Abstract
Targeting of cancer stem cells is believed to be essential for curative therapy of cancers, but supporting evidence is limited. Few selective target genes in cancer stem cells have been identified. Here we identify the arachidonate 5-lipoxygenase (5-LO) gene (Alox5) as a critical regulator for leukemia stem cells (LSCs) in BCR-ABL-induced chronic myeloid leukemia (CML). In the absence of Alox5, BCR-ABL failed to induce CML in mice. This Alox5 deficiency caused impairment of the function of LSCs but not normal hematopoietic stem cells (HSCs) through affecting differentiation, cell division, and survival of long-term LSCs (LT-LSCs), consequently causing a depletion of LSCs and a failure of CML development. Treatment of CML mice with a 5-LO inhibitor also impaired the function of LSCs similarly by affecting LT-LSCs, and prolonged survival. These results demonstrate that a specific target gene can be found in cancer stem cells and its inhibition can completely inhibit the function of these stem cells.
Cancer stem cells in many types of hematologic malignancies and solid tumors are believed to be a cell population required for cancer initiation, and must be targeted for effective treatment of the diseases 1–7; however, the direct supporting evidence is still lacking. The challenge includes identification of differences between cancer stem cells and their normal stem cell counterparts, and demonstration of complete control of cancer by targeting these stem cells. Success of an anti-stem cell strategy relies on complete inhibition of function of a gene required for the maintenance of cancer stem cells but not normal stem cells. A number of genes have been found to promote or inhibit cancer cell proliferation, but they also play similar roles in regulating normal stem cells. Examples include pathways involved in signaling through Wnt/β-catenin, Hedgehog and Notch 3,4,8–11, Bim-1 12,13, p53 8, p16 INK4a 14, p19ARF 15, etc. The role of Pten in the inhibition of cancer stem cells of acute myeloid leukemia (AML) and in the maintenance of normal HSCs is unique, and provides an example that distinguishes AML stem cells from normal HSCs, although Pten has an effect on normal HSCs 16. Because cancer stem cells express markers similar to those on normal stem cells 17,18, the major difference between them should be related to the cancer-initiating genetic changes such as acquiring an oncogene or accumulating a DNA mutation. It is reasonable to hypothesize that these genetic changes cause aberrant expression of genes, consequently turning a normal stem cell into a cancer stem cell. It is essential to identify genes that are functionally required by cancer stem cells but not by normal stem cell counterparts. Some clues for identifying genes that play roles in cancer stem cells could come from studies of normal stem cell counterparts and different lineages of cancer cells 19–22. It is reasonable to think that biological features of cancer stem cells are reflected by the deference in gene expression between cancer and normal stem cells. The list of aberrantly expressed genes could be huge, and it is critical to identify key genes or pathways that are required for initiating and maintaining cancer stem cells and can be used as targets for inhibiting these cells.
We have tested our hypothesis using BCR-ABL-induced CML as a disease model system, because CML is a stem cell disease and we have previously identified LSCs for CML in mice 23. In addition, CML stem cells are insensitive to BCR-ABL kinase inhibitors 24,25, calling for the development of new therapeutic strategies. Here we identify Alox5 as a key gene that regulates the function of LSCs but not normal HSCs in mice. Alox5 has been shown to be involved in numerous physiological and pathological processes, including oxidative stress response, inflammation, and cancer 26–33. Here we also show that Alox5 deficiency or inhibition of the function of this gene completely prevent the initiation of BCR-ABL-induced CML.
Results
Alox5 is essential for the induction of CML by BCR-ABL
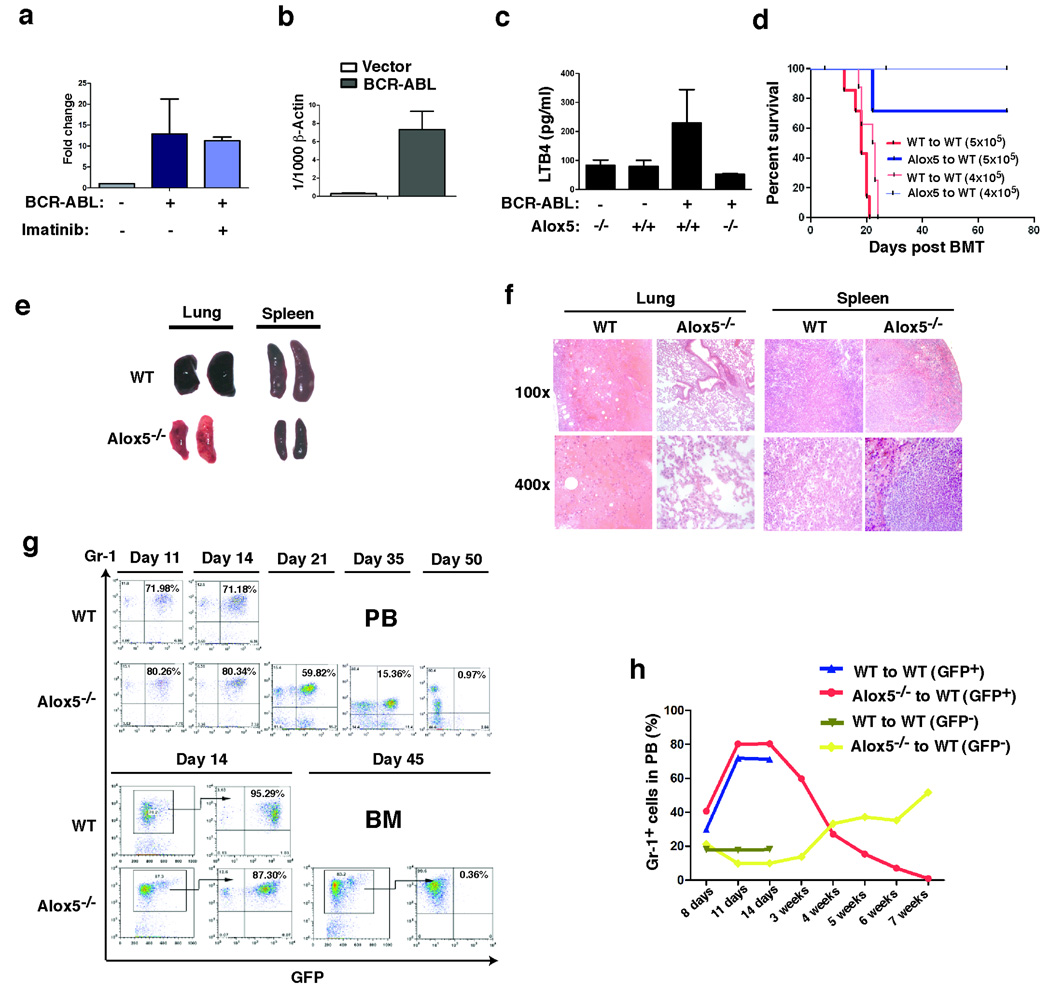
LSCs in CML are insensitive to BCR-ABL kinase inhibitors 24, and we believe that this insensitivity would correspond to unresponsiveness of some genes to the kinase inhibitors. To identify genes that are regulated by BCR-ABL in LSCs, we used the bone marrow transplantation (BMT) mouse model of CML as an assay system, in which bone marrow cells from donor mice pre-treated with 5-fluorouracil (5-FU) and transduced with BCR-ABL result in development of CML in recipient mice 34. We transduced bone marrow cells from C57BL/6 (B6) mice with retrovirus containing BCR-ABL/GFP or GFP alone under conditions for the induction of CML, followed by transplantation of the transduced cells into B6 recipient mice. Some mice were treated with the BCR-ABL kinase inhibitor imatinib to allow identification of genes that were altered by BCR-ABL in LSCs, but this alteration was not restored by inhibition of BCR-ABL kinase activity with imatinib. This approach to identifying pathways that are activated by BCR-ABL but are insensitive to inhibition by imatinib is based on our previous observation that BCR-ABL kinase activity does not relate to all signaling pathways activated by BCR-ABL 23. 14 days after BMT, bone marrow cells were isolated and subsequently sorted by FACS for LSCs (GFP+Lin−c-Kit+Sca-1+) 23. Total RNA was isolated from these BCR-ABL-expressing LSCs or from the GFP+Lin−c-Kit+Sca-1+ cells that only expressed GFP, and DNA microarray analysis was carried out to compare gene expression between BCR-ABL-expressing and non-BCR-ABL-expressing Lin−c-Kit+Sca-1+ cells. The Alox5 gene was up-regulated and this up-regulation was not abolished by imatinib treatment (Fig. 1a). There were several genes that were also up-regulated by BCR-ABL and not changed in expression following imatinib treatment (Supplementary Fig. 1). The up-regulation of Alox5 by BCR-ABL in LSCs was confirmed by RT-PCR (Fig. 1b). To further demonstrate the regulation of Alox5 by BCR-ABL, we tested whether BCR-ABL up-regulates Alox5 function by measuring levels of leukotriene B4 (LTB4), which is synthesized and metabolized through the 5-LO pathway, in peripheral blood of the transplanted mice. Plasma level of LTB4 was increased in CML mice (Fig. 1c)
Figure 1. Alox5 is essential for the induction of CML induced by BCR-ABL.
a, Bone marrow cells from C57BL/6 mice (B6) were transduced with retrovirus containing BCR-ABL/GFP or GFP alone (BCR-ABL-IRES-GFP-pMSCV or IRES-GFP-pMSCV), and then transferred into B6 recipient mice to induce CML. One group of CML mice was treated with imatinib (150 mg/kg body weight/ per dose, once every 4 hours) for 5 doses beginning at day 13 post bone marrow transplantation (BMT). Bone marrow cells were isolated from CML mice, and were sorted by FACS for GFP+Lin−c-Kit+Sca-1+ cells (normal or CML stem cells). Total RNA was isolated from these sorted cells for DNA micorarray analysis. Expression of the Alox5 gene was up-regulated by BCR-ABL in CML stem cells as compared to the sorted GFP+Lin−c-Kit+Sca-1+ cells that did not express BCR-ABL, and this up-regulation was not prevented by imatinib treatment. b, Bone marrow cells from C57BL/6 mice (B6) were transduced with retrovirus containing BCR-ABL/GFP or GFP alone to induce CML as described in a. Bone marrow cells were isolated from the mice were sorted by FACS for GFP+Lin−c-Kit+Sca-1+ cells, and then total RNA was isolated from these sorted cells for comparing Alox5 expression between GFP vector-transduced normal stem cells and BCR-ABL-transduced LSCs by RT-PCR. Expression of the Alox5 gene was significantly up-regulated by BCR-ABL in LSCs as compared to the sorted GFP vector-transduced normal stem cells (p< 0.001). c, The plasma level of LTB4 in recipients of BCR-ABL-transduced bone marrow cells was significantly higher than that in recipients of bone marrow cells transduced with GFP-containing retrovirus (p< 0.05), and this increased level of LTB4 was not observed in recipients of Alox5−/− bone marrow cells transduced by BCR-ABL. These results indicated that BCR-ABL up-regulates LTB4 through Alox5. d, Kaplan-Meier survival curves for recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice (10 mice per group). All recipients of BCR-ABL-transduced bone marrow cells from wild type donor mice developed CML and died within 4 weeks after bone marrow transplantation (days post BMT), whereas recipients of BCR-ABL-transduced bone marrow cells from Alox5−/− donor mice survived. e, Gross appearance of the lungs and spleens showed severe lung hemorrhages and splenomegaly of recipients of BCR-ABL-transduced bone marrow cells from wild type but not Alox5−/− donor mice. f, Photomicrographs of haematoxylin and eosin-stained lung and spleen sections from recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice. g, FACS analysis showed gradual disappearance of GFP+Gr-1+ cells in peripheral blood (PB) and bone marrow (BM) of recipients of BCR-ABL-transduced bone marrow cells from Alox5−/− but not wild type donor mice. h, In recipients of BCR-ABL-transduced bone marrow cells from Alox5−/− donor mice, GFP+Gr-1+ cells in PB gradually decreased with time, whereas the GFPGr-1+ cells that did not express BCR-ABL gradually increased, showing that Alox5 deficiency significantly inhibited engraftment of BCR-ABL-expressing but not normal BM cells in the same animals. Mean percentage for each group (n=5) was shown.
Next, we studied the role of Alox5 in regulation of LSC function using Alox5 homozygous knockout (Alox5−/−) mice. Wild type or Alox5−/− donor bone marrow cells in B6 background were used to induce CML. We first investigated whether Alox5 is required for the induction of CML by BCR-ABL. Recipients of BCR-ABL-transduced bone marrow cells from 5-FU-treated wild type donor mice developed and died of CML within 4 weeks, whereas recipients of BCR-ABL-transduced bone marrow cells from Alox5−/− donor mice were resistant to the induction of CML (Fig. 1d). This defective disease phenotype correlated with much less severe infiltration of myeloid leukemia cells in the lung and spleen (Fig. 1e, f). In addition, FACS analysis of CML cells in peripheral blood and bone marrow showed that Gr-1+ myeloid leukemia cells grew initially, reached a peak after 2 weeks, then started to decline, and eventually disappeared after 7 weeks in peripheral blood and bone marrow of recipients receiving BCR-ABL-transduced Alox5−/− donor bone marrow cells (Figs. 1 g , h). Alox5 deficiency mainly affected growth of BCR-ABL-expressing (GFP+) but not non-BCR-ABL-expressing (GFP−) donor bone marrow cells (Fig. 1h), suggesting that Alox5 signaling is much more critical for the function of LSCs but not normal HSCs. Together, these results demonstrate that Alox5 pathway is essential for the induction of CML by BCR-ABL.
Alox5 transgene rescues defective CML
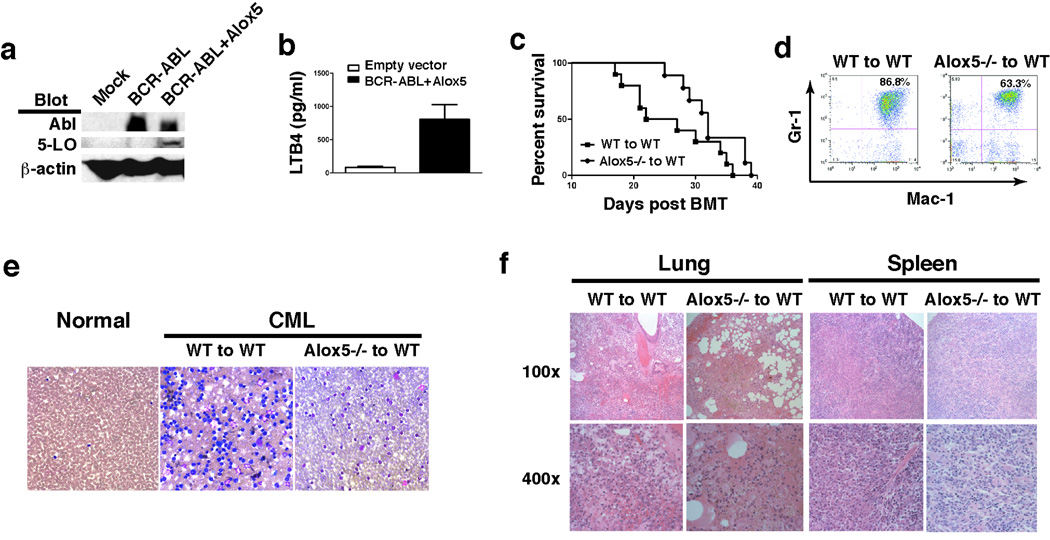
To further confirm the role of Alox5 in CML development, we co-expressed BCR-ABL and Alox5 in Alox5−/− and wild type bone marrow cells, respectively, by retroviral transduction, followed by transplantation of the transduced cells into recipient mice. The BCR-ABL-IRES-Alox5-pMSCV construct expressed BCR-ABL and 5-LO in 293T cells (Fig. 2a), and induced LTB4 production in mice (Fig. 2b). In contrast to no CML induction by BCR-ABL in the absence of Alox5 (Fig. 1), ectopically expressed Alox5 in Alox5−/− bone marrow cells rescued defective CML phenotype, and all mice receiving the BCR-ABL-IRES-Alox5-pMSCV transduced Alox5−/− bone marrow cells died (Fig. 2c). In this experiment, we included the control mice that received BCR-ABL transduced Alox5−/− bone marrow cells as shown in Fig. 1, and all these mice survived (data not shown). FACS analysis (Fig. 2d) and peripheral blood smears (Fig. 2e) showed the development of typical CML after the expression of the rescue gene Alox5, consistent with the severe infiltration of myeloid leukemia cells in the lung and spleen (Figs. 2f). The mice receiving the BCR-ABL-IRES-Alox5-pMSCV transduced wild type bone marrow cells died faster than those receiving the BCR-ABL-IRES-Alox5-pMSCV transduced Alox5−/− bone marrow cells (Fig. 2c), correlating with more myeloid cells in peripheral blood (Fig. 2d, e) and more severe infiltration of myeloid leukemia cells in the lung and spleen (Fig. 2f). The results from this rescue experiment definitively confirmed that Alox5 plays a critical role in CML development.
Figure 2. Alox5 transgene rescues defective CML phenotype.
a, The rescue construct (BCR-ABL-IRES-Alox5-pMSCV) was used to transfect 293T cells to test for expression of 5-LO with mock- or BCR-ABL-IRES-GFP-pMSCV-transfected cells as controls. BCR-ABL and 5-LO were detected by Western blotting using antibodies against Abl and 5-LO. 5-LO protein was detected in cells transfected with BCR-ABL-IRES-Alox5-pMSCV. b, Bone marrow cells from B6 mice were transduced with retrovirus containing IRES-GFP-pMSCV (empty vector) or BCR-ABL-IRES-Alox5-pMSCV, and then transferred into B6 recipient mice to induce CML. The plasma level of LTB4 in recipients of BCR-ABL-IRES-Alox5-pMSCV-transduced bone marrow cells was significantly higher than that in recipients of bone marrow cells transduced with empty vector-containing retrovirus (p< 0.01), confirming that the BCR-ABL-IRES-Alox5-pMSCV construct induced LTB4 production in mice. c, Kaplan-Meier survival curves for recipients of BCR-ABL-IRES-Alox5-pMSCV-transduced bone marrow cells from wild type (n=10) or Alox5−/− (n=9) donor mice. All recipient mice died. d, FACS analysis showed appearance of GFP+Gr-1+ cells in peripheral blood of recipients of BCR-ABL-IRES-Alox5-pMSCV-transduced bone marrow cells from both wild type and Alox5−/− donor mice. e, Peripheral blood smears showed accumulation of neutrophils, indicating high white blood cell counts in these CML mice. f, Photomicrographs of haematoxylin and eosin-stained lung and spleen sections from recipients of BCR-ABL-IRES-Alox5-pMSCV-transduced bone marrow cells from wild type or Alox5−/− donor mice.
Alox5 deficiency impairs the function of LSCs
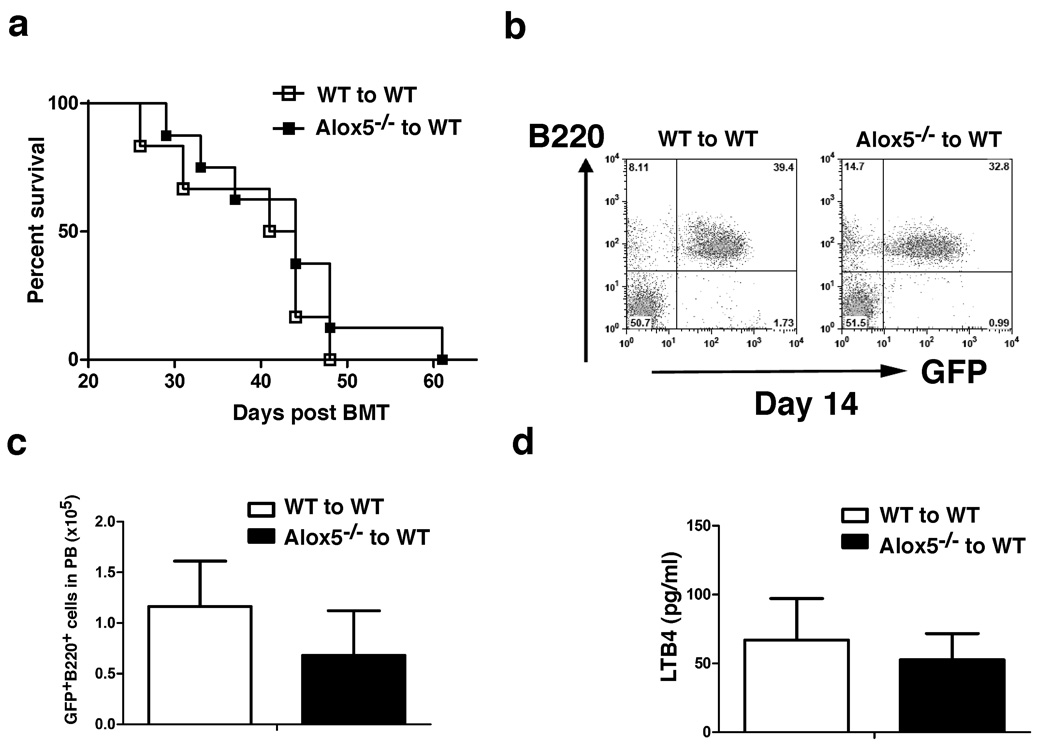
The eventual disappearance of myeloid leukemia cells in CML mice in the absence of Alox5 (Fig. 1h) prompted us to examine whether Alox5 is required for self-renewal of LSCs. A biological assay for LSCs is to examine their ability to transfer disease to secondary recipient mice 17,18. We transferred bone marrow cells from primary recipients of BCR-ABL-transduced wild type or Alox5−/− donor bone marrow cells to secondary recipient mice. BCR-ABL-expressing wild type bone marrow cells transferred lethal CML, whereas BCR-ABL-expressing Alox5−/− bone marrow cells failed to induce CML in secondary recipient mice (Supplementary Fig. 2). This result suggests that Alox5 deficiency causes the impairment of the function of LSCs.
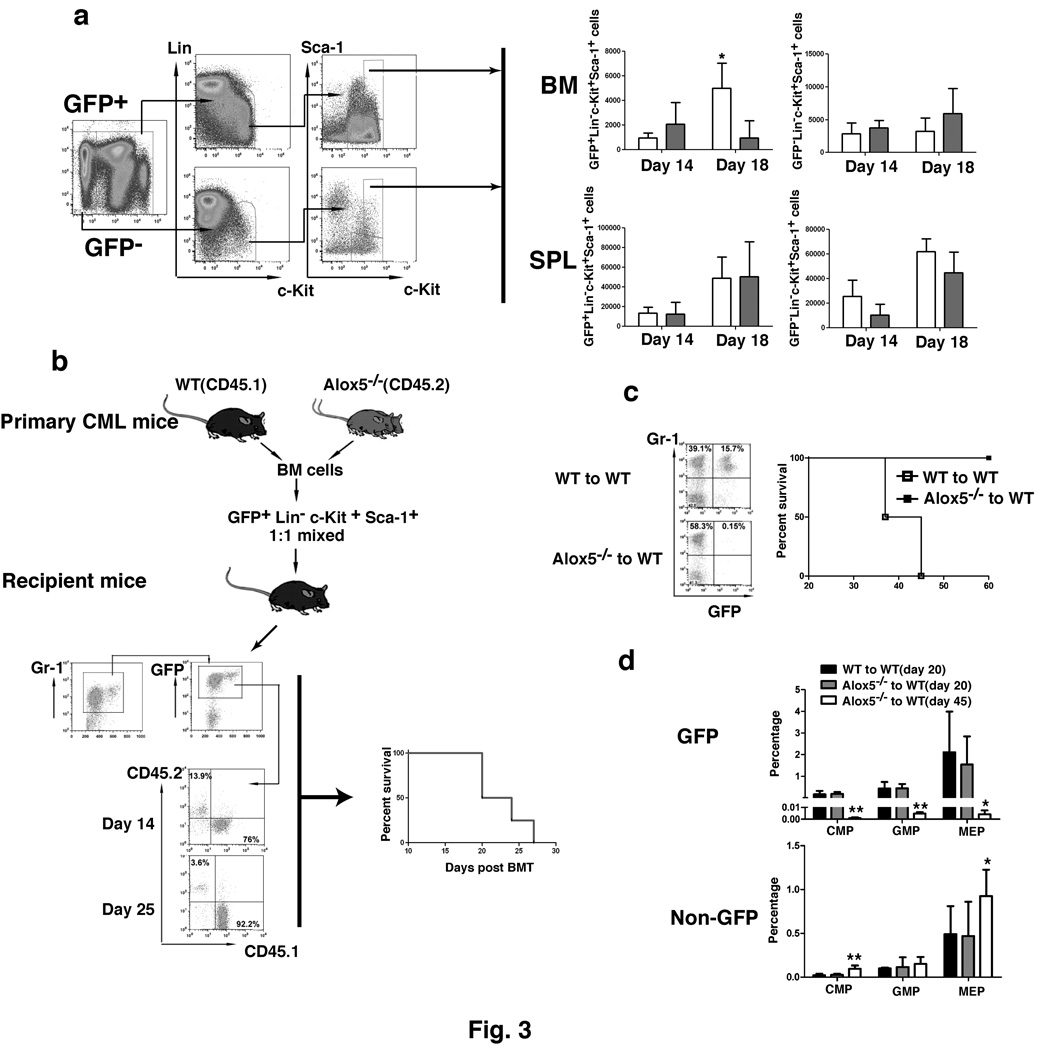
The impaired CML development in the absence of Alox5 could be caused by decreased number of LSCs. To test this hypothesis, we quantified LSCs (GFP+Lin−c-Kit+Sca-1+) and normal HSCs (GFP−Lin−c-Kit+Sca-1+) in bone marrow and the spleens of CML mice at day 14 and day 18 after the induction of CML by BCR-ABL. At day 14, Alox5 deficiency did not cause a reduction of LSCs in bone marrow and the spleens, but did so in bone marrow not in the spleens at day 18, as compared to LSCs in wild type CML mice (Fig. 3a). In the spleen of these mice, the number of Alox5−/− LSCs at day 18 was similar to that of wild type LSCs (Fig. 3a), indicating that the reduction of LSCs in bone marrow was not due to the migration of bone marrow LSCs to the spleens but due to an intrinsic defect caused by Alox5 deficiency in LSCs. Alox5 deficiency did not cause a significant reduction of normal HSCs (GFP−) in bone marrow and the spleens of the same animals (Fig. 3a), suggesting that Alox5 is functionally required by LSCs but not by normal HSCs. In addition, we did not observe a homing defect of HSCs lacking Alox5 (Supplementary Fig. 3), which could cause the impaired CML development (Fig. 1). Furthermore, Alox5 deficiency did not appear to cause a homing defect on LSCs, as the total number of Alox5−/− LSCs was not lower than that of wild type LSCs at day 14 after the induction of CML by BCR-ABL (Fig. 3a).
Figure 3. Loss of Alox5 impairs the function of CML stem cell.
a, BCR-ABL-expressing (GFP+) and non-BCR-ABL-expressing (GFP) Lin−c-Kit+Sca-1+ cells (CML stem cells) in BM and the spleens (SPL) were analyzed by FACS in recipients of BCR-ABL-transduced BM cells from wild type or Alox5−/− donor mice (n=4 for each group at two time points after BMT). Total number of GFP+Lin−c-Kit+Sca-1+ cells for each mouse was calculated as percentage of GFP+Lin−c-Kit+Sca-1+ cells x total cell count for the cells from femurs and tabias. Loss of Alox5 caused significant reduction of CML stem cells in BM (p< 0.05). b, Equal numbers of the sorted GFP+Lin−c-Kit+Sca-1+ cells from recipients of BCR-ABL-transduced bone marrow cells (5000 cells each) from wild type (CD45.1) or Alox5−/−(CD45.2) donor mice were mixed, followed by transplantation into lethally irradiated wild type mice. At days 14 and 25 after BMT, FACS analysis showed that the percentages of CD45.1+ cells were much higher than those of CD45.2+ cells. All these mice died of CML, presumably due to the development of CML from CD45.1+ cells. c, GFP+Lin−c-kit+Sca-1+ cells sorted by FACS from BCR-ABL-transduced BM cells from wild type or Alox5−/− mice were injected into lethally irradiated wild type recipient mice (15000 GFP+Lin−c-kit+Sca-1+ cells per recipient mouse). At day 14 after BMT, GFP+ Gr-1+ cells in peripheral blood of the mice were analyzed by FACS. Alox5-deficient GFP+Lin−c-kit+Sca-1+ poorly engrafted. The mice receiving the Alox5-deficient GFP+Lin−c-kit+Sca-1+ cells survived (n=4), whereas the mice receiving the wild type GFP+Lin−c-kit+Sca-1+ cells died of CML (n=2). d, FACS analysis indicated the percentages of BCR-ABL-expressing (GFP+) and non-BCR-ABL-expressing (GFP) CMP, GMP, and MEP cells in BM of recipients of BCR-ABL-transduced wild type or Alox5−/− donor BM cells (n=4). The results showed that loss of Alox5 caused depletion of BCR-ABL-expressing but not non-BCR-ABL-expressing CMP, GMP, and MEP cells in BM of the mice.
Engraftment of donor bone marrow cells in lethally irradiated recipient mice reflects stem cell function. Alox5 deficiency could cause a decreased engraftment of LSCs, leading to a decrease in LSCs. To test this idea, LSCs were sorted by FACS from bone marrow of CML induced by transplanting BCR-ABL-transduced wild type (CD45.1) or Alox5−/− (CD45.2) donor bone marrow cells. The sorted wild type and Alox5−/− LSCs were 1:1 mixed, followed by transplantation into lethally irradiated recipient mice. At day 14 or 25 after transplantation, more than 70% or 90% of GFP+Gr-1+ cells in peripheral blood of the mice were wild type (CD45.1+) leukemia cells, and all these mice developed CML and died (Fig. 3b). Total wild type and Alox5−/− bone marrow cells that were not sorted by FACS were also 1:1 mixed, followed by transplantation into lethally irradiated recipient mice. At 40 day after BMT, more than 80% of GFP+Gr-1+ cells in peripheral blood were wild type (CD45.1+) leukemia cells, and all these mice died of CML (Supplementary Fig. 4). To further compare biological function between wild type and Alox5−/− LSCs, BCR-ABL-transduced wild type or Alox5−/− bone marrow cells were sorted by FACS for Lin−c-Kit+Sca-1+ cells, and equal number of the sorted wild type or Alox5−/− cells were transplanted into each lethally irradiated recipient mouse. At day 14 after transplantation, GFP+Gr-1+ cells were detected in peripheral blood of recipient mice receiving BCR-ABL-transduced wild type bone marrow cells but barely in peripheral blood of mice receiving BCR-ABL-transduced Alox5−/− bone marrow cells (Fig. 3c). By day 50 after transplantation, all mice receiving BCR-ABL-transduced sorted wild type bone marrow cells died of CML, whereas all mice receiving the transduced sorted Alox5−/− bone marrow cells survived (Fig. 3c). These results indicated that Alox5 deficiency caused impairment of the function of LSCs, presumably leading to reduced production of leukemia progenitor cells over time. This assumption was consistent with our finding that at day 20 after the induction of CML by BCR-ABL, similar numbers of GFP+ CMP (common myeloid progenitor), GMP (granulocyte-macrophage progenitor), and MEP (megakaryocyte-erythroid progenitor) cells were detected in bone marrow of mice receiving BCR-ABL-transduced Alox5−/− donor bone marrow cells (Fig. 3d), and at day 45 the number of these cells were much less (Fig. 3d). By contrast, Alox5−/− deficiency did not cause a reduction of non-BCR-ABL-expressing myeloid progenitor cells in the same animals (Fig. 3d), providing an indirect evidence that Alox5 deficiency only causes a functional defect in LSCs without affecting normal HSCs. The conclusion that Alox5 deficiency does not cause a functional defect in normal HSCs was further confirmed by comparing the engraftment of wild type (CD45.1) and Alox5−/− (CD45.2) bone marrow cells by injecting the same number of each type of bone marrow cells into lethally irradiated recipient mice. At 30 day after BMT, the percentage of wild type or Alox5−/− bone marrow cells was similar (Supplementary Fig. 5). A control experiment showed that after lethal irradiation, 100 % of cells in peripheral blood of recipient mice were donor-derived when assayed at day 30 after BMT (data not shown), allowing the direct analysis of CD45.1 or CD45.2 cells to reflect the donor-derived wild type (CD45.1) and Alox5−/− (CD45.2) bone marrow cells in the same animal.
Alox5 deficiency affects differentiation, cell division and survival of LT-LSCs
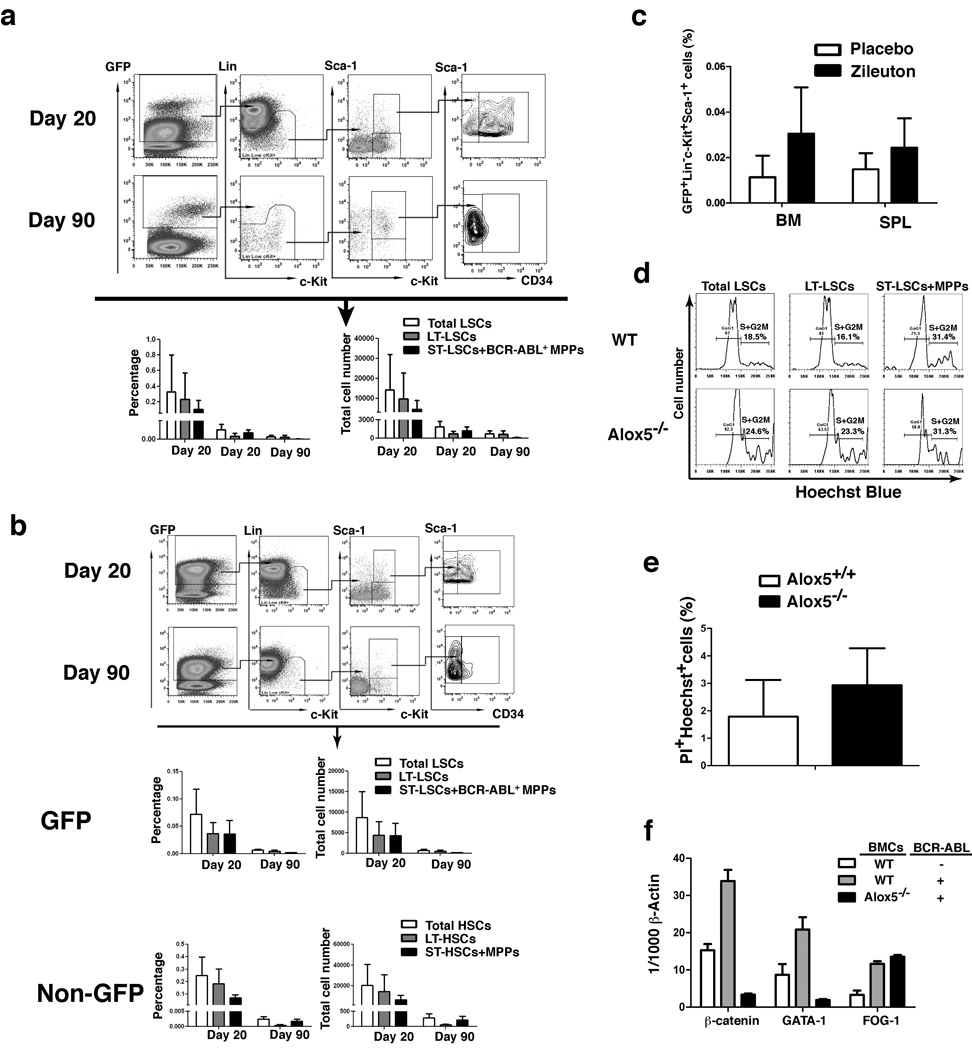
Alox5 deficiency caused the impairment of the function of LSCs (Fig. 3), and these cells were Lin−c-Kit+Sca-1+ phenotypically, containing long-term (LT) HSCs, short-term (ST) HSCs, and multipotent progenitor (MPP) cells. We further investigated which of these cell populations is affected by Alox5 deficiency. BCR-ABL-transduced Alox5−/− bone marrow cells were transplanted into recipient mice to induce CML, and bone marrow cells from these mice were analyzed by FACS for the percentages of total HSCs (Lin−c-Kit+Sca-1+), LT-HSCs (Lin−c-Kit+Sca-1+CD34−), and ST-HSCs /MPP cells (Lin−c-Kit+Sca-1+CD34+). At day 20 after the induction of CML by BCR-ABL, the percentage or total number of bone marrow LT-LSCs (GFP+Lin−c-Kit+Sca-1+CD34−) was about 1/2 of those of ST-LSCs/MPP cells (GFP+Lin−c-Kit+Sca-1+CD34+) (Fig. 4a). However, at day 90, the percentage or total number of LT-LSCs was about 8 fold higher than that of ST-LSCs/MPP cells (Fig. 4a). These results suggest that Alox5 deficiency blocks differentiation of LT-LSCs, preventing these cells from developing CML. In these mice, the percentage of GFP− LT-HSCs was much lower than that of GFP− ST-HSCs/MPP cells (Supplementary Fig. 6), demonstrating that Alox5 deficiency did not similarly affect differentiation of normal LT-HSCs. Although loss of Alox5 caused a relative high percentage of LT-LSCs than that of ST-LSCs/MPP cells, the total number of LT-LSCs declined with time (Fig. 4a), suggesting that Alox5 deficiency caused a gradual depletion of LSCs.
Figure 4. Loss of Alox5 function blocks differentiation of LT-LSCs.
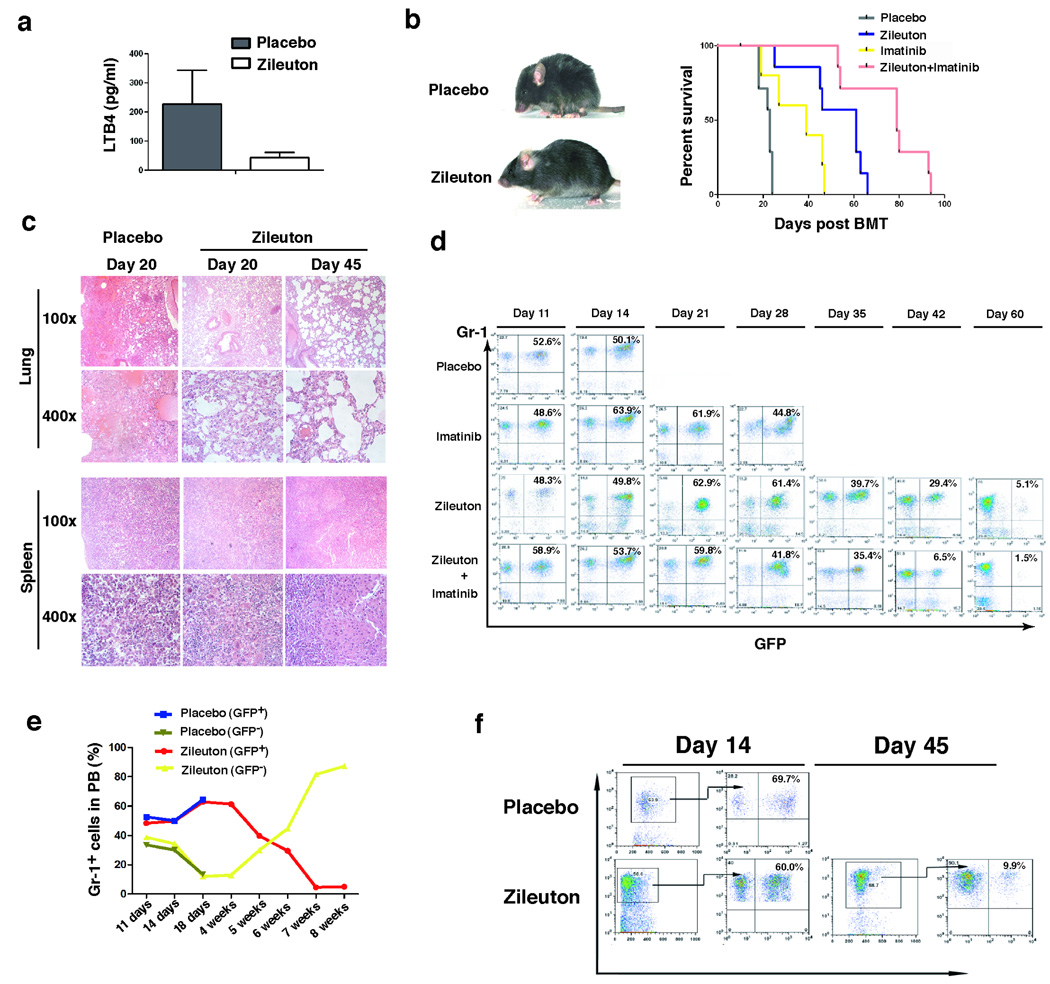
a, Bone marrow cells were isolated from recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice, and FACS analysis showed that the percentages and total numbers of LT-LSCs (GFP+Lin−c-Kit+Sca-1+CD34-) and ST-LSCs /BCR-ABL-expressing MPP cells (Lin−c-Kit+Sca-1+CD34+) in recipients of BCR-ABL-transduced Alox5−/− (middle and right panels) donor BM cells were much lower than those in recipients of BCR-ABL-transduced wild type (left panel) donor BM cells (n=4). In addition, LT-LSCs in recipients of BCR-ABL-transduced Alox5−/− donor BM cells relatively accumulated due to the blockade of differentiation, resulting in depletion of ST-LSCs /BCR-ABL-expressing MPP cells as assayed at day 90 post BMT. b, Recipients of BCR-ABL-transduced bone marrow cells from wild type donor mice were treated with Zileuton (300 mg/kg, twice a day) beginning at 8 days after BMT. At days 20 and 90 post BMT, FACS analysis showed the depletion of ST-LSCs /BCR-ABL-expressing MPP cells in Zileuton-treated CML mice, indicating a blockade of differentiation of LT-LSCs. In contrast, in the same animals the percentages and total numbers of ST-HSCs /MPP cells were higher than those of LT-LSCs, indicating that Zileuton treatment did not lead to a blockade of differentiation of normal LT-HSCs in Zileuton-treated CML mice. c, Mice receiving wild type donor BM cells transduced with MSCV-IRES-GFP retrovirus were treated with a placebo or Zileuton as described in b. At day 14 after BMT, the numbers of GFP+Lin−c-Kit+Sca-1+ cells in BM and the spleens (SPL) of placebo- and Zileuton-treated mice were compared. Zileuton treatment did not result in a reduction of normal HSCs in the mice. d, At day 14 after BMT, bone marrow cells were isolated from recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice. The cells were stained with Hoechst Blue, and DNA contents, represented by the percentages of three LSC populations (total LSCs, LT-LSCs, and ST-LSCs+MPPs) in the S+G2M phase of the cell cycle, was examined by FACS. Mean percentage for each cell population (n=5) was shown. e, At day 14 after BMT, bone marrow cells were isolated from recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice. The cells were stained with PI and Hoechst Blue, and the percentages of LSCs positive for PI and Hoechst Blue, representing apoptotic cells, were determined by F ACS. f, Bone marrow GFP+Lin−c-Kit+Sca-1+ cells were sorted by FACS from recipients of GFP vector- or BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice for isolation of total RNA, and expression of β-catenin, GATA-1 and FOG were detected by RT-PCR. Each bar represents the mean value of three samples. BMCs: bone marrow cells.
The effect of Alox5 deficiency on LSCs was further demonstrated in experiments showing that Alox5 function was inhibited by a selective 5-LO inhibitor Zileuton 35. BCR-ABL-transduced wild type bone marrow cells were transplanted into recipient mice to induce CML, and LSCs in bone marrow of these mice treated with Zileuton (300 mg/kg of body weight, twice a day) were analyzed by FACS at days 20 and 90 after the induction of CML by BCR-ABL. As the treatment went on, the ratio between the percentage of LT-LSCs and that of ST-LSCs/MPP cells became increasing (Fig. 4b), suggesting a blockade of differentiation of LT-LSCs. Zileuton treatment did not similarly affect differentiation of GFP− LT-HSCs in the same animals (Fig. 4b), demonstrating that inhibition of 5-LO did not suppress normal HSCs. These findings were consistent with those from the studies using Alox5−/− mice (Fig. 4a). No suppression of normal HSCs was further demonstrated in placebo- or Zileuton-treated mice receiving wild type donor bone marrow cells transduced with MSCV-IRES-GFP retrovirus. At day 14 after BMT, the numbers of GFP+Lin−c-Kit+Sca-1+ cells in bone marrow of placebo- and Zileuton-treated mice were compared. Zileuton treatment did not result in a reduction of GFP+Lin−c-Kit+Sca-1+ cells in bone marrow and the spleens of the mice (Fig. 4c).
The blockade of LT-LSC differentiation was correlated with biological and molecular changes in LSCs. Cell cycle analysis of LSCs in bone marrow of CML mice showed that there was higher percentage of LT-LSCs in the S+G2M phase of the cell cycle in mice receiving BCR-ABL-transduced Alox5−/− bone marrow cells than in mice receiving BCR-ABL-transduced wild type bone marrow cells (Fig. 4d); however, the percentages of LT-LSCs and ST-LSCs/MPPs in the S+G2M phase of the cell cycle were similar (Fig. 4d), suggesting that this differentiation blockade caused a compensatory response of Alox5 deficient LSCs to the shortage of downstream cell lineages. However, the higher percentage of LT-LSCs in the S+G2M phase of the cell cycle in the absence of Alox5 may also be explained by asymmetric cell division of LT-LSCs. In addition, Alox5 deficiency caused slightly increased apoptosis of LSCs (p> 0.05) (Fig. 4e), but the apoptotic ratio between ST-LSCs/MPPs and LT-LSCs in the absence of Alox5 was similar to that in the presence of Alox5 (Supplementary Fig. 7). To study the underlying mechanism for the defective function of LSCs at molecular level, we examined the expressive levels of three regulatory genes of hematopoiesis, β-catenin, GATA-1 and FOG-1 9,36,37. We found that the defective function of LSCs in the absence of Alox5 was correlated with the reduction of β-catenin and GATA-1 but not FOG-1 (Fig. 4f).
Inhibition of 5-LO prolongs survival of CML mice
Zileuton treatment suppressed LSCs in CML mice (Fig. 4). We examined whether 5-LO serves as a potential target in LSCs for treating CML. Mice with BCR-ABL-induced CML were treated with a placebo, the 5-LO inhibitor Zileuton, imatinib alone, or two agents in combination. All placebo-treated mice developed and died of CML within 4 weeks after the induction of CML by BCR-ABL. Zileuton inhibited Alox5 function in CML mice, as plasma level of LTB4 was decreased compared to placebo-treated CML mice (Fig. 5a). As expected, imatinib treatment was effective in treating CML, but Zileuton treatment was even more effective (Fig. 5b). Treatment of CML mice with both Zileuton and imatinib had a better therapeutic effect than with either Zileuton or imatinib alone in prolonging survival of the mice (Fig. 5b). Prolonged survival of Zileuton-treated CML mice correlated with less severe leukemia cell infiltration to the lungs and the spleens (Fig. 5c). In peripheral blood of CML mice treated with Zileuton and imatinib, GFP+Gr-1+ leukemia cells gradually decreased with treatment, and dropped from over 50% to less than 2%, as analyzed at day 60 after the induction of CML (Fig. 5d). Although these mice eventually died (Fig. 5d), FACS analysis barely detected any GFP+Gr-1+ myeloid leukemic cells in peripheral blood at death (data not shown), indicating that myeloid leukemia was eliminated. Instead, these mice developed ALL, as shown by the presence of GFP+B220+ leukemic cells in peripheral blood (data not shown). Zileuton treatment did not have an inhibitory effect on normal myeloid cells (GFP−Gr-1+) in peripheral blood of the same animals, as the number of these non-leukemia cells increased during the treatment (Fig. 5e). In bone marrow of Zileuton-treated CML mice, GFP+Gr-1+ myeloid leukemia cells also dropped to low levels during the treatment (Fig. 5f). Prolonged survival of CML mice by Zileuton treatment is consistent with the inhibitory effect of Zileuton on LSCs (Fig. 4b). Zileuton treatment caused a reduction of white blood cell counts less dramatically than did imatinib treatment (Supplementary Fig. 8), presumably because Zileuton targeted LSCs and imatinib inhibited more differentiated leukemic cells. Zileuton treatment also prolonged survival of mice with CML induced by BCR-ABL-T315I (Supplementary Fig. 9).
Figure 5. Inhibition of Alox5 prolongs survival of CML mice.
a, Zileuton treatment resulted in a reduction of the plasma LTB4 level in CML mice. b, Kaplan-Meier survival curves for CML mice treated with a placebo, Zileuton alone, imatinib alone, or both Zileuton and imatinib in combination. Zileuton-treated CML mice were much healthier than placebo-treated CML mice, and inhibition of Alox5 by Zileuton significantly prolonged survival of CML mice. c, Photomicrographs of haematoxylin and eosin-stained lung and spleen sections from CML mice treated with a placebo or Zileuton. d, FACS analysis showed gradual disappearance of GFP+Gr-1+ cells in PB of CML mice treated with Zileuton. e, In CML mice treated with Zileuton, GFP+Gr-1+ cells in PB gradually decreased with time, whereas the GFP−Gr-1+ cells that did not express BCR-ABL gradually increased, showing that inhibition of Alox5 with Zileuton significantly inhibited engraftment of BCR-ABL-expressing but not normal BM cells in the same animals. f, FACS analysis showed gradual disappearance of GFP+Gr-1+ cells in BM of CML mice treated with Zileuton.
Elevation of LTB4 level was observed in bone marrow cells of CML patients 38. However, a later report from the same group showed that the level of leukotriene C4 (LTC4) synthase, which is a key enzyme in the biosynthesis of LTC4 and represents a different metabolic pathway from LTB4, was elevated in human CML cells 39. Therefore, we examined whether BCR-ABL stimulates LTC4 production in CML mice using an ELISA method. We did not observe an increase of plasma LTC4 levels in mice receiving BCR-ABL-expressing wild type bone marrow cells (BCR-ABL+Alox5+/+) as compared to mice receiving BCR-ABL-expressing Alox5 deficient bone marrow cells (BCR-ABL+Alox5−/−). The mean level of LTC4 was 128.63 pg/ml for the BCR-ABL+Alox5+/+ group and 154.84 pg/ml for the BCR-ABL+Alox5−/− group, respectively.
Alox5 deficiency does not significantly affect normal HSCs
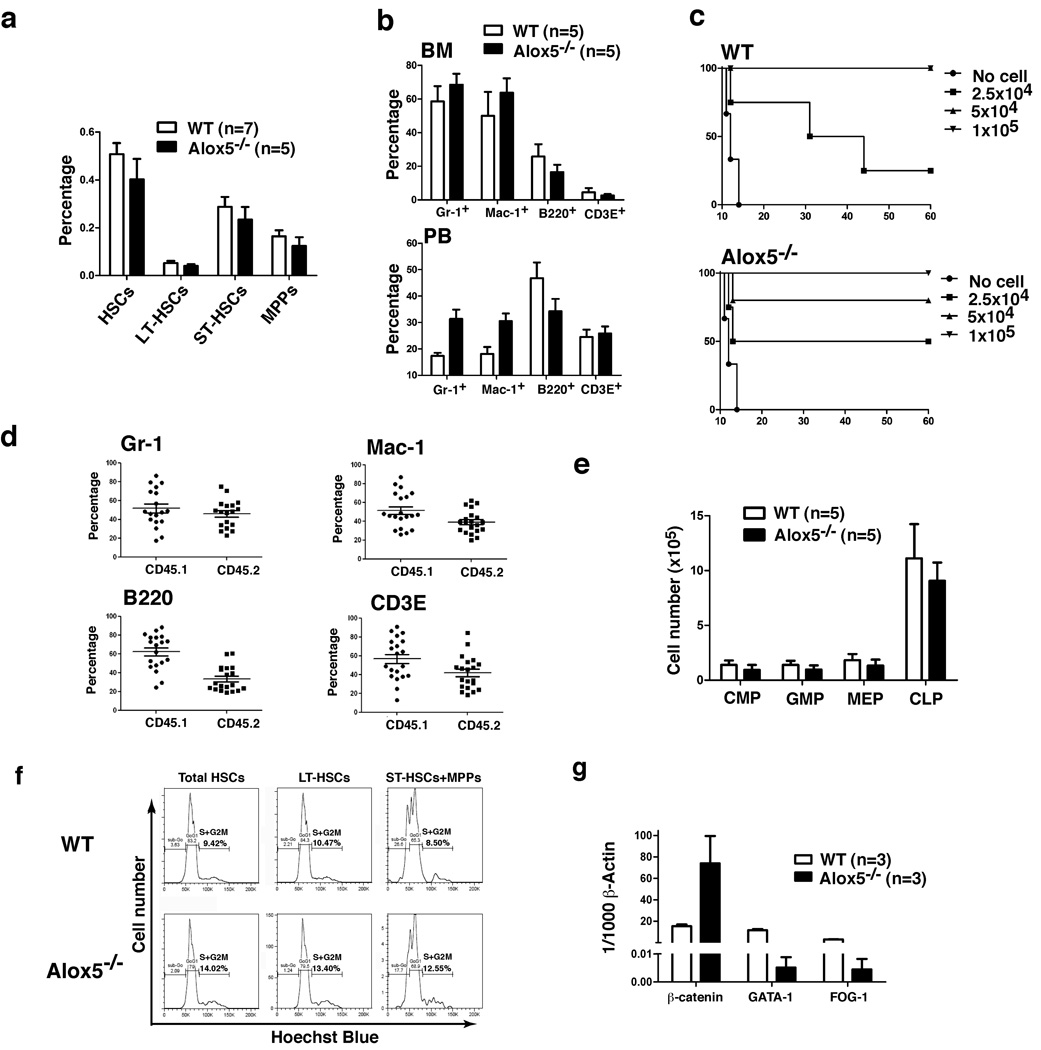
Alox5 deficiency or inhibition of 5-LO by Zileuton specifically impaired the function of Alox5−/− LSCs but not non-BCR-ABL-expressing Alox5−/− HSCs in CML mice (Fig. 4, Fig. 5), showing that these Alox5−/− HSCs had stronger stem cell ability to engraft recipient mice than did Alox5−/− LSCs in the same animals. However, these results did not clearly show whether Alox5 deficiency affected the function of normal HSCs. This question is important, because it is critical to know whether Alox5 deficiency or inhibition of 5-LO lowers the function of HSCs below their normal level, especially if Alox5 were considered as a therapeutic target. We characterized hematopoietic cell lineages in bone marrow and peripheral blood of Alox5−/− mice in comparison with those of wild type B6 mice. The percentages of HSCs (Lin−c-Kit+Sca-1+), LT-HSCs (Lin−c-Kit+Sca-1+CD34−), ST-HSCs (Lin−c-Kit+Sca-1+CD34+Flt3−) and MPPs (Lin−c-Kit+Sca-1+CD34+Flt3+) in bone marrow of Alox5−/− mice were only slightly lower than those of wild type mice (Fig. 6a), and the percentages of Gr-1+, Mac-1+, B220+, or CD3E+ cell populations in bone marrow of these mice were similar to those of wild type mice (Fig. 6b). In peripheral blood, the numbers of Gr-1+ and Mac-1+ cells in Alox5−/− mice were even higher than those in wild type mice (Fig. 6b). These results demonstrated that Alox5 deficiency did not lead to significant decrease in the numbers of hematopoietic cell lineages.
Figure 6. Alox5 deficiency does not significantly affect normal HSCs.
a, Bone marrow cells from wild type and Alox5−/− mice were analyzed by FACS for the percentages of total HSCs (Lin−c-Kit+Sca-1+), LT-HSCs (Lin−c-Kit+Sca-1+CD34−), ST-HSCs (Lin−c-Kit+Sca-1+CD34+Flt3), and MPPs (Lin−c-Kit+Sca-1+CD34+Flt3+). b, Cells from bone marrow and peripheral blood of wild type and Alox5−/− mice were analyzed by FACS for the percentages of Gr-1+, Mac-1+, B220+, and CD3E+ cells. c, Three doses (1×105, 5×104, and 2.5×104) of wild type or Alox5−/− BM cells were injected into lethally irradiated recipient mice. Survival curves showed that there was only a minor engraftment defect of BM in Alox5−/− mice. d, Alox5−/−(CD45.2) and wild type (CD45.1) BM cells were 1:1 mixed and then transferred into recipient mice (n=20). 12 weeks after BMT, FACS analysis was carried out to compare the percentages of wild type and Alox5−/−Gr-1+, Mac-1+, B220+, and CD3E+ cells in PB of recipient mice. e, BM cells from BM of wild type and Alox5−/− mice were analyzed by FACS for the numbers of CMPs, GMPs, MEP, and CLPs. f, Bone marrow cells were isolated from wild type or Alox5−/− mice, and stained with Hoechst Blue. DNA contents in total HSCs, LT-HSCs, and ST-HSCs+MPPs in the S+G2M phase of the cell cycle were examined by FACS. g, Bone marrow HSCs were sorted by FACS from wild type or Alox5−/− mice for isolation of total RNA, and expression of β-catenin, GATA-1 and FOG were detected by RT-PCR. Each bar represents the mean value of three samples.
To compare the function of HSCs between Alox5−/− and wild type mice, we first performed engraftment assay, in which several doses of Alox5−/− or wild type bone marrow cells were transplanted into lethally irradiated wild type B6 mice. The engraftment ability of Alox5−/− bone marrow cells was slightly lower than that of wild type bone marrow cells, as 5×104 wild type bone marrow cells completely protected death of lethally irradiated recipient mice, whereas the same number of Alox5−/− bone marrow cells partially rescued the irradiated mice (Fig. 6c). The minor engraftment defect of Alox5−/− bone marrow cells was further examined in a competitive reconstitution analysis, in which Alox5−/− (CD45.2) and wild type (CD45.1) bone marrow cells were 1:1 mixed and then transferred into recipient mice. 12 weeks after the transplantation, the percentages of CD45.2 Gr-1+ and Mac-1+ cells were slightly lower than those of CD45.1 Gr-1+ and Mac-1+ cells in peripheral blood (Fig. 6d), indicating that Alox5−/− HSCs had slightly lower stem cell function as compared to wild type HSCs. However, the effect of Alox5 deficiency on normal HSCs is much less than that on LSCs, as the competitive engraftment capability of the sorted Alox5−/− LSCs was about thirty fold lower than that of wild type LSCs as assayed at day 25 after CML induction (Fig. 3c), comparing to the one fold difference between Alox5−/− and normal HSCs (Fig. 6d). This was further supported by the observation that Alox5−/− LSCs failed to induce CML (Fig. 3c). We also compared the numbers of bone marrow CMP, GMP, MEP, and CLP between wild type and Alox5−/− mice, and no significant differences were found (Fig. 6e), suggesting that there were no significant functional defects in these progenitor cells. The minor functional defect of Alox5−/− HSCs prompted us to examine whether there were any cellular changes in cell cycle and gene expression of β-catenin, GATA-1 and FOG-1 in these cells. We found that there were slightly higher percentages of both LT-HSCs and ST-HSCs/MPPs in the S+G2M phase of the cell cycle in Alox5−/− bone marrow cells than in wild type bone marrow cells (Fig. 6f), in contrast to the only increase in LT-LSCs in S+G2M phase of the cell cycle in BCR-ABL-expressing Alox5−/− bone marrow cells (Fig. 4d). This further indicates that there is no differentiation blockade in Alox5−/− HSCs and that Alox5 deficiency has distinct biological effect on normal HSCs and LSCs. To support this idea, we compared the expressive levels of β-catenin, GATA-1 and FOG-1 in HSCs of wild type and Alox5−/− mice. We found that the minor functional defect of Alox5−/− HSCs was correlated with a decreased expression of GATA-1 and FOG-1, and that β-catenin expression was increased in Alox5−/− HSCs compared to that in wild type HSCs (Fig. 6g). Different expression patterns of β-catenin, GATA-1 and FOG-1 in Alox5−/− HSCs (Fig. 6g) and LSCs (Fig. 4e) indicate that these three genes function differently in LSCs and normal HSCs.
Alox5 is not required for the induction of lymphoid leukemia by BCR-ABL
We tested whether Alox5 plays a role in BCR-ABL-induced acute lymphoblastic leukemia (ALL), which originates from committed lymphoid progenitors 34. We have previously shown that BCR-ABL-expressing pro-B cells serve as LSCs for this disease 23. Wild type or Alox5−/− donor bone marrow cells were transduced by BCR-ABL retrovirus, followed by transplantation of the transduced cells into lethally irradiated wild type B6 recipient mice. Both groups of mice developed and died of ALL with similar disease latency and survival time (Fig. 7a). FACS analysis of lymphoid leukemia cells showed that both group of ALL mice had similar percentages (Fig. 7b) and numbers (Fig. 7c) of B220+ leukemia cells in peripheral blood of these mice. Together these results showed that Alox5 did not contribute to the development of ALL induced by BCR-ABL, and suggest that Alox5 is not required by ALL stem cells, but is specifically required by CML stem cells. Consistent with these findings, BCR-ABL did not up-regulate Alox5 function in ALL, as the plasma level of LTB4 in recipients of BCR-ABL-transduced wild type bone marrow cells was not significantly higher compared to recipients of BCR-ABL-transduced Alox5−/− bone marrow cells (Fig. 7d).
Figure 7. Alox5 is not required for the induction of lymphoid leukemia by BCR-ABL.
a, Kaplan-Meier survival curves for recipients of BCR-ABL-transduced bone marrow cells from wild type (n=6) or Alox5−/− (n=8) donor mice. Both groups of mice developed and died of ALL. b, At day 14 post BMT, FACS analysis showed no difference in the percentages of GFP+B220+ cells in PB of recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice. c, FACS analysis showed similar numbers of GFP+B220+ cells in PB of recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice. d, The plasma levels of LTB4 in recipients of BCR-ABL-transduced bone marrow cells from wild type or Alox5−/− donor mice were compared. Loss of Alox5 did not result in a reduction of the plasma LTB4 level in ALL mice.
Disccusion
The specific role of the Alox5 gene in regulating the function of LSCs but not normal HSCs shows a new mechanism for how cancer and normal stem cells distinctly self-renew and differentiate, and provides a potential novel strategy for curative therapy of CML induced by BCR-ABL. Although Alox5 and its related pathways were identified in LSCs of CML in this study, they may play a critical role in regulating LSCs of myeloid leukemias induced by other types of oncogenes. This idea is supported by our preliminary finding that the development of Tel-PDGFR-beta induced myeloid leukemia was also largely prevented in the absence of the Alox5 gene (data not shown). In addition, similar phenomenon that a gene specifically regulates cancer stem cells but not normal stem cells may likely be found in other types of cancers.
The mechanism for the specific role of the Alox5 gene in regulating function of LSCs but not normal HSCs remains to be further elucidated. We showed the differential regulation of the beta-catenin gene in wild type and Alox5 deficient LSCs, partially explaining the specific inhibitory effect of Alox5 deficiency on LSCs but not on normal HSCs. It is reasonable to think that these two types of stem cells utilize different pathways for self-renewal and differentiation, and Alox5 represents a critical difference in these stem cells. It is likely that BCR-ABL stimulates transcription of Alox5 to jointly form a unique pathway critical for LSC function. Because the transcriptional activation of Alox5 by BCR-ABL is not inhibited by the BCR-ABL kinase inhibitor imatinib, this at least partially explains why imatinib does not inhibit LSCs in CML mice 23. It is hopeful that targeting Alox5 in combination with a BCR-ABL inhibitor that eliminates BCR-ABL protein, such as a heat shock protein 90 inhibitor 40, would lead to a better control of CML. 5-LO inhibitor and imatinib could also be a good combination, with one inhibiting LSCs and another eliminating differentiated leukemia cells.
We show that Alox5 deficiency causes the depletion of LSCs in CML mice, and we believe that three biological changes (blockade of differentiation, asymmetric cell division and apoptosis) contribute to the eradication of these stem cells. At present, it is difficult to know which of the three biological changes plays a major role in depleting LSCs, and further mechanistic studies are required to answer this question.
No studies have shown a role of Alox5 in regulation of LSCs. Our findings in this study first demonstrate that Alox5 is a critical regulator of LSCs in CML, providing an in vivo system for investigating underlying molecular mechanisms. Human CML microarray studies have shown that Alox5 is differentially expressed in CD34+ CML cells 41,42, suggesting a role of Alox5 in human CML stem cells. An in vitro study also supports the role of Alox5 in CML, and in this study the treatment of CML blast cells in culture with 5-LO inhibitors reduced cell proliferation 43, although genetic approach is required to rule out any off-target effects. Our study shows complete eradication of myeloid leukemia in mice by removing and inhibiting 5-LO, prompting us to test this novel therapeutic strategy in human CML patients in the future. Alox5 function has been linked to many important signaling pathways such as p53 26, NF-kB 31, and PI3K 31. PI3K is regulated by Pten, which plays a critical role in AML stem cells in mice 16. Thus, inhibition of Alox5 function may hold a promise for treating other types of malignant diseases. In addition, the impairment of LSCs in CML development by inhibiting Alox5 function also suggests that Alox5 may activate unknown pathways, and investigation of this Alox5 network is critical to understanding the mechanisms by which LSCs survive, self-renew, and differentiate. Besides LSCs in CML, specific signaling networks are likely found in other cancer stem cells, relative to their normal stem cell counterparts.
Methods
Mice
C57BL/6J–CD45.1, C57BL/6J–CD45.2, and homozygous Alox5 knockout (Alox5−/−) mice in C57BL/6 background were obtained from The Jackson Laboratory. Mice were maintained in a temperature- and humidity-controlled environment and given unrestricted access to 6% chow diet and acidified water. Alox5−/− mice have normal hematopoietic cell counts in bone marrow and peripheral blood 27.
Flow cytometry and identification of leukemia and normal hematopoietic cell lineages
Hematopoietic cells were collected from bone marrow and peripheral blood of the normal and diseased mice, and red blood cells were lysed with NH4Cl red blood cell lysis buffer (pH 7.4). The cells were washed with PBS, and stained with B220-PE for B cells, Gr-1-APC for neutrophils, Mac-1-PE for macrophage, CD3E–APC for T cells, and Sca-1-PE-cy7/c-Kit-APC-cy7/CD34-APC/CD135 (Flt3)-PE for hematopoietic stem cells. After staining, the cells were washed once with PBS and subjected to FACS analysis.
Bone marrow transduction/transplantation
The retroviral vector MSCV-IRES-eGFP carrying the p210 BCR-ABL cDNA and retroviral transduction and transplantation of mouse bone marrow cells for induction of CML and ALL by BCR-ABL had been described previously 40,44.
Measurement of plasma LTB4
Plasma of BCR-ABL induced CML mice were collected on day 20 post induction of the disease, and stored at the −80 °C before use. An enzyme immunoassay was carried out to measure the LTB4 levels in plasma using an ELISA kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instruction. Briefly, 50 ul of each standard or plasma sample was added to each well of 96-well plates, followed by adding 50 ul of LTB4 AchE trancer to each well. Then, 50 ul of LTB4 EIA antiserum was added to each well, and incubated the plates at 4 °C overnight. Next day, the plates were read and the results were analyzed.
Western blot analysis and antibodies
Antibodies against c-ABL, β-actin, and 5−LO were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Protein lysates were prepared by lysing cells in RIPA buffer, and Western blotting was carried out as described previously 45.
Real-time PCR
Total mRNA was isolated from HSCs or LSCs isolated by the FACS sorting. cDNA was synthesized using the Ovation-Pico cDNA synthesis method. Real-time PCR (RT-PCR) was performed to detect expression of β-catenin, GATA-1 and FOG-1 with the primers shown in the Supplementary Table 1.
Drug treatment
Zileuton (Critical Therapeutics. Inc) and Imatinib (Novartis) were dissolved in water. The drugs were given orally in a volume of less than 0.3 ml by gavage (300 mg/kg, twice a day for Zileuton, and 100 mg/kg, twice a day for Imatinib) beginning at 8 days after bone marrow transplantation, and continuing until the morbidity or death of the leukemia mice. Water was used as placebo.
Supplementary Material
Acknowledgements
This work was supported by the grants from the Leukemia & Lymphoma Society and the National Institute of Health (R01-CA122142, R01-CA114199) to S.L. S.L. is a Scholar of the Leukemia & Lymphoma Society. uthor contributions
Footnotes
Accession number
DNA microarray GSE10912
REFERENCES
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 3.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 7.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 10.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 13.Park IK, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 14.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe SW, Sherr CJ. Tumor suppression by Ink4a–Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz OH, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 18.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 19.Bowie MB, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dierks C, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Huntly BJ, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Neering SJ, et al. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood. 2007;110:2578–2585. doi: 10.1182/blood-2007-02-073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y. Targeting multiple kinase pathways in leukemic prognitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham SM, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 25.Marley SB, Deininger MW, Davidson RJ, Goldman JM, Gordon MY. The tyrosine kinase inhibitor STI571, like interferon-alpha, preferentially reduces the capacity for amplification of granulocyte-macrophage progenitors from patients with chronic myeloid leukemia. Exp Hematol. 2000;28:551–557. doi: 10.1016/s0301-472x(00)00142-9. [DOI] [PubMed] [Google Scholar]
- 26.Catalano A, Rodilossi S, Caprari P, Coppola V, Procopio A. 5-Lipoxygenase regulates senescence-like growth arrest by promoting ROS-dependent p53 activation. Embo J. 2005;24:170–179. doi: 10.1038/sj.emboj.7600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 28.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Soberman RJ, Christmas P. The organization and consequences of eicosanoid signaling. J Clin Invest. 2003;111:1107–1113. doi: 10.1172/JCI18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor PM, et al. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene. 2002;21:5765–5772. doi: 10.1038/sj.onc.1205702. [DOI] [PubMed] [Google Scholar]
- 31.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 32.Yokomizo T, Izumi T, Shimizu T. Leukotriene B4: metabolism and signal transduction. Arch Biochem Biophys. 2001;385:231–241. doi: 10.1006/abbi.2000.2168. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Ilaria RL, Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210,, p230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J. Exp. Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knapp HR. Reduced allergen-induced nasal congestion and leukotriene synthesis with an orally active 5-lipoxygenase inhibitor. N Engl J Med. 1990;323:1745–1748. doi: 10.1056/NEJM199012203232506. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira R, Ohneda K, Yamamoto M, Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. 2005;25:1215–1227. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang AP, et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 38.Stenke L, Lauren L, Reizenstein P, Lindgren JA. Leukotriene production by fresh human bone marrow cells: evidence of altered lipoxygenase activity in chronic myelocytic leukemia. Exp Hematol. 1987;15:203–207. [PubMed] [Google Scholar]
- 39.Tornhamre S, et al. Inverse relationship between myeloid maturation and leukotriene C4 synthase expression in normal and leukemic myelopoiesis-consistent overexpression of the enzyme in myeloid cells from patients with chronic myeloid leukemia. Exp Hematol. 2003;31:122–130. doi: 10.1016/s0301-472x(02)01026-3. [DOI] [PubMed] [Google Scholar]
- 40.Peng C, et al. Inhibition of heat shock protein 90 prolongs survival of mice with BCR-ABL-T315I–induced leukemia and suppresses leukemic stem cells. Blood. 2007;110:678–685. doi: 10.1182/blood-2006-10-054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham SM, Vass JK, Holyoake TL, Graham GJ. Transcriptional analysis of quiescent and proliferating CD34+ human hemopoietic cells from normal and chronic myeloid leukemia sources. Stem Cells. 2007;25:3111–3120. doi: 10.1634/stemcells.2007-0250. [DOI] [PubMed] [Google Scholar]
- 42.Radich JP, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson KM, et al. Selective inhibitors of 5-lipoxygenase reduce CML blast cell proliferation and induce limited differentiation and apoptosis. Leuk Res. 1995;19:789–801. doi: 10.1016/0145-2126(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet. 2004;36:453–461. doi: 10.1038/ng1343. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Couvillon AD, Brasher BB, Van Etten RA. Tyrosine phosphorylation of Grb2 by Bcr/Abl and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling. EMBO J. 2001;20:6793–6804. doi: 10.1093/emboj/20.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.