Abstract
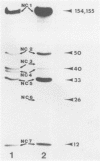
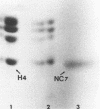
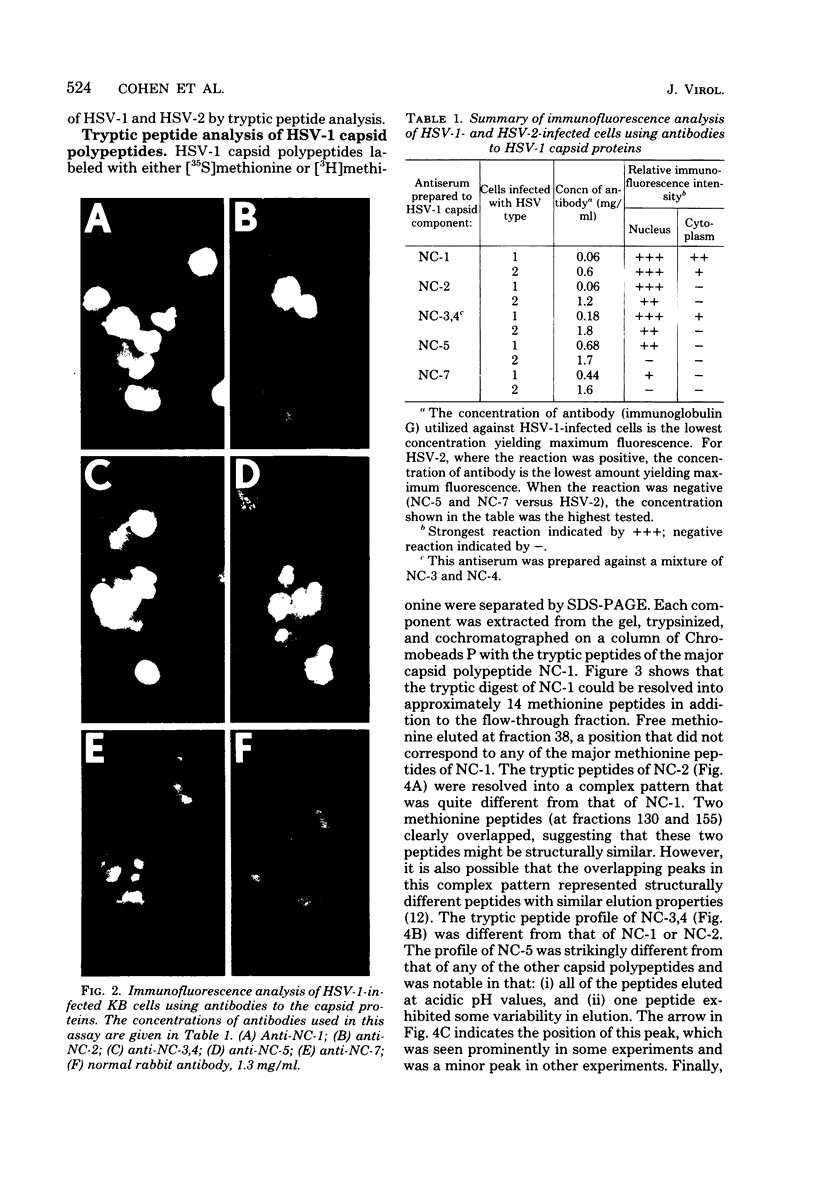
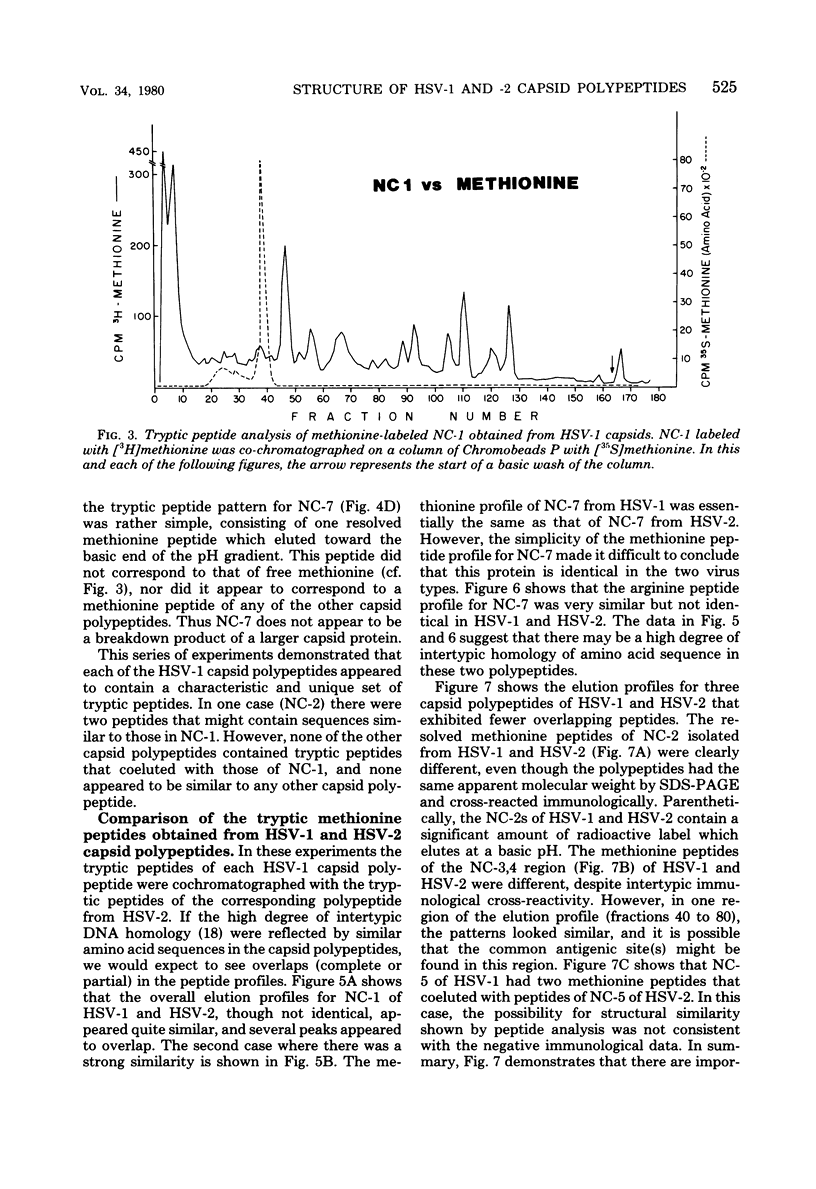
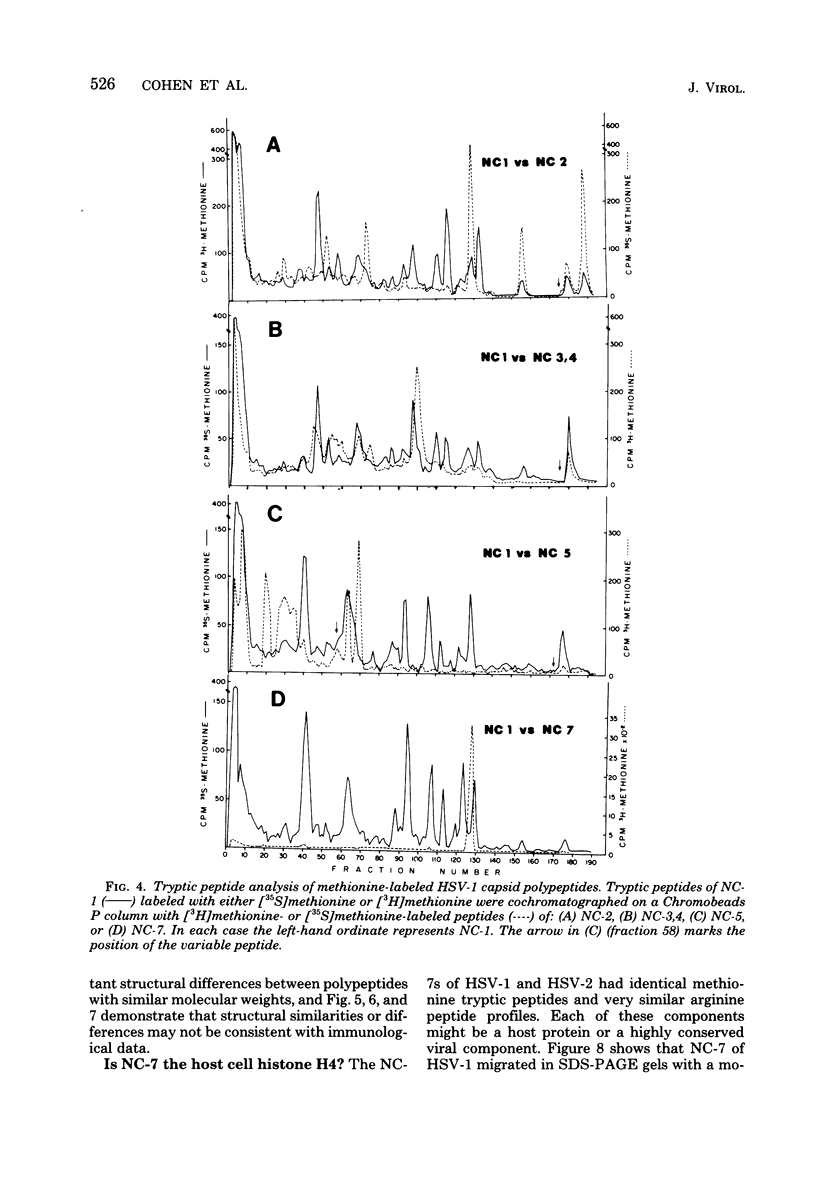
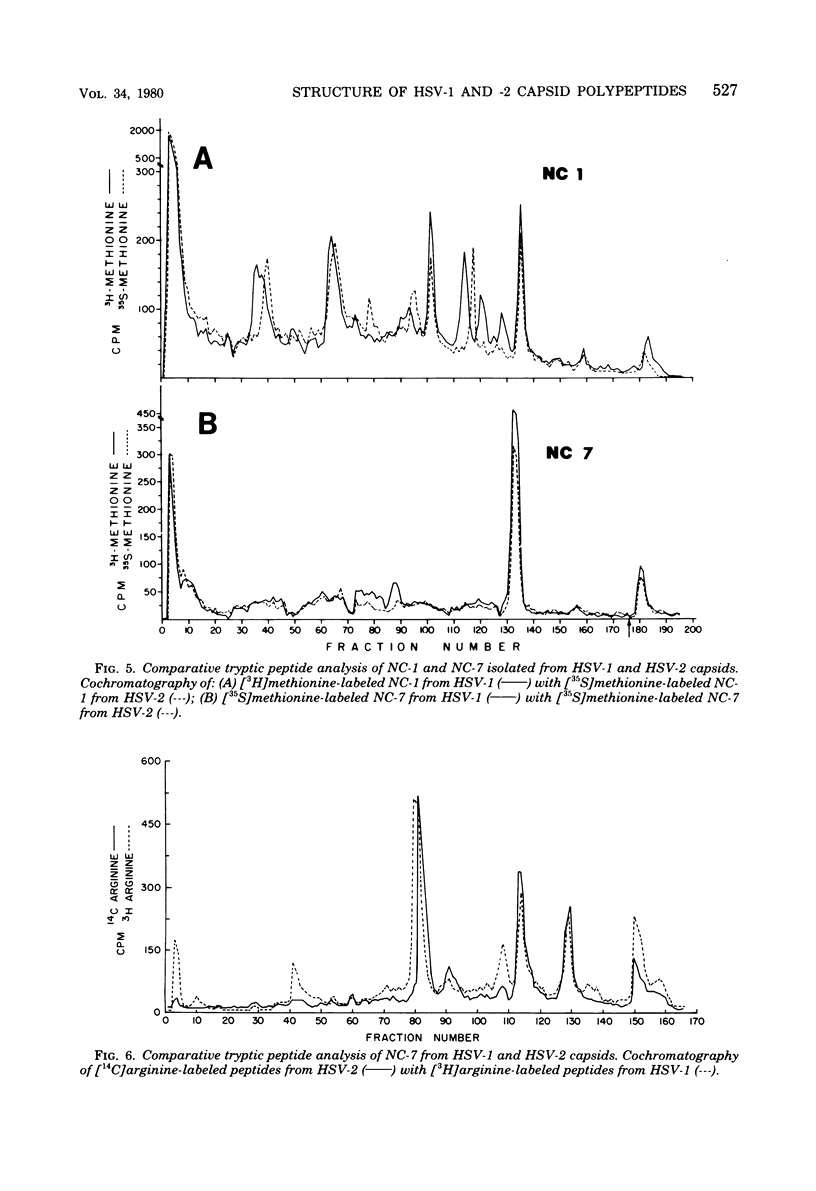
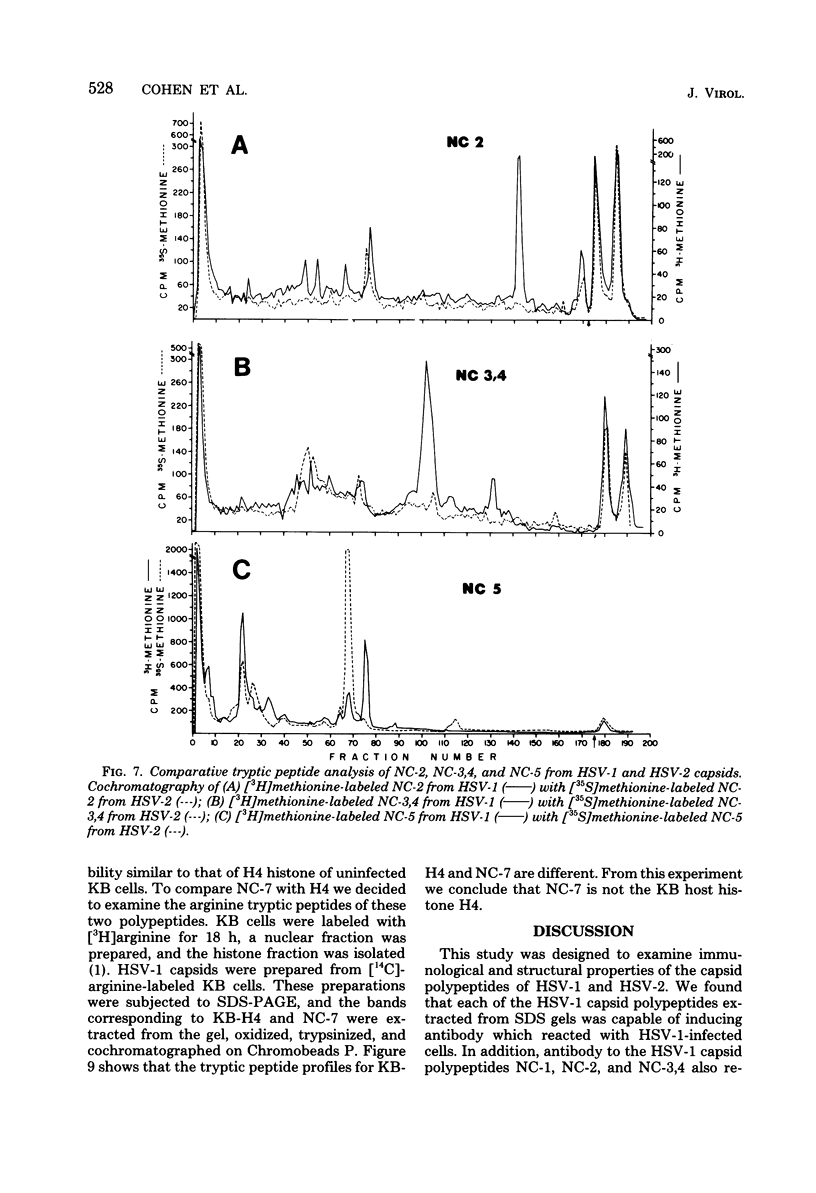
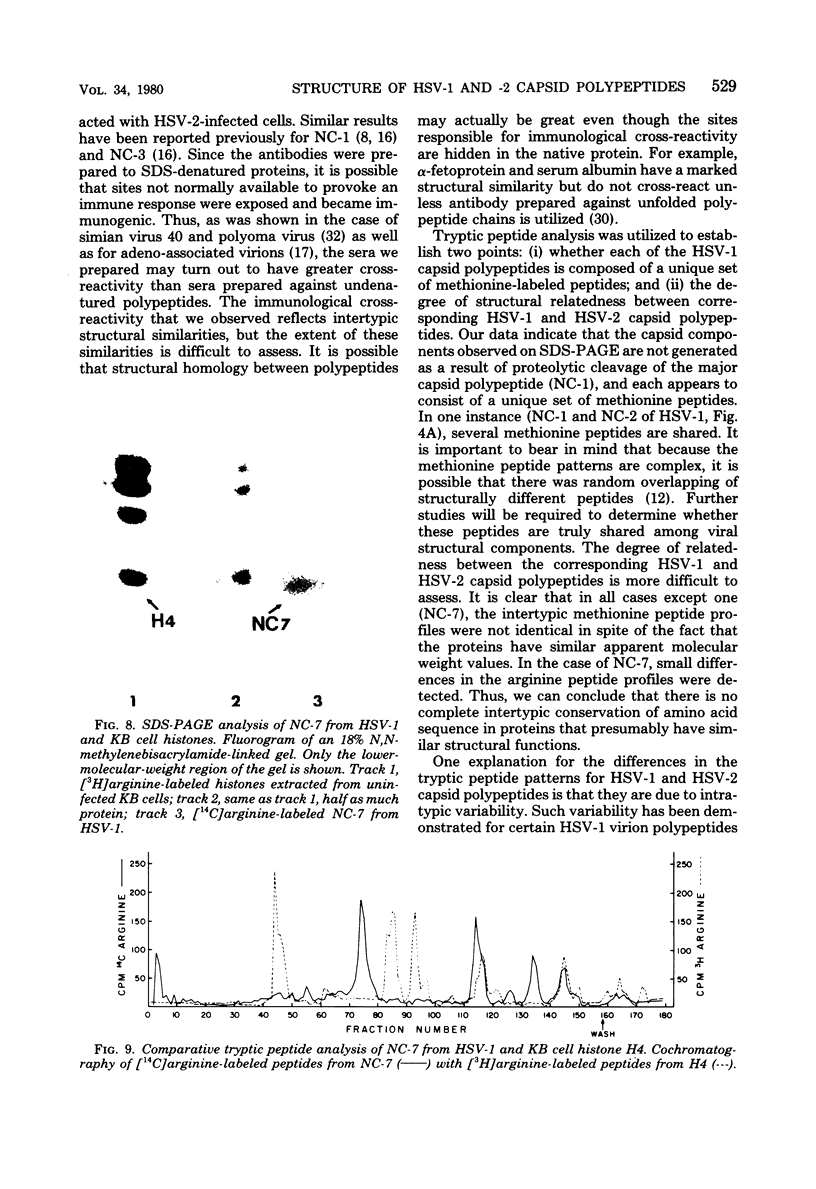
Capsids of herpes simplex virus (HSV) types 1 and 2 contain seven polypeptides ranging in molecular weight from 154,000 to 12,000 (termed NC-1 through NC-7 in order of descending molecular weight). Antibodies prepared to HSV-1 capsid polypeptides isolated from sodium dodecyl sulfate-polyacrylamide gels reacted in an immunofluorescence assay against HSV-1-infected KB cells. Three of the antibodies (anti-NC-1, anti-NC-2, and anti-NC-3,4) also reacted with HSV-2-infected cells. Tryptic peptide analysis showed that each of the HSV-1 capsid polypeptides had a unique methionine peptide profile, and none appeared to be derived from the major capsid polypeptide. Comparative peptide analysis of HSV-1 and HSV-2 showed that one polypeptide (NC-7, 12,000 molecular weight) had an identical methionine peptide profile and a very similar arginine peptide profile in both virus types. The arginine peptide profile of NC-7 of HSV-1 was very different from the arginine profile of KB histone H4. Although there were certain intertypic similarities in the methionine peptide profiles of the other capsid components especially in NC-1 (the major capsid protein), there was no case where the tryptic peptides were identical in the two virus types.
Full text
PDF



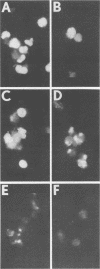
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler J. K., Stevely W. S. Virus-induced proteins in pseudorabies-infected cells. I. Acid-extractable proteins of the nucleus. J Virol. 1973 Jun;11(6):815–822. doi: 10.1128/jvi.11.6.815-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Factor M. N., Ponce de Leon M. Inhibition of herpes simplex virus type 2 replication by thymidine. J Virol. 1974 Jul;14(1):20–25. doi: 10.1128/jvi.14.1.20-25.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Nichols C. Isolation of a herpes simplex virus-specific antigenic fraction which stimulates the production of neutralizing antibody. J Virol. 1972 Nov;10(5):1021–1030. doi: 10.1128/jvi.10.5.1021-1030.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Powell K. L. Immunological and biochemical characterization of polypeptides induced by herpes simplex virus types 1 and 2. IARC Sci Publ. 1975;(11 Pt 1):63–73. [PubMed] [Google Scholar]
- Dreesman G. R., Suriano J. R., Swartz S. K., McCombs R. M. Characterization of the herpes virion. I. Purification and amino acid composition of nucleocapsids. Virology. 1972 Nov;50(2):528–534. doi: 10.1016/0042-6822(72)90404-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Philipson L. Structural proteins of adenoviruses. XI. Purification of three low molecular weight virion proteins of adenovirus type 2 and their synthesis during productive infection. Virology. 1974 Nov;62(1):253–269. doi: 10.1016/0042-6822(74)90320-1. [DOI] [PubMed] [Google Scholar]
- Fey G., Lewis J. B., Grodzicker T., Bothwell A. Characterization of a fused protein specified by the adenovirus type 2-simian virus 40 hybrid Ad2+ND1 dp2. J Virol. 1979 Apr;30(1):201–217. doi: 10.1128/jvi.30.1.201-217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman C. J., Jr, Zweig M., Stephenson J. R., Hampar B. Isolation of a nucleocapsid polypeptide of herpes simplex virus types 1 and 2 possessing immunologically type-specific and cross-reactive determinants. J Virol. 1979 Jan;29(1):34–42. doi: 10.1128/jvi.29.1.34-42.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. B., Blacklow N. R., Hoggan M. D. Immunological reactivity of antisera prepared against the sodium dodecyl sulfate-treated structural polypeptides of adenovirus-associated virus. J Virol. 1972 Jun;9(6):1017–1026. doi: 10.1128/jvi.9.6.1017-1026.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery P. C., Dorrington K. J., Rockey J. H. Equine antihapten antibody. The molecular weights of the subunits of equine immunoglobulins. Biochemistry. 1969 Mar;8(3):1247–1258. doi: 10.1021/bi00831a060. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Herpes simplex virus structural proteins. Virology. 1970 Apr;40(4):948–960. doi: 10.1016/0042-6822(70)90141-8. [DOI] [PubMed] [Google Scholar]
- Pereira L., Cassai E., Honess R. W., Roizman B., Terni M., Nahmias A. Variability in the structural polypeptides of herpes simplex virus 1 strains: potential application in molecular epidemiology. Infect Immun. 1976 Jan;13(1):211–220. doi: 10.1128/iai.13.1.211-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Mirkovic R., Courtney R. J. Comparative analysis of polypeptides induced by type 1 and type 2 strains of herpes simplex virus. Intervirology. 1977;8(1):18–29. doi: 10.1159/000148873. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Watson D. H. Some structural antigens of herpes simplex virus type 1. J Gen Virol. 1975 Nov;29(2):167–178. doi: 10.1099/0022-1317-29-2-167. [DOI] [PubMed] [Google Scholar]
- Reed C. L., Cohen G. H., Rapp F. Detection of a virus-specific antigen on the surface of herpes simplex virus-transformed cells. J Virol. 1975 Mar;15(3):668–670. doi: 10.1128/jvi.15.3.668-670.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. J., Watson D. H. Structural proteins of herpes simplex virus. J Gen Virol. 1971 Feb;10(2):163–171. doi: 10.1099/0022-1317-10-2-163. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Immunological crossreaction between alpha-fetoprotein and albumin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4641–4644. doi: 10.1073/pnas.73.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Kado-Boll G. J., Haven C. B. Changes in nuclear basic proteins during pseudorabies virus infection. J Virol. 1969 May;3(5):490–497. doi: 10.1128/jvi.3.5.490-497.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon S. K., Lawrence W. C., Cohen G. H., Durso M., Rubin B. A. Morphological components of herpesvirus. II. Preservation of virus during negative staining procedures. J Gen Virol. 1976 May;31(2):183–191. doi: 10.1099/0022-1317-31-2-183. [DOI] [PubMed] [Google Scholar]
- Vernon S. K., Lawrence W. C., Cohen G. H. Morphological components of herpesvirus. I. Intercapsomeric fibrils and the geometry of the capsid. Intervirology. 1974;4(4):237–248. doi: 10.1159/000149968. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Hampar B. Identification of disulfide-linked protein complexes in the nucleocapsids of herpes simplex virus type 2. Virology. 1979 Apr 30;94(2):442–450. doi: 10.1016/0042-6822(79)90474-4. [DOI] [PubMed] [Google Scholar]