysis, and inhibits respiration, whereas growth on glycerol disinhibits catabolite repression and stimulates respiration via the conversion of glycerol to pyruvate. Thus glycerol might have been expected to lengthen, not shorten, the lifespan of C. elegans. It is not known to what extent the pathways of carbohydrate metabolism differ between worms and yeast. However, we found that worms fed glucose contain elevated levels of glycerol but not vice versa, suggesting that glycerol may be an important intermediate in the pathway through which glucose shortens lifespan.
One metabolite whose level could be elevated by glucose and/or glycerol feeding is fat, especially because glycerol is an essential building block of triglyceride. Consistent with this idea, Schulz et al. showed that glucose feeding increases the level of triglyceride in C. elegans (Schulz et al., 2007). These results raise the possibility that the increased fat in the glucose-fed animals could shorten lifespan directly. However, increased fat alone is not sufficient to shorten lifespan, as some mutants that contain high levels of fat, such as daf-2 mutants live long (Kimura et al, 1997). Moreover, since we found that down-regulation of the DAF-16/FOXO transcription factor, a condition that does not elevate fat levels (Kimura et al, 1997), mediates the lifespan-shortening effect of glucose feeding, it seems unlikely that the increased fat level is responsible for the shortened lifespan caused by glucose feeding.
The fact that we identified a glycerol channel as a mediator for the effect of glucose on lifespan further lends support to the idea that glycerol plays an important role in the glucose metabolic pathway. We speculate that glucose feeding may increase glycerol level in C. elegans, which may down-regulate the AQP-1 glycerol channel, and that this in turn may lead to further changes in glycerol metabolism and to short lifespan. Future studies using traceable glycerol, which does not exist at present, are required to test this model.
The AQP-1 channel appears to act cell non-autonomously
In daf-2 mutants, DAF-16 appears to influence lifespan by regulating multiple antioxidant, chaperone, immunity, metabolic and other genes that act in a cumulative way to influence lifespan (Murphy et al., 2003). Thus we were surprised to find that loss of a single metabolic gene, aqp-1, could apparently account for the effect of glucose on lifespan [at least in a daf-2(+) background]. Our finding that aqp-1 acts as a regulatory gene that is required for the expression of diverse DAF-16-regulated genes provides a possible explanation for this finding. By changing the expression of a variety of genes that individually have smaller effects on lifespan, loss of aqp-1 could have a relatively large impact.
The mechanism by which this glycerol channel influences DAF-16-target gene expression is not known, but our findings indicate that AQP-1, which is present only in the intestine and pharynx (Huang et al., 2007), is required for normal sod-3 expression in the head muscles. Thus aqp-1 can function cell non-autonomously. The finding that aqp-1 activity regulates ins-7, which is expressed in the intestine and known to affect lifespan (Murphy et al., 2003; Murphy et al., 2007), suggests a model in which aqp-1 mutations change the level of glycerol in the intestine, which in turn somehow affects the expression of ins-7 in that tissue. In addition, since AQP-1 is a glycerol channel, it is also possible that glycerol itself is an intercellular signal that can act on other tissues to influence the expression of DAF-16 target genes.
Roles of mammalian glycerol channels in glucose metabolism
In mammals, aquaporins (AQP) 3, 7, 9 and 10 encode glycerol channels (reviewed in Maeda et al., 2008). Among them, AQP7 and AQP9 have interesting features that seem related to those of C. elegans aqp-1. First, AQP7 and AQP9 are predominantly expressed in adipocytes and hepatocytes, respectively (Maeda et al., 2008). In worms, aqp-1 is expressed in the intestine, which performs at least some functions of these two tissues (fat storage and yolk production). (C. elegans has no distinct adipose tissue or liver.) The mRNA levels of AQP7 and AQP9 decrease upon insulin secretion (Kishida et al., 2001; Kuriyama et al., 2002). Moreover, both of these proteins influence insulin-dependent physiology: AQP7 knockout mice exhibit insulin resistance and obesity (Hibuse et al., 2005), whereas AQP9 deletion decreases blood glucose levels in mice that are obese and diabetic (Rojek et al., 2007). Fourth, recent human studies showed that AQP7 gene expression was significantly reduced in obese women (Ceperuelo-Mallafre et al., 2007) and that AQP7 polymorphisms were associated with risk of obesity and diabetes (Prudente et al., 2007). Together these studies suggest that mammalian aquaporin/glycerol channels have important roles in glucose metabolism. We showed that the C. elegans aqp-1/glycerol-channel is a glucose-responsive gene regulated by the insulin/IGF-1 pathway and that it is crucial for mediating the effect of glucose on lifespan. These findings suggest that C. elegans aqp-1 may be a functional homolog of mammalian AQP7 and/or AQP9.
Potential benefits of diets with a low glycemic index on human aging
The typical American diet contains high levels of sugar. A typical candy bar contains ~75% sugar, and the National Academy of Sciences Dietary Reference Intake recommends limiting the level of added sugar to 25% of total calories. Although we do not fully understand the mechanism by which glucose shortens the lifespan of C. elegans, the fact that the two mammalian aquaporin glycerol-transporting channels are down-regulated by insulin raises the possibility that glucose may have a lifespan-shortening effect in humans, and, conversely, that a diet with a low glycemic index may extend human lifespan. The finding that FOXO3A variants have now been associated with exceptional longevity in at least six human cohorts (Willcox et al., 2008; Flachsbart et al., 2009; Anselmi et al., 2009; Pawlikowska et al., 2009) makes this possibility seem realistic. It is particularly interesting to think about low-GI diets in the context of our findings with the C. elegans insulin/IGF-1 receptor/daf-2 mutants. These animals, which are predicted to be somewhat insulin resistant, have very long lifespans in the absence of glucose, but not in its presence. In multiple studies, lowering the dietary glycemic index has been shown to benefit humans with insulin resistance associated with the metabolic syndrome or type 2 diabetes (Aston, 2006; Venn and Green, 2007). Our findings raise the unorthodox but nevertheless conceivable possibility that humans with partially defective insulin receptors on low-glycemic index diets may be even more healthy and long-lived than normal people on low glycemic-index diets. Finally, because they block gluconeogenesis, drugs that inhibit glycerol aquaporin channels are now under development pharmaceutically for the treatment of diabetes (Fruhbeck et al., 2006). Our findings suggest that while these drugs might be beneficial for curtailing glucose production in diabetics, they may have additional, less beneficial effects.
Experimental Procedures
Strains
See Table S5 for Strains that were analyzed in this study.
Lifespan analysis
All lifespan measurements were performed at 20°C as described previously (Apfeld and Kenyon, 1999), starting with day 1 adults. In some experiments, D-Glucose, L-glucose, D-sorbitol, or glycerol was added to standard NG (normal growth) agar plates. The chemical 2’fluoro-5’deoxyuridine (FUDR, Sigma, 75 μM) was added to pre-fertile young-adult animals to prevent their progeny from developing unless stated otherwise. For the RNAi experiments, eggs were added to plates seeded with bacteria expressing double strand RNA of given genes.
Progeny profiles
Single L4 stage wild-type (N2) worms grown on OP50 bacteria with or without 2% glucose were placed on individual plates at 20°C (36 worms per condition). The animals were then transferred to new plates every 24 hours for 7 days while they produced progeny. Bagged or ruptured worms were censored. All the plates containing progeny were incubated for 2-3 days after removing the parental worms, and the number of worms that developed was counted.
Dauer assay
Animals were grown at 22.5°C or 27°C as indicated. After three days of incubation, dauers were scored under a dissecting scope.
Microarray analysis
Synchronized day 1 adult animals that were cultured at 20°C were harvested, washed 3 times in M9 and frozen immediately using liquid nitrogen. Total RNA extraction, mRNA purification, reverse transcription of mRNA, labelling and hybridization were performed as described previously (Murphy et al., 2003). Six biological replicate samples of glucose-fed vs. control wild-type adult animals were used to identify the glucose-responsive genes (Table 1, and Table S2 and S3). Four biological replicate samples of daf-2(e1370) vs. daf-16(RNAi); daf-2(e1370) and four biological replicate samples of fer-15(b26); daf-2(mu150); fem-1(hc17) vs. daf-16(RNAi); fer-15(b26); daf-2(mu150); fem-1(hc17) animals were used for identification of genes that are regulated by DAF-16 in daf-2 mutants (Table S2 and S4).
Microarray raw data were normalized using Acuity 4.0 (Molecular Devices, USA). Significance Analysis of Microarray (SAM) (Tusher et al., 2001) was performed to identify genes that were differentially regulated in each of the comparisons. For glucose-responsive genes, 5% median false positive discovery rate was used (Table 1 and Table S2). For DAF-16 regulated genes, 1% median false positive discovery rate was used (Table S2).
DAF-16 regulated genes in daf-2(-) mutants were compared with previously published data (McElwee et al., 2003; Murphy et al., 2003). The overlap between DAF-16-target genes in this study and the genes from the previous reports (McElwee et al., 2003; Murphy et al., 2003) were highly significant in both comparisons (See Figure S18).
Upstream sequence analysis
Upstream sequences for the glucose-responsive genes were extracted from Wormbase (www.wormbase.org). Regulatory Sequence Analysis Tools (RSAT, http://rsat.ulb.ac.be/rsat/) was used to search the sequence 2kb upstream of the translation start site of each open reading frame for overrepresented sequences. DAF-16 and GATA transcription factor binding sites in the 5kb upstream of glucose-responsive genes were identified using RSAT (Table 1).
Generation of ΔPTS OP50 double mutant E. coli
A ΔPTS mutation, which prevents glucose uptake in E. coli (Deutscher et al., 2006), was introduced from the GI698 ΔPTS strain into OP50 bacteria using a standard phage transduction method (Sambrook et al., 1989). The double mutant was selected by picking colonies on kanamycin-containing LB plates and confirmed by the formation of white colonies on McConkey agar medium plates containing 0.5% glucose (Figure S3).
Quantitative RT-PCR
RNA extraction, purification and reverse transcription were performed as described (Taubert et al., 2006). Quantitative RT-PCR was carried out in a 7300 Real Time PCR System (Applied Biosystems) and analyzed using the Ct method (Applied Biosystems Prism 7700 Users Bulletin No. 2
http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf). mRNA levels of act-1, nhr-23 and/or ama-1 were used for normalization as indicated. The average of at least 2 technical repeats was used for each biological data point. Primer sequences are available on request.
Measurement of glucose and glycerol
Glycerol levels were quantified as described (Lamitina et al., 2004) using Free Glycerol Reagent (Sigma, USA). Glucose levels were determined essentially as described (Lamitina and Strange, 2005) with modifications. Briefly, neutralized perchloric acid worm extracts prepared as described (Lamitina et al., 2004) were used for quantification of glucose concentration using Amplex® Red Glucose/Glucose Oxidase Kit (Molecular Probes, USA). Protein content was measured with BCA assay kit (Pierce, USA) and used for the normalization of glucose and glycerol levels. Approximately 10,000 synchronized day 1 adult worms were used for one data set.
Measurement of oxygen consumption rates
Oxygen consumption rates were determined using the Oxytherm (Hansatech, UK), which is a DW1/AD Clark-type oxygen electrode, as described (Schulz et al., 2007). Approximately 5000 synchronized day 1 adult worms were collected, quickly washed three times in M9 buffer, resuspended in 1 ml of M9 and transferred into the chamber. Respiration was measured at 20°C and the oxygen consumption rates were automatically calculated by using the ‘Rate Cursors’ function in the Oxygraph program (Hansatech, UK).
Fluorescence microscopy
All pictures of transgenic animals were captured using a Retiga EXi Fast1394 CCD digital camera (QImageing, Burnaby, BC, Canada) attached to a Zeiss Axioplan 2 compound microscope (Zeiss Corporation, Germany). The expression of sod-3::GFP was assayed as described previously (Libina et al., 2003). Briefly, well-fed 3-day-old adult animals grown at 20°C were mounted on 2% agarose slides (~10 per slide). The level of sod-3::GFP in the head muscle was scored blindly. P values were calculated using the χ2 test as described (Tullet et al., 2008). The expression level of ins-7::GFP was examined as described previously (Murphy et al., 2007). Subcellular localization of DAF-16::GFP was determined as previously described (Lin et al., 2001).
Supplementary Material
Acknowledgments
We thank Drs. T. Lamitina and G. Ruvkun, and the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources, for providing some strains, and all Kenyon lab members for sharing outcrossed strains and for helpful comments on the experiments, data analysis and manuscript. We also thank Dr. T. Lamitina for valuable suggestions on osm-8 and gpdh-1; gpdh-2 mutant experiments, Dr. M. Gaglia for initiating experiments on dauer formation, Vera Tenberg for performing some lifespan experiments, A. B. Hwang and J. S. Yang for technical helps during revision, and Drs. B. Koo and Y. J. Seok for providing ΔPTS E. coli. S.J.L. was an Ellison Medical Foundation fellow of the Life Sciences Research Foundation and was also supported by a postdoctoral fellowship from the American Heart Association, Western States Affiliate. This work was supported by NIH grant #AG11816 to C.K., who is the director of the UCSF Hillblom Center for the Biology of Aging, an American Cancer Society Professor, and a co-founder of the biotechnology company Elixir Pharmaceuticals. The data shown in Figure S7E, additional experiments requested by reviewers, and the salary of S.J.L during revision were supported by Korean government grant World Class University Program #R31-2008-000-10100-0. Author contributions: The majority of the experiments were performed by S.J.L. C.T.M. discovered that glucose affected insulin gene expression using an ins-7::GFP fusion strain. C.K. performed the initial glucose experiments involving wild type and daf-16 mutants. S.J.L and C.K. designed experiments and wrote the paper.
References
- Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, Bellazzi R, Puca AA. Association of the FOXO3A locus with extreme longevity in a southern italian centenarian study. Rejuvenation Res. 2009;12:95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Aston LM. Glycaemic index and metabolic disease risk. Proc Nutr Soc. 2006;65:125–134. doi: 10.1079/pns2005485. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Brisson D, Vohl MC, St-Pierre J, Hudson TJ, Gaudet D. Glycerol: a neglected variable in metabolic processes? Bioessays. 2001;23:534–542. doi: 10.1002/bies.1073. [DOI] [PubMed] [Google Scholar]
- Budovskaya YV, Wu K, Southworth LK, Jiang M, Tedesco P, Johnson TE, Kim SK. An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C. elegans. Cell. 2008;134:291–303. doi: 10.1016/j.cell.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceperuelo-Mallafre V, Miranda M, Chacon MR, Vilarrasa N, Megia A, Gutierrez C, Fernandez-Real JM, Gomez JM, Caubet E, Fruhbeck G, Vendrell J. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:3640–3645. doi: 10.1210/jc.2007-0531. [DOI] [PubMed] [Google Scholar]
- Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeck G, Catalan V, Gomez-Ambrosi J, Rodriguez A. Aquaporin-7 and glycerol permeability as novel obesity drug-target pathways. Trends Pharmacol Sci. 2006;27:345–347. doi: 10.1016/j.tips.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Khattra JS, McKay S, Pouzyrev A, Stott JM, Yang GS, Holt RA, Jones SJ, Marra MA, Brooks-Wilson AR, Riddle DL. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15:603–615. doi: 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S, Uchida S, Verkman AS. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem. 2005;280:15493–15496. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci. 2006;63:1386–1392. doi: 10.1007/s00018-006-6028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, Kishida K, Inoue K, Kuriyama H, Nakamura T, Fushiki T, Kihara S, Shimomura I. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci U S A. 2005;102:10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. Faseb J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hu PJ. Dauer. WormBook; 2007. pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CG, Lamitina T, Agre P, Strange K. Functional analysis of the aquaporin gene family in Caenorhabditis elegans. Am J Physiol Cell Physiol. 2007;292:C1867–1873. doi: 10.1152/ajpcell.00514.2006. [DOI] [PubMed] [Google Scholar]
- Katic M, Kahn CR. The role of insulin and IGF-1 signaling in longevity. Cell Mol Life Sci. 2005;62:320–343. doi: 10.1007/s00018-004-4297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Murphy CT. Enrichment of regulatory motifs upstream of predicted DAF-16 targets. Nat Genet. 2006;38:397–398. doi: 10.1038/ng0406-397. author reply 398. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kishida K, Shimomura I, Kondo H, Kuriyama H, Makino Y, Nishizawa H, Maeda N, Matsuda M, Ouchi N, Kihara S, Kurachi Y, Funahashi T, Matsuzawa Y. Genomic structure and insulin-mediated repression of the aquaporin adipose (AQPap), adipose-specific glycerol channel. J Biol Chem. 2001;276:36251–36260. doi: 10.1074/jbc.M106040200. [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, Maeda N, Matsuda M, Nagaretani H, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes. 2002;51:2915–2921. doi: 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- Lamitina ST, Morrison R, Moeckel GW, Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol Cell Physiol. 2004;286:C785–791. doi: 10.1152/ajpcell.00381.2003. [DOI] [PubMed] [Google Scholar]
- Lamitina ST, Strange K. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am J Physiol Cell Physiol. 2005;288:C467–474. doi: 10.1152/ajpcell.00451.2004. [DOI] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300:644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Maeda N, Funahashi T, Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab. 2008;4:627–634. doi: 10.1038/ncpendmet0980. [DOI] [PubMed] [Google Scholar]
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, Lee SJ, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:19046–19050. doi: 10.1073/pnas.0709613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Dixit BL, Raha T, Green MR, Tissenbaum HA. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- Ookuma S, Fukuda M, Nishida E. Identification of a DAF-16 transcriptional target gene, scl-1, that regulates longevity and stress resistance in Caenorhabditis elegans. Curr Biol. 2003;13:427–431. doi: 10.1016/s0960-9822(03)00108-8. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Prudente S, Flex E, Morini E, Turchi F, Capponi D, De Cosmo S, Tassi V, Guida V, Avogaro A, Folli F, Maiani F, Frittitta L, Dallapiccola B, Trischitta V. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes. 2007;56:1468–1474. doi: 10.2337/db06-1389. [DOI] [PubMed] [Google Scholar]
- Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, Nielsen S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci U S A. 2007;104:3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schlotterer A, Kukudov1 G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, Ibrahim Y, Pfisterer F, Rabbani N, Thornalley P, Sayed A, Fleming T, Humpert P, Schwenger V, Zeier M, Hamann A, Stern D, Brownlee M, Bierhaus A, Nawroth P, Morcos M. C. elegans as model for the study of high glucose mediated lifespan reduction. Diabetes. 2009 Aug 12; doi: 10.2337/db09-0567. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Stepanyan Z, Hughes B, Cliche DO, Camp D, Hekimi S. Genetic and molecular characterization of CLK-1/mCLK1, a conserved determinant of the rate of aging. Exp Gerontol. 2006;41:940–951. doi: 10.1016/j.exger.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006;20:1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr. 2007;61(Suppl 1):S122–131. doi: 10.1038/sj.ejcn.1602942. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.