Abstract
In MDCK cells inoculated with an appropriate dilution of influenza virus, single hemadsorbing cells could be counted 8 h postinfection against a background of nonadsorbing cells. Standard virus preparation exhibited a linear relationship between the virus dilution and the number of hemadsorbing cells. With incomplete virus preparations obtained by passages of undiluted virus in chicken embryo, the dependence was nonlinear. A ts mutant (ts-29) of A/FPV/Weybridge (Hav1 Neq1) failed to convert MDCK cells into a hemadsorbing state at 42 degrees C. The ability of ts-29 to produce hemadsorbing cells could be rescued by incomplete wild-type virus. The capacity of incomplete virus for this partial functional complementation was inactivated by UV irradiation with one-hit kinetics. The size of the target was estimated to be 5.5 times smaller than that of the virus genome. The results suggest that at least some of the influenza virus genes in defective interfering particles are functional.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G., Maber H. B. Plaque formation by influenza viruses in the presence of trypsin. J Gen Virol. 1974 Dec;25(3):351–357. doi: 10.1099/0022-1317-25-3-351. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Transcriptase activity and genome composition of defective influenza virus. J Virol. 1976 Apr;18(1):365–369. doi: 10.1128/jvi.18.1.365-369.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
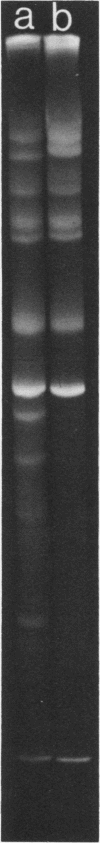
- Crumpton W. M., Dimmock N. J., Minor P. D., Avery R. J. The RNAs of defective-interfering influenza virus. Virology. 1978 Oct 15;90(2):370–373. doi: 10.1016/0042-6822(78)90322-7. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P. Sequence relationships among defective interfering influenza viral RNAs. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3092–3096. doi: 10.1073/pnas.76.7.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger U., Palese P. Molecular weights of RNA segments of influenza A and B viruses. Virology. 1978 Jul 15;88(2):394–399. doi: 10.1016/0042-6822(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Floyd R. W., Stone M. P., Joklik W. K. Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. Anal Biochem. 1974 Jun;59(2):599–609. doi: 10.1016/0003-2697(74)90313-3. [DOI] [PubMed] [Google Scholar]
- Ghendon Y. Z., Markushin S. G. Studies on mutation lesions and physiology of fowl plague virus ts mutants. Philos Trans R Soc Lond B Biol Sci. 1980 Feb 25;288(1029):383–392. doi: 10.1098/rstb.1980.0015. [DOI] [PubMed] [Google Scholar]
- Hirst G. K., Pons M. W. Mechanism of influenza recombination. II. Virus aggregation and its effect on plaque formation by so-called noninfective virus. Virology. 1973 Dec;56(2):620–631. doi: 10.1016/0042-6822(73)90063-9. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Davis A. R., Nayak D. P., De B. K. Diversity and generation of defective interfering influenza virus particles. Virology. 1979 May;95(1):48–58. doi: 10.1016/0042-6822(79)90400-8. [DOI] [PubMed] [Google Scholar]
- Kaverin N. V., Varich N. L., Smirnov Y. A. Sedimentational pattern of virus-specific RNA synthesized in Newcastle disease virus-infected cells treated with amino acid analogues. Arch Gesamte Virusforsch. 1973;41(3):191–198. doi: 10.1007/BF01252765. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. I. Isolation and preliminary characterization of RNA from virus particles. J Mol Biol. 1966 Jun;18(1):195–203. doi: 10.1016/s0022-2836(66)80085-2. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Sugiura A. Isolation of an influenza virus temperature-sensitive mutant of a new recombination-complementation group. Virology. 1977 May 15;78(2):365–374. doi: 10.1016/0042-6822(77)90114-3. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Ueda M., Sugiura A. Origin of small RNA in von Magnus particles of influenza virus. J Virol. 1979 Mar;29(3):1142–1148. doi: 10.1128/jvi.29.3.1142-1148.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Tobita K., Janda J. M., Davis A. R., De B. K. Homologous interference mediated by defective interfering influenza virus derived from a temperature-sensitive mutant of influenza virus. J Virol. 1978 Oct;28(1):375–386. doi: 10.1128/jvi.28.1.375-386.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M., Hirst G. K. The single- and double-stranded RNA's and the proteins of incomplete influenza virus. Virology. 1969 May;38(1):68–72. doi: 10.1016/0042-6822(69)90128-7. [DOI] [PubMed] [Google Scholar]
- VON MAGNUS P. Incomplete forms of influenza virus. Adv Virus Res. 1954;2:59–79. doi: 10.1016/s0065-3527(08)60529-1. [DOI] [PubMed] [Google Scholar]