Abstract
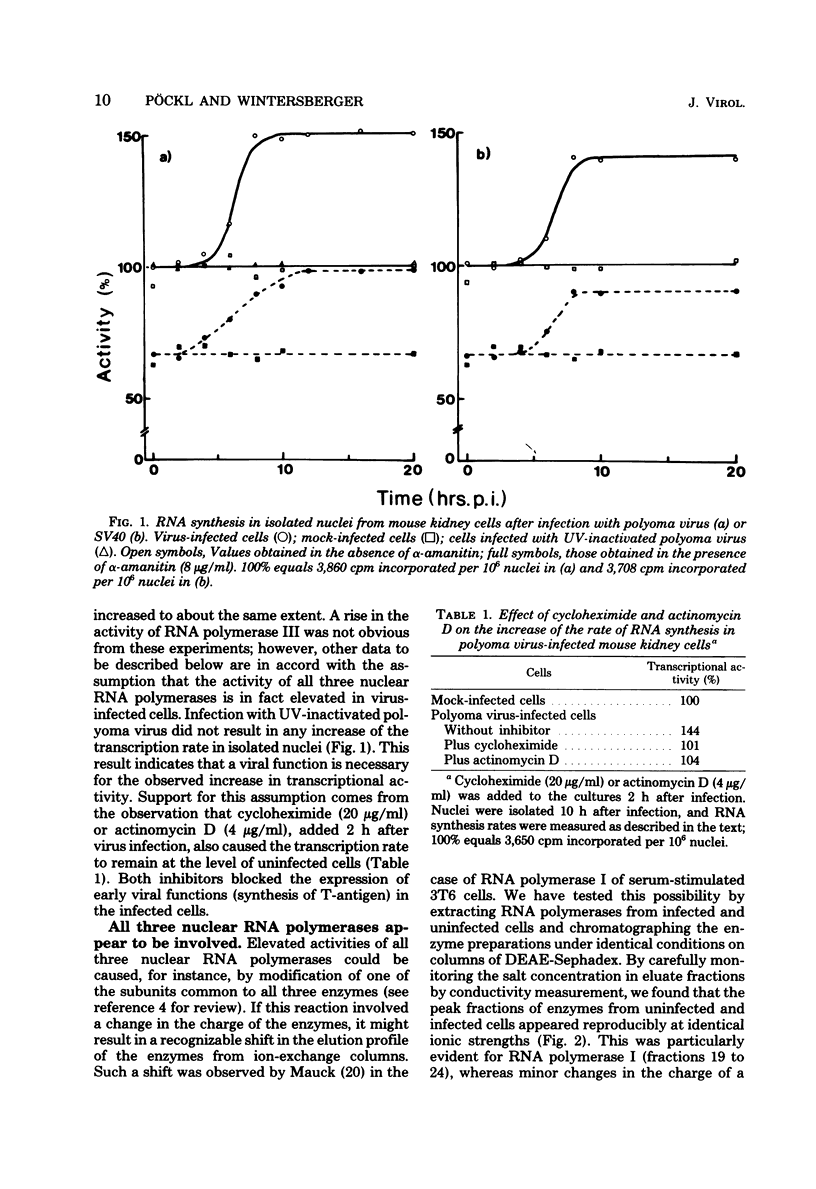
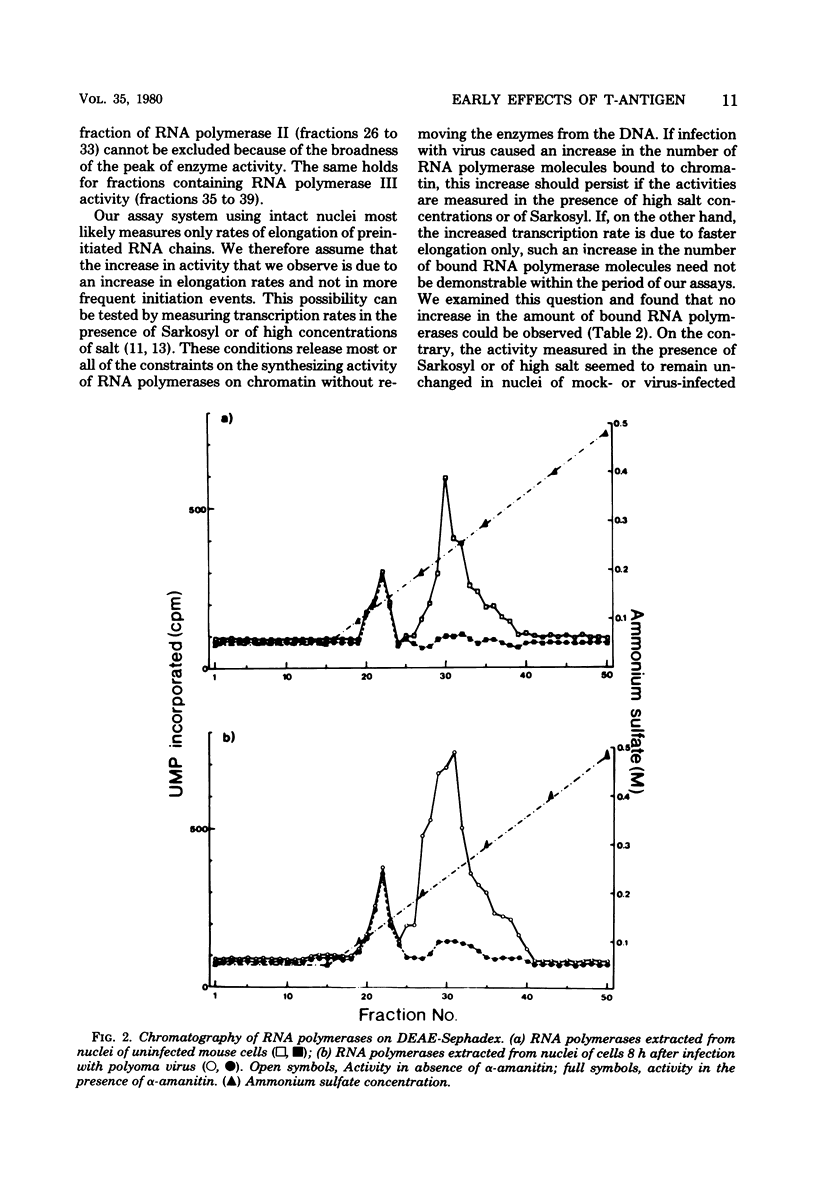
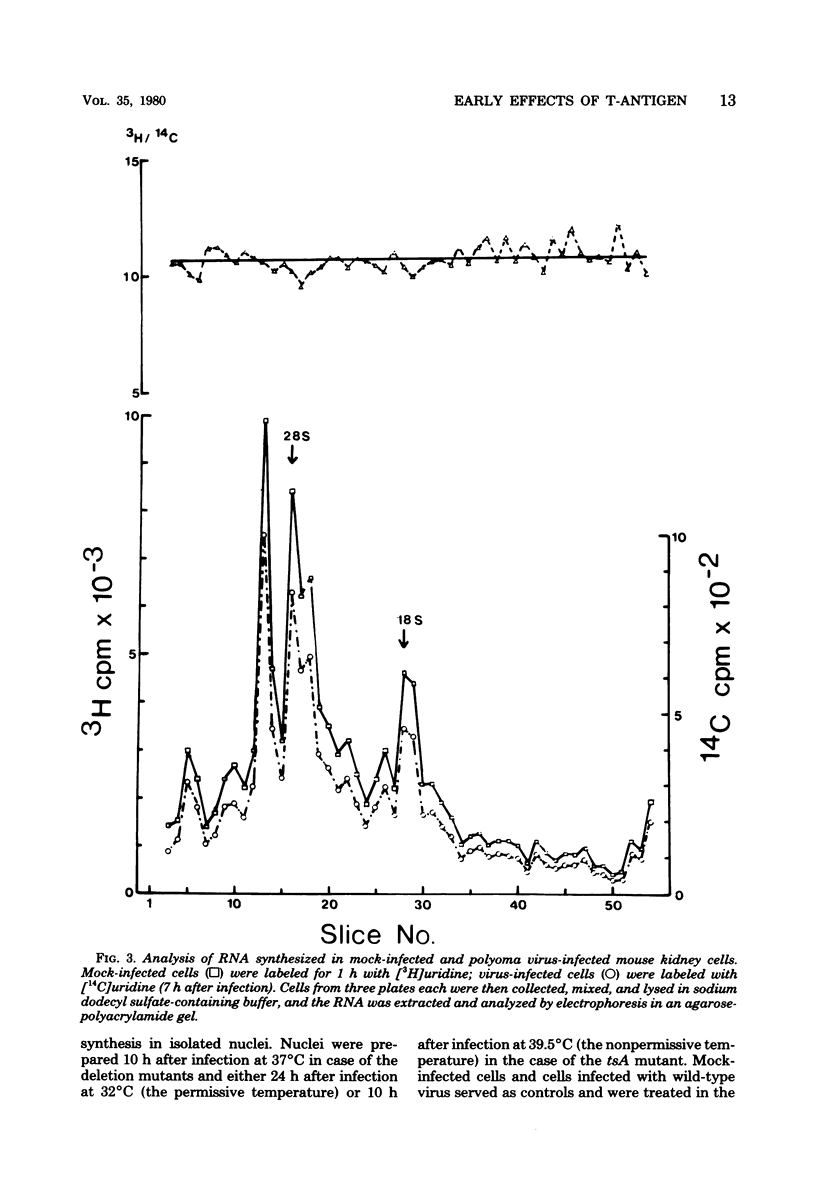
The rate of transcription in isolated nuclei of primary mouse kidney cells increases by a factor of about 1.5 between 5 and 8 h after infection with polyoma virus or simian virus 40. This process requires intact virus and is inhibited by cycloheximide and actinomycin D. It appears to involve the activity of all three nuclear RNA polymerases, and evidence was obtained for an increase in the rate of synthesis of most, if not all, species of RNA that were already produced in resting cells before infection. The additional synthesis of a few new RNA species not made in uninfected cells, however, cannot be excluded. The increase in the rate of transcription seems to precede an increase in the rate of translation in infected cells. Our additional finding that large and small tumor antigens (T-antigens) are synthesized at the same time after infection suggests that these cellular reactions are early consequences of the action of one or both of these T-antigens in the infected cell. Experiments with simian virus 40 mutants provided strong evidence for an involvement of large-T-antigen but not of small t-antigen. These studies furthermore indicate that the increase in the transcription rate is a prerequisite for the induction of DNA replication in simian virus 40-infected mouse kidney cells.
Full text
PDF


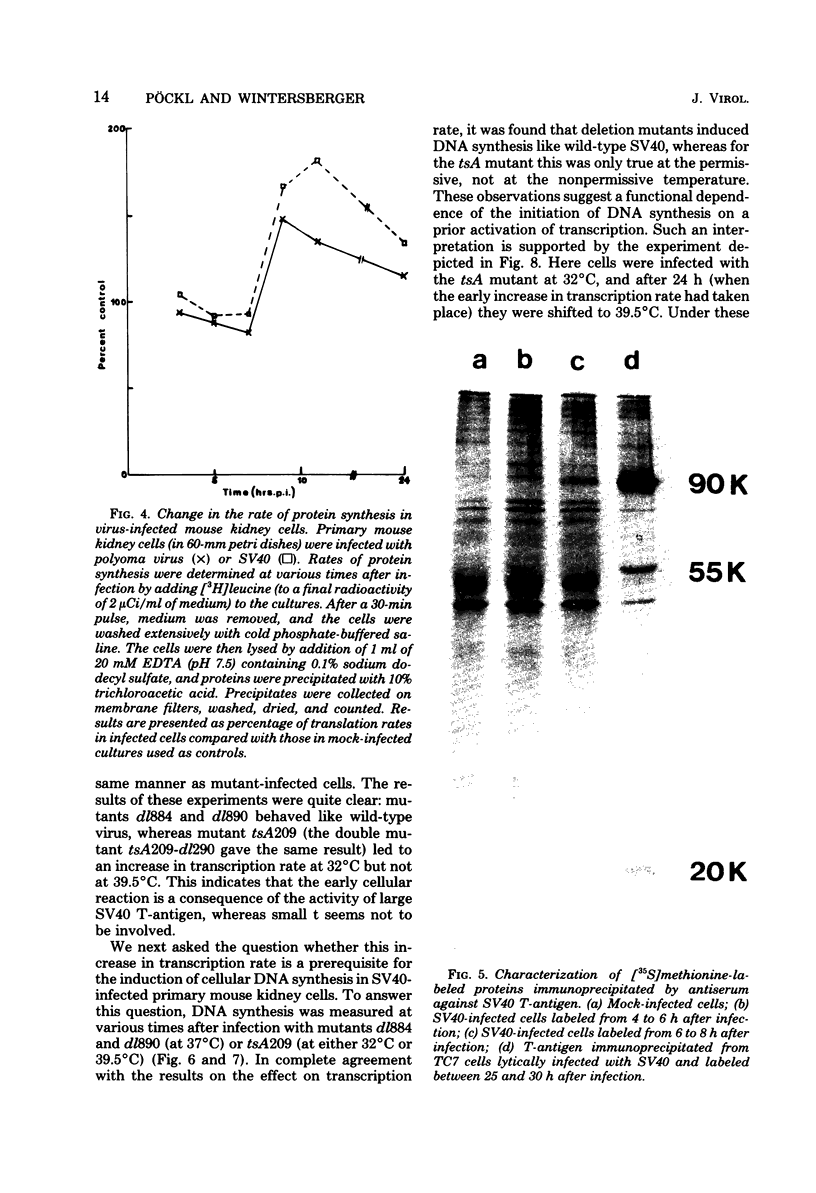
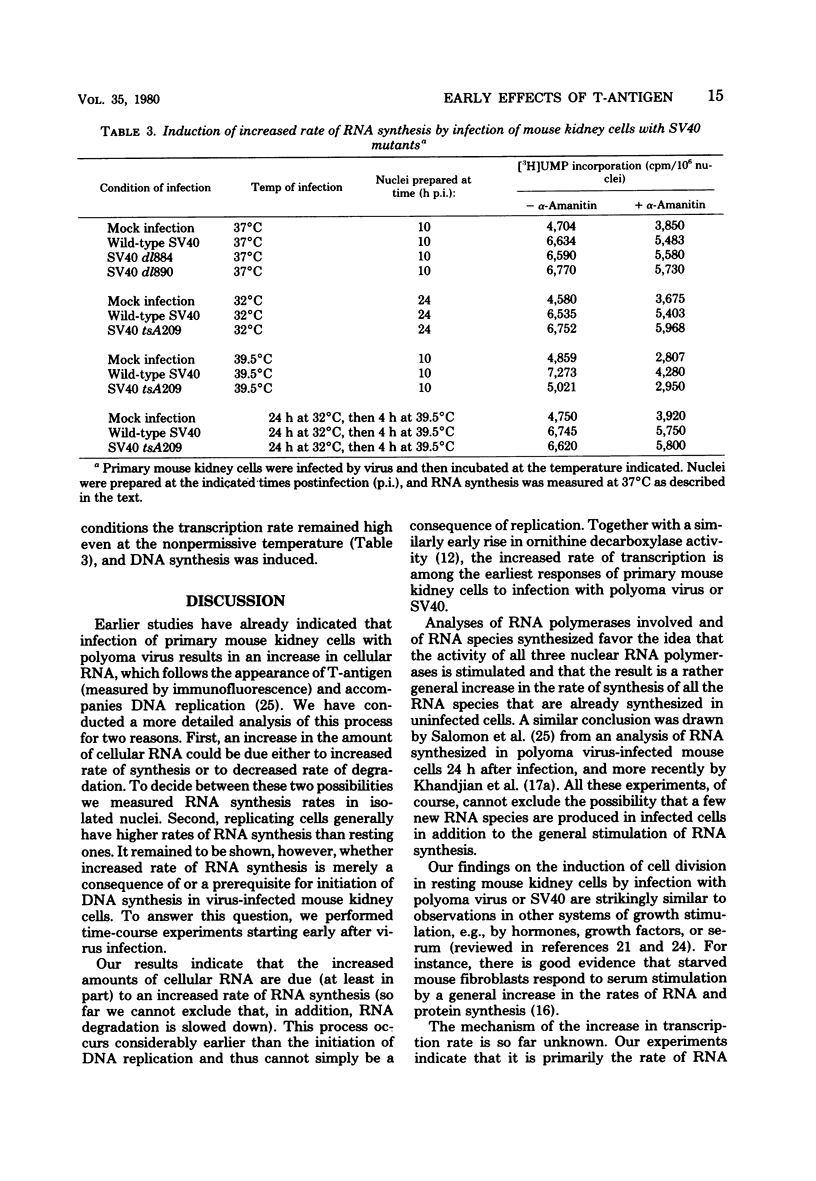
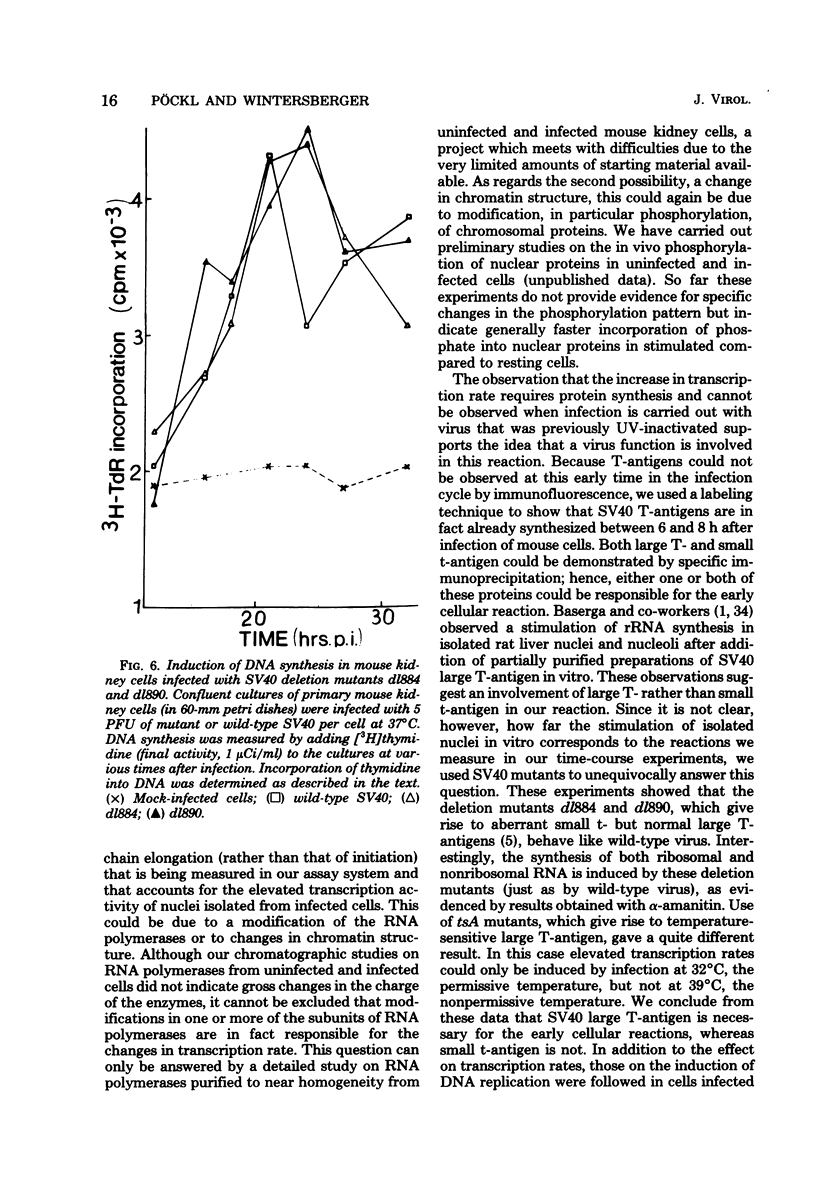
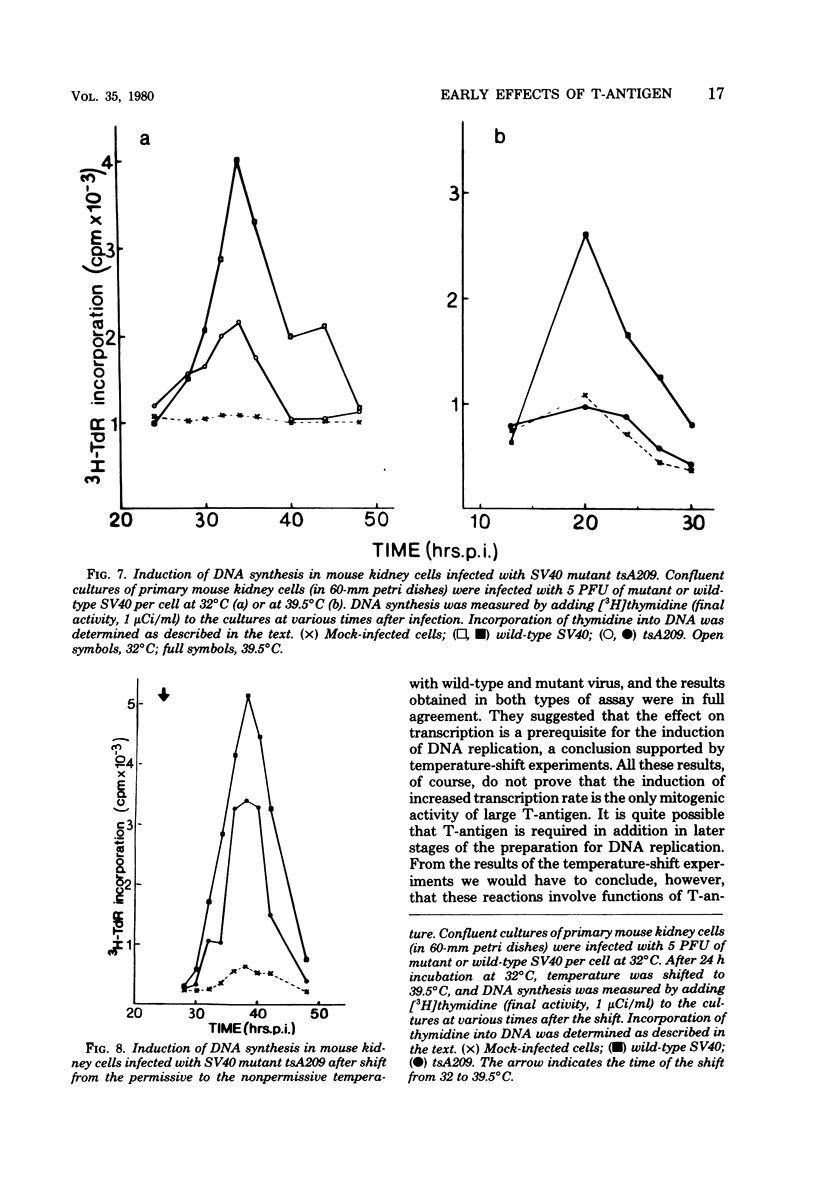


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baserga R., Ide T., Whelly S. Stimulation of ribosomal RNA synthesis in isolated nuclei and nucleoli by partially purified preparations of SV40 T antigen. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):685–691. doi: 10.1101/sqb.1978.042.01.070. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Strominger J. L. Viral and cellular DNA synthesis in nuclei from human lymphocytes transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2413–2417. doi: 10.1073/pnas.72.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Brugge J. S., Noonan C. A. Transformation of primate and rodent cells by temperature-sensitive mutants of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):25–36. doi: 10.1101/sqb.1974.039.01.006. [DOI] [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Trkula D., Kit S. T antigen and initiation of cell DNA synthesis in a temperature-sensitive mouse line transformed by an SV40tsA mutant and in heterokaryons of the transformed cells and chick erythrocytes. Somatic Cell Genet. 1978 Jan;4(1):95–110. doi: 10.1007/BF01546495. [DOI] [PubMed] [Google Scholar]
- FREARSON P. M., KIT S., DUBBS D. R. DEOXYTHYMIDYLATE SYNTHETASE AND DEOXYTHYMIDINE KINASE ACTIVITIES OF VIRUS-INFECTED ANIMAL CELLS. Cancer Res. 1965 Jun;25:737–744. [PubMed] [Google Scholar]
- Frearson P. M., Kit S., Dubbs D. R. Induction of dihydrofolate reductase activity by SV40 and polyoma virus. Cancer Res. 1966 Aug;26(8):1653–1660. [PubMed] [Google Scholar]
- Gariglio P., Buss J., Green M. H. Sarkosyl activation of RNA polymerase activity in mitotic mouse cells. FEBS Lett. 1974 Aug 30;44(3):330–333. doi: 10.1016/0014-5793(74)81170-1. [DOI] [PubMed] [Google Scholar]
- Goldstein D. A., Heby O., Marton L. J. Biphasic stimulation of polyamine biosynthesis in primary mouse kidney cells by infection with polyoma virus:uncoupling from DNA and rRNA synthesis. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4022–4026. doi: 10.1073/pnas.73.11.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Vogt M., Dulbecco R. Induction of cellular DNA synthesis by polyoma virus. II. Increase in the rate of enzyme synthesis after infection with polyoma virus in mouse kidney cells. Virology. 1965 Nov;27(3):262–272. doi: 10.1016/0042-6822(65)90105-4. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. I. In permissive cells. J Virol. 1979 May;30(2):590–599. doi: 10.1128/jvi.30.2.590-599.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Matter J. M., Léonard N., Weil R. Simian virus 40 and polyoma virus stimulate overall cellular RNA and protein synthesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1476–1480. doi: 10.1073/pnas.77.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kára J., Weil R. Specific activation of the DNA-synthesizing apparatus in contact-inhibited mouse kidney cells by polyoma virus. Proc Natl Acad Sci U S A. 1967 Jan;57(1):63–70. doi: 10.1073/pnas.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y., Avila J., Saral R. The semiautonomous replicon: a molecular model for the oncogenicity of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):17–24. doi: 10.1101/sqb.1974.039.01.005. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Oppenheim A. Initiation points for DNA replication in nontransformed and simian virus 40-transformed Chinese hamster lung cells. Cell. 1977 Aug;11(4):859–869. doi: 10.1016/0092-8674(77)90297-5. [DOI] [PubMed] [Google Scholar]
- Mauck J. C. Solubilized DNA-dependent RNA polymerase activities in resting and growing fibroblast. Biochemistry. 1977 Feb 22;16(4):793–796. doi: 10.1021/bi00623a035. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Ponta H., Ponta U., Wintersberger E. DNA-dependent RNA polymerases from yeast. Partial characterization of three nuclear enzyme activities. FEBS Lett. 1971 Nov 1;18(2):204–208. doi: 10.1016/0014-5793(71)80445-3. [DOI] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S., Jimenez de Asua L. Action of growth factors in the cell cycle. Biochim Biophys Acta. 1979 Feb 4;560(1):91–133. doi: 10.1016/0304-419x(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Salomon C., Türler H., Weil R. Polyoma-induced stimulation of cellular RNA synthesis is paralleled by changed expression of the viral genome. Nucleic Acids Res. 1977;4(5):1483–1503. doi: 10.1093/nar/4.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow V. P., Persico-Dilauro M., Edwards C. A., Martin R. G. The isolation of SV40 tsA/deletion, double mutants and the induction of host DNA synthesis. Virology. 1980 Feb;101(1):250–260. doi: 10.1016/0042-6822(80)90500-0. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Weil R., Michel M. R., Ruschmann G. K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R., Salomon E., May E., May P. A simplifying concept in tumor virology: virus-specific "pleiotropic effectors". Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):381–395. doi: 10.1101/sqb.1974.039.01.050. [DOI] [PubMed] [Google Scholar]
- Weil R. Viral 'tumor antigens': A novel type of mammalian regulator protein. Biochim Biophys Acta. 1978 Nov 17;516(3):301–388. doi: 10.1016/0304-419x(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Whelly S., Ide T., Baserga R. Stimulation of RNA synthesis in isolated nucleoli by preparations of simian virus 40 T antigen. Virology. 1978 Jul 1;88(1):82–91. doi: 10.1016/0042-6822(78)90112-5. [DOI] [PubMed] [Google Scholar]
- Wintersberger E., Wintersberger U. Induction of DNA polymerase in polyoma virus-infected mouse cells requires transcription and translation. J Virol. 1976 Aug;19(2):291–295. doi: 10.1128/jvi.19.2.291-295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. DNA polymerases in polyoma virus-infected mouse kidney cells. J Virol. 1975 Nov;16(5):1095–1100. doi: 10.1128/jvi.16.5.1095-1100.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]