Abstract
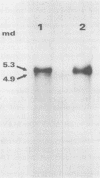
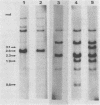
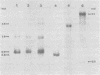
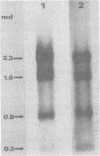
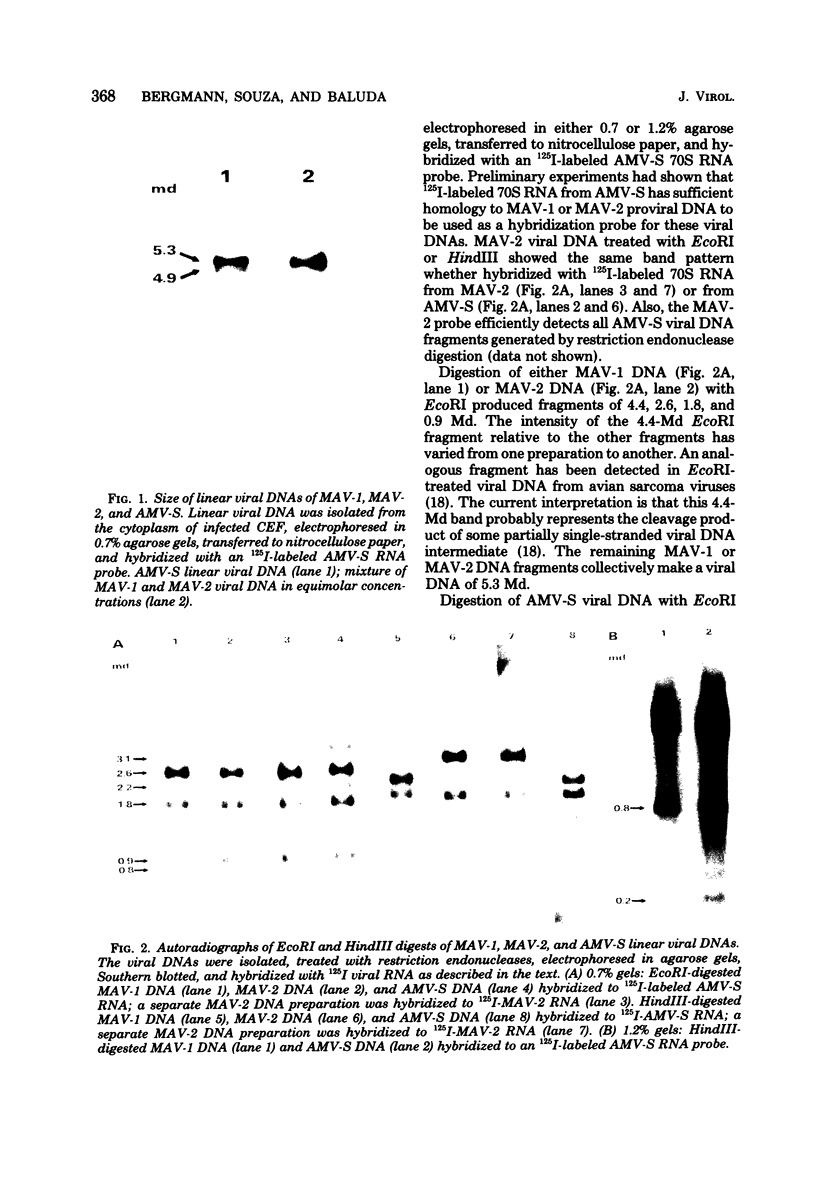
The major species of unintegrated linear viral DNA identified in chicken embryonic fibroblasts infected with either the avian myeloblastosis-associated viruses (MAV-1, MAV-2) or the standard avian myeloblastosis virus complex (AMV-S) has a mass of 5.3 X 10(6) daltons. An additional minor DNA component observed only in AMV-S-infected cells has a mass of 4.9 X 10(6) daltons. The unintegrated linear viral DNAs and integrated proviruses of MAV-1 and MAV-2 have been analyzed by digestion with the restriction endonucleases EcoRI and HindIII. MAV-2 lacks a HindIII site present in MAV-1. These fragments have been compared to those generated by EcoRI and HindIII digestion of linear viral DNAs of AMV-S. Restriction enzyme digestion of AMV-S viral DNA produced unique fragments not found with either MAV-1 or MAV-2 viral DNAs. The major viral component present in AMV-S stocks has the HindIII restriction pattern of MAV-1. Restriction enzyme analysis of the 5.3 X 10(6)-dalton unintegrated MAV viral DNAs and their integrated proviruses suggests that the DNAs have a direct terminal redundancy of approximately 0.3 megadaltons and integrate colinearly with respect to the unintegrated linear DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Baluda M. A. Synthesis of avian oncornavirus DNA in infected chicken cells. J Virol. 1974 May;13(5):1005–1013. doi: 10.1128/jvi.13.5.1005-1013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARD J. W. AVIAN VIRUS GROWTHS AND THEIR ETIOLOGIC AGENTS. Adv Cancer Res. 1963;7:1–127. doi: 10.1016/s0065-230x(08)60982-3. [DOI] [PubMed] [Google Scholar]
- Bacheler L. T., Fan H. Multiple integration sites for Moloney murine leukemia virus in productively infected mouse fibroblasts. J Virol. 1979 Jun;30(3):657–667. doi: 10.1128/jvi.30.3.657-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. DNA complementary to viral RNA in leukemic cells induced by avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):329–336. doi: 10.1073/pnas.66.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Keshet E., O'Rear J. J., Temin H. M. DNA of noninfectious and infectious integrated spleen necrosis virus (SNV) is colinear with unintegrated SNV DNA and not grossly abnormal. Cell. 1979 Jan;16(1):51–61. doi: 10.1016/0092-8674(79)90187-9. [DOI] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Sites of integration of reticuloendotheliosis virus DNA in chicken DNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3372–3376. doi: 10.1073/pnas.75.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham P. D., Baluda M. A. Integrated state of oncornavirus DNA in normal chicken cells and in cells transformed by avian myeloblastosis virus. J Virol. 1973 Oct;12(4):721–732. doi: 10.1128/jvi.12.4.721-732.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Vogt P. K. Effects of genetic cellular resistance on cell transformation and virus replication in chicken hematopoietic cell cultures infected with avian myeloblastosis virus (BAI-A). Virology. 1968 Aug;35(4):487–497. doi: 10.1016/0042-6822(68)90278-x. [DOI] [PubMed] [Google Scholar]
- Prensky W., Steffensen D. M., Hughes W. L. The use of iodinated RNA for gene localization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1860–1864. doi: 10.1073/pnas.70.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Hsu T. W., Lai M. M. Restriction enzyme sites on the avian RNA tumor virus genome. J Virol. 1978 May;26(2):479–484. doi: 10.1128/jvi.26.2.479-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. The RNA tumor viruses--background and foreground. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1016–1020. doi: 10.1073/pnas.69.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]