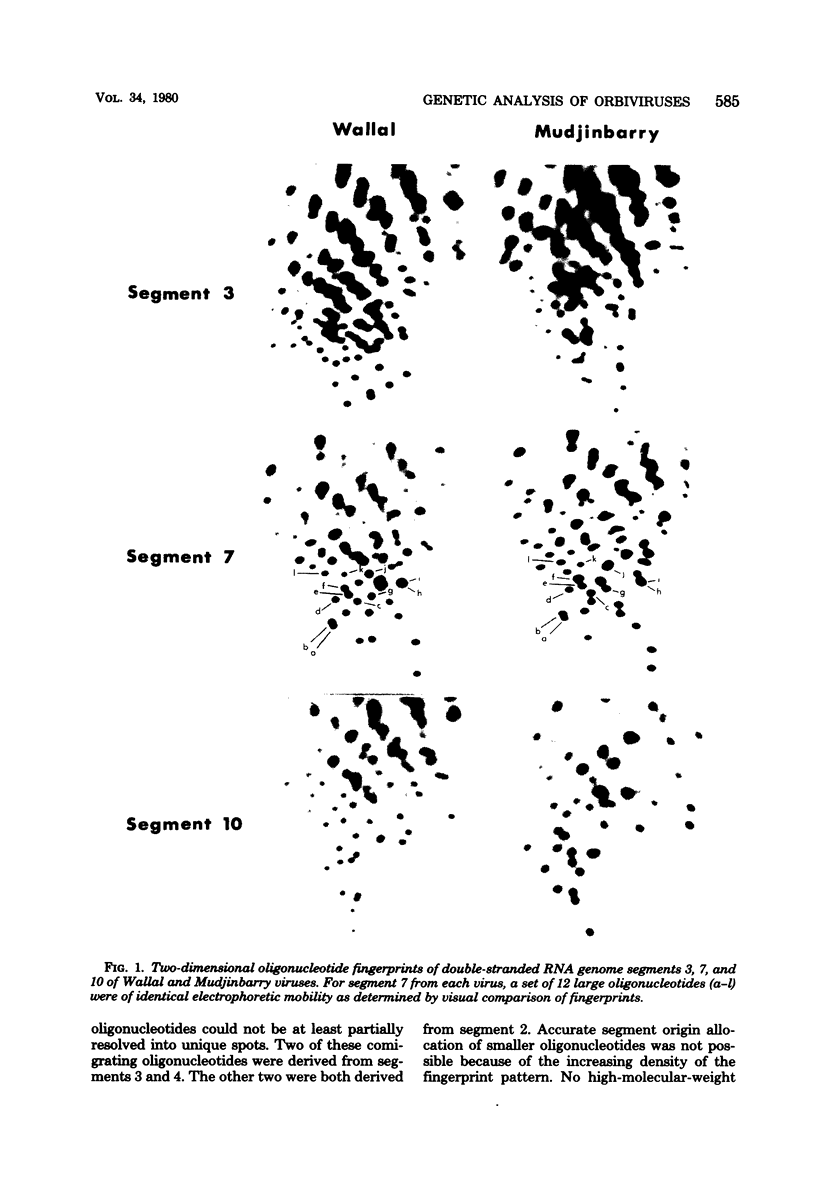
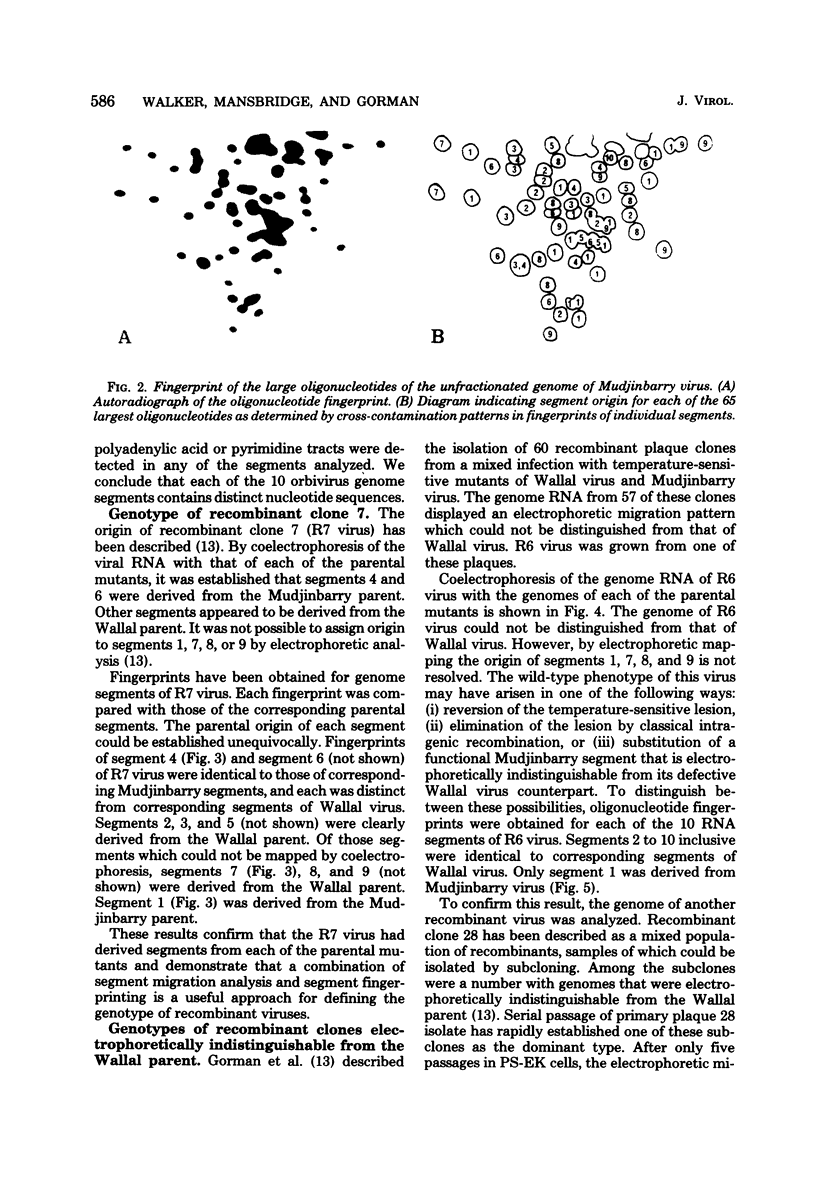
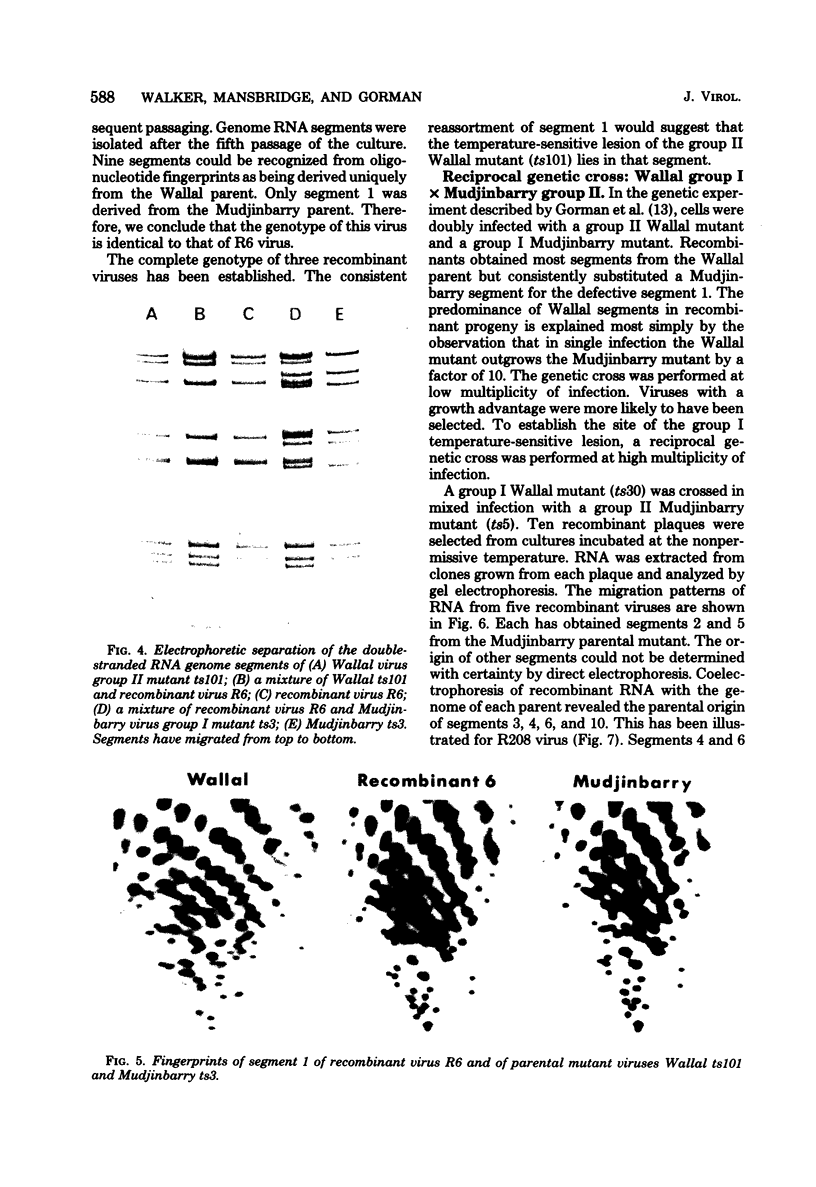
Abstract
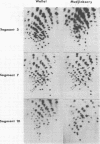
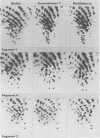
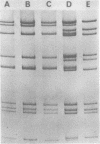
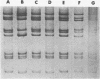
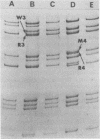
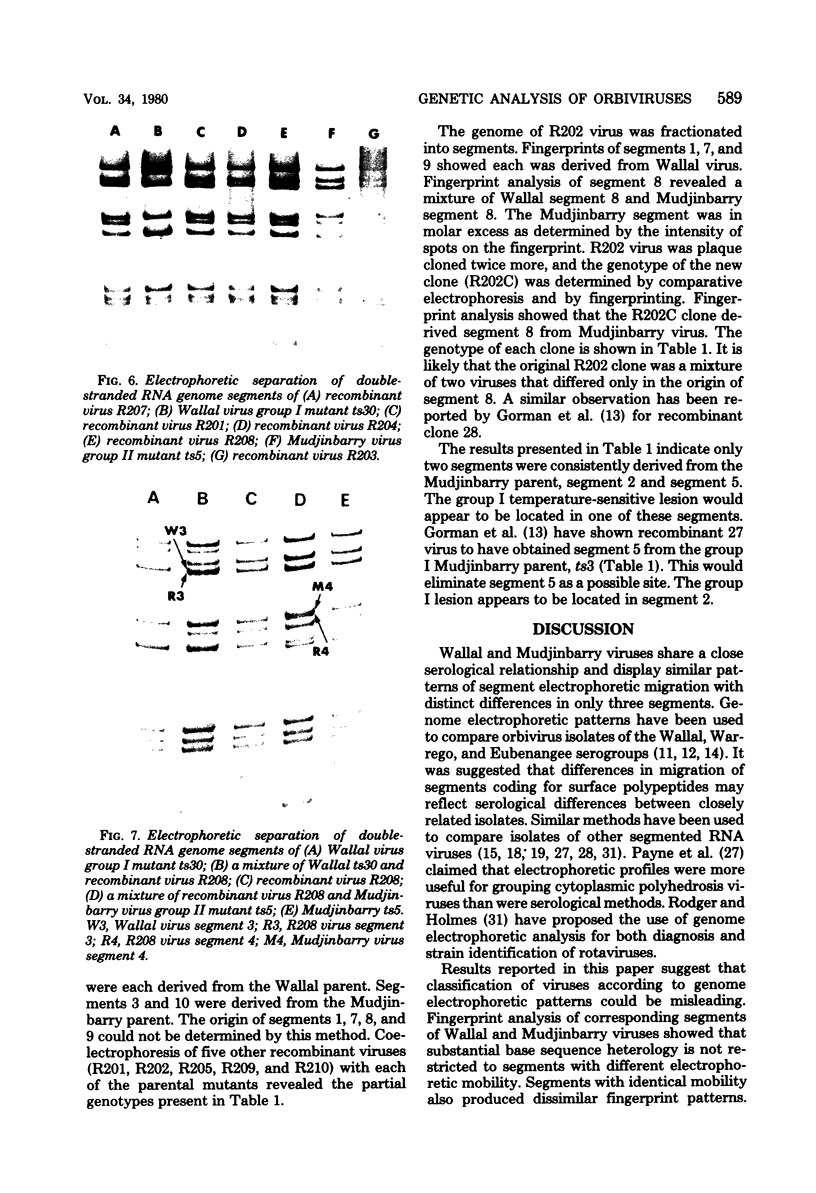
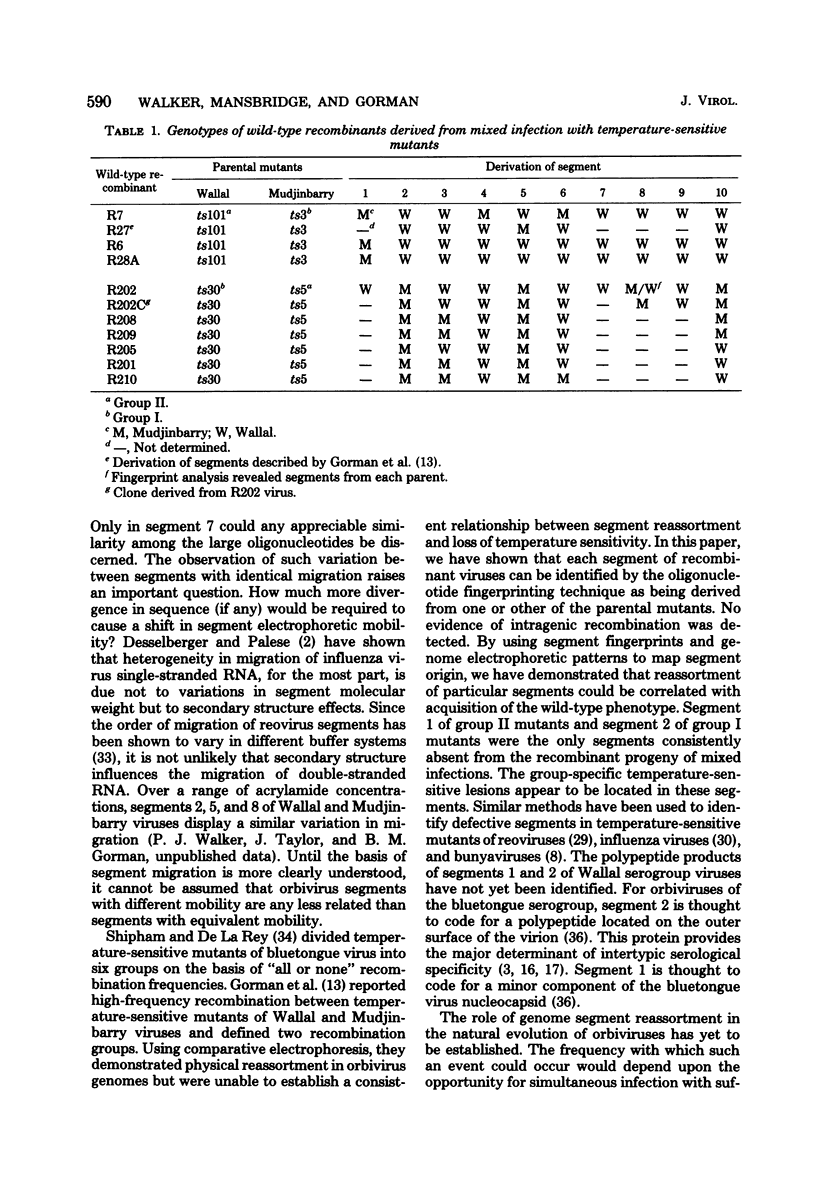
Corresponding double-stranded RNA segments of the related orbiviruses Wallal and Mudjinbarry produced distinctly different RNase T1 fingerprint patterns. No extensive sequence reiteration was observed between segments of Mudjinbarry virus. Fingerprint analysis of the genome of recombinant orbiviruses confirmed segment reassortment as a mechanism of interchange of genetic information. When temperature-sensitive mutants of each virus were crossed in mixed infection, a consistent pattern of segment reassortment was correlated with generation of the wild-type phenotype. Thus, the temperature-sensitive lesion of group II Wallal serogroup mutants was mapped to segment 1. The group I mutant lesion appears to be located in segment 2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avital S., Elson D. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):297–307. doi: 10.1016/0005-2787(69)90038-0. [DOI] [PubMed] [Google Scholar]
- De Villiers E. M. Comparison of the capsid polypeptides of various bluetongue virus serotypes. Intervirology. 1974;3(1-2):47–53. doi: 10.1159/000149741. [DOI] [PubMed] [Google Scholar]
- Desselberger U., Palese P. Molecular weights of RNA segments of influenza A and B viruses. Virology. 1978 Jul 15;88(2):394–399. doi: 10.1016/0042-6822(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Doherty R. L., Carley J. G., Standfast H. A., Dyce A. L., Kay B. H., Snowdon W. A. Isolation of arboviruses from mosquitoes, biting midges, sandflies and vertebrates collected in Queensland, 1969 and 1970. Trans R Soc Trop Med Hyg. 1973;67(4):536–543. doi: 10.1016/0035-9203(73)90084-9. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J., Wynne L. R., Clewley J. P., Shope R. E., Bishop D. H. Formation of recombinants between snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 Dec;24(3):893–902. doi: 10.1128/jvi.24.3.893-902.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman B. M., Taylor J. The RNA genome of Tilligerry virus. Aust J Exp Biol Med Sci. 1978 Jun;56(3):369–371. doi: 10.1038/icb.1978.40. [DOI] [PubMed] [Google Scholar]
- Gorman B. M., Taylor J., Walker P. J., Young P. R. The isolation of recombinants between related orbiviruses. J Gen Virol. 1978 Nov;41(2):333–342. doi: 10.1099/0022-1317-41-2-333. [DOI] [PubMed] [Google Scholar]
- Gorman B. M. Variation in orbiviruses. J Gen Virol. 1979 Jul;44(1):1–15. doi: 10.1099/0022-1317-44-1-1. [DOI] [PubMed] [Google Scholar]
- Gorman B. M., Walker P. J., Taylor J. Electrophoretic separation of double-stranded RNA genome segments from Warrego and Mitchell River viruses. Arch Virol. 1977;54(1-2):153–158. doi: 10.1007/BF01314388. [DOI] [PubMed] [Google Scholar]
- Hrdy D. B., Rosen L., Fields B. N. Polymorphism of the migration of double-stranded RNA genome segments of reovirus isolates from humans, cattle, and mice. J Virol. 1979 Jul;31(1):104–111. doi: 10.1128/jvi.31.1.104-111.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huismans H., Howell P. G. Molecular hybridization studies on the relationships between different serotypes of bluetongue virus and on the difference between the virulent and attenuated strains of the same serotype. Onderstepoort J Vet Res. 1973 Sep;40(3):93–103. [PubMed] [Google Scholar]
- Huismans H. Protein synthesis in bluetongue virus-infected cells. Virology. 1979 Jan 30;92(2):385–396. doi: 10.1016/0042-6822(79)90143-0. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Garon C. F., Wyatt R. G., Mebus C. A., van Kirk D. H., Chanock R. M., Kapikian A. Z. Differentiation of human and calf reoviruslike agents associated with diarrhea using polyacrylamide gel electrophoresis of RNA. Virology. 1976 Oct 1;74(1):86–92. doi: 10.1016/0042-6822(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Sereno M. M., Wyatt R. G., Mebus C. A., Chanock R. M., Kapikian A. Z. Comparison of human and animal rotavirus strains by gel electrophoresis of viral RNA. Virology. 1978 Jun 15;87(2):247–255. doi: 10.1016/0042-6822(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Ecology of influenza viruses in lower mammals and birds. Br Med Bull. 1979 Jan;35(1):29–33. doi: 10.1093/oxfordjournals.bmb.a071537. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Wimmer E. "Fingerprinting" high molecular weight RNA by two-dimensional gel electrophoresis: application to poliovirus RNA. Nucleic Acids Res. 1976 Jul;3(7):1647–1658. doi: 10.1093/nar/3.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson H. G., Wagenaar E. B. Thermal elution chromatography and the resolution of nucleic acids on hydroxylapatite. Anal Biochem. 1974 Sep;61(1):144–154. doi: 10.1016/0003-2697(74)90341-8. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C. C., Piasecka-Serafin M., Pilley B. The properties of two recent isolates of cytoplasmic polyhedrosis viruses. Intervirology. 1977;8(3):155–163. doi: 10.1159/000148890. [DOI] [PubMed] [Google Scholar]
- Payne C. C., Rivers C. F. A provisional classification of cytoplasmic polyhedrosis viruses based on the sizes of the RNA genome segments. J Gen Virol. 1976 Oct;33(1):71–85. doi: 10.1099/0022-1317-33-1-71. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Mustoe T. A., Sharpe A. H., Fields B. N. A genetic map of reovirus. II. Assignment of the double-stranded RNA-negative mutant groups C, D, and E to genome segments. Virology. 1978 Apr;85(2):531–534. doi: 10.1016/0042-6822(78)90459-2. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P. Identification of the defective genes in three mutant groups of influenza virus. J Virol. 1977 Mar;21(3):1196–1204. doi: 10.1128/jvi.21.3.1196-1204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Holmes I. H. Comparison of the genomes of simian, bovine, and human rotaviruses by gel electrophoresis and detection of genomic variation among bovine isolates. J Virol. 1979 Jun;30(3):839–846. doi: 10.1128/jvi.30.3.839-846.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerch A. R., Mitchell W. R., Joklik W. K. Isolation of intact individual species of single- and double-stranded RNA after fractionation by polyacrylamide gel electrophoresis. Anal Biochem. 1975 May 12;65(1-2):331–345. doi: 10.1016/0003-2697(75)90517-5. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Ramig R. F., Mustoe T. A., Fields B. N. A genetic map of reovirus. 1. Correlation of genome RNAs between serotypes 1, 2, and 3. Virology. 1978 Jan;84(1):63–74. doi: 10.1016/0042-6822(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Shipham S. O., de la Rey M. The isolation and preliminary genetic classification of temperature-sensitive mutants of bluetongue virus. Onderstepoort J Vet Res. 1976 Dec;43(4):189–192. [PubMed] [Google Scholar]
- Summers W. C. A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. Anal Biochem. 1970 Feb;33(2):459–463. doi: 10.1016/0003-2697(70)90316-7. [DOI] [PubMed] [Google Scholar]
- Verwoerd D. W., Els H. J., De Villiers E. M., Huismans H. Structure of the bluetongue virus capsid. J Virol. 1972 Oct;10(4):783–794. doi: 10.1128/jvi.10.4.783-794.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]