Abstract
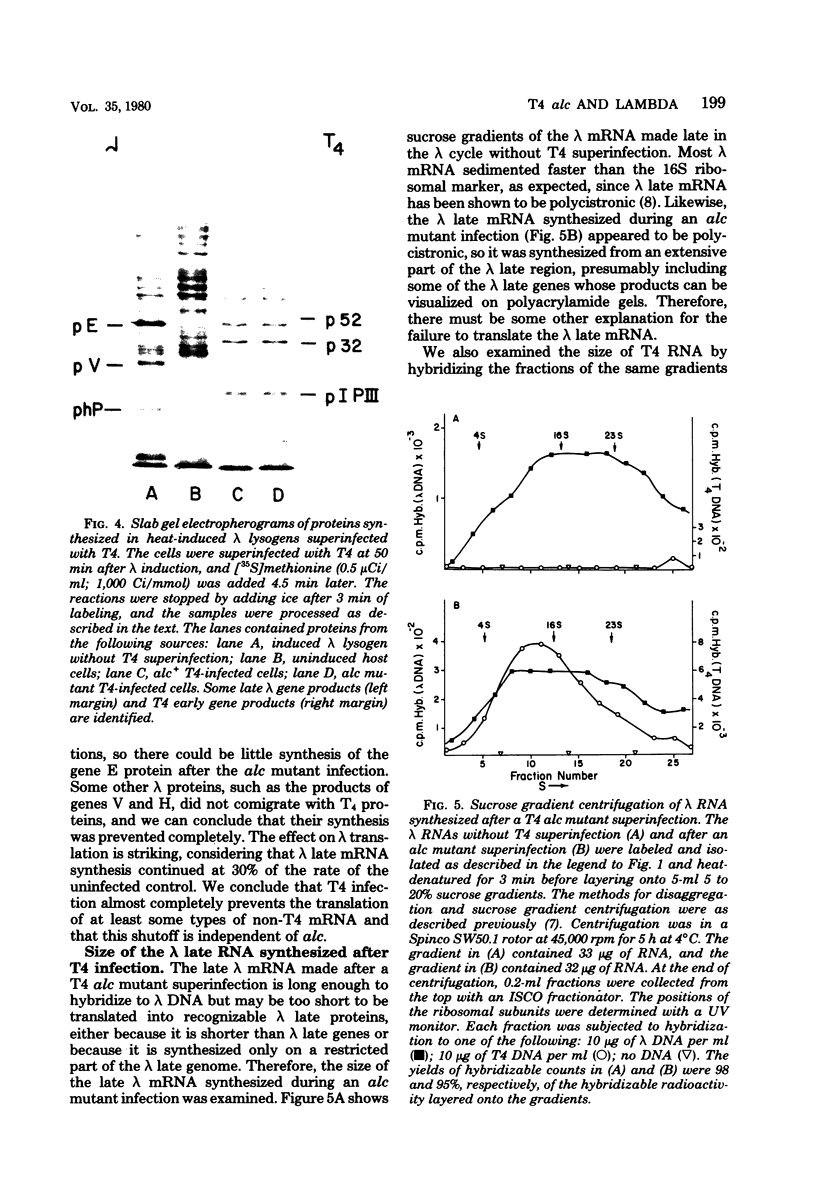
Bacteriophage T4 normally contains 5-hydroxymethylcytosine instead of cytosine in its DNA. Multiple mutants of T4 which synthesize DNA with cytosine do not transcribe their late genes due to the action of the T4 alc gene (Snyder et al., Proc. Natl. Acad. Sci. U.S.A. 73:3098--3102, 1976), which is also responsible for unfolding the host nucleoid after T4 infection (Sirotkin et al., Nature [London] 265:28--32, 1977; Tigges et al., J. Virol. 24:775--785, 1977). It seems reasonable that T4 alc function plays a role in shutting off host transcription, and the observation that some of the RNA made after infection with a T4 alc mutant hybridizes to Escherichia coli DNA (Sirotkin et al., Nature [London] 265:28--32, 1977; Tigges et al., J. Virol. 24:775--785, 1977) supports this hypothesis. Although it is likely that the roles of the alc function in the blocking of some types of transcription and in the unfolding of the host nucleoid are related, it is not known how these effects are achieved or, in fact, whether all types of transcription are affected equally by the alc function. In an attempt to answer these questions, we studied the effect of T4 alc function on bacteriophage lambda transcription and on the structure of intracellular lambda DNA. We found that the alc function is responsible for the shutoff of lambda late transcription but probably not for the shutoff of lambda early transcription. We also found that alc does not block lambda transcription by directly removing the supercoils from circular lambda DNA via either a nicking or topoisomerase activity. Furthermore, we conclude that T4 infection also prevents the translation of non-T4 mRNA because late lambda mRNA's were made after superinfection by a T4 alcs mutant and were of normal length but were not translated into lambda late proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair D. G., Clewell D. B., Sheratt D. J., Helinski D. R. Strand-specific supercoiled DNA-protein relaxation complexes: comparison of the complexes of bacterial plasmids ColE1 and ColE2. Proc Natl Acad Sci U S A. 1971 Jan;68(1):210–214. doi: 10.1073/pnas.68.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Bruner R., Souther A., Suggs S. Stability of cytosine-containing deoxyribonucleic acid after infection by certain T4 rII-D deletion mutants. J Virol. 1972 Jul;10(1):88–92. doi: 10.1128/jvi.10.1.88-92.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F. Host mutant (tabD)-induced inhibition of bacteriophage T4 late transcription. II. Genetic characterization of mutants. J Mol Biol. 1975 Aug 25;96(4):601–624. doi: 10.1016/0022-2836(75)90141-2. [DOI] [PubMed] [Google Scholar]
- Dove W. F. Action of the lambda chromosome. I. Control of functions late in bacteriophage development. J Mol Biol. 1966 Aug;19(1):187–201. doi: 10.1016/s0022-2836(66)80060-8. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H. The metabolism of T4 phage ghost-infected cells. I. Macromolecular synthesis and ransport of nucleic acid and protein precursors. Virology. 1970 Mar;40(3):673–684. doi: 10.1016/0042-6822(70)90212-6. [DOI] [PubMed] [Google Scholar]
- Frederick R. J., Snyder L. [Regulation of anti-late RNA synthesis in bacteriophage T4: a delayed early control]. J Mol Biol. 1977 Aug 25;114(4):461–476. doi: 10.1016/0022-2836(77)90172-3. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Green M. H. Characterization of polycistronic late lambda messenger RNA. Virology. 1973 Jun;53(2):392–404. doi: 10.1016/0042-6822(73)90219-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. Regulation of the expression of the N gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1973 Feb;70(2):421–424. doi: 10.1073/pnas.70.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Green M. H. Inhibition of Escherichia coli and bacteriophage lambda messenger RNA synthesis by T4. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1675–1678. doi: 10.1073/pnas.54.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants in a nonessential gene of bacteriophage T4 which are defective in the degradation of Escherichia coli deoxyribonucleic acid. J Virol. 1971 Jan;7(1):95–105. doi: 10.1128/jvi.7.1.95-105.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. II. Induction of host messenger ribonucleic acid and its exclusion from polysomes. J Virol. 1970 Aug;6(2):208–217. doi: 10.1128/jvi.6.2.208-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Kutter E., Beug A., Sluss R., Jensen L., Bradley D. The production of undegraded cytosine-containing DNA by bacteriophage T4 in the absence of dCTPase and endonucleases II and IV, and its effects on T4-directed protein synthesis. J Mol Biol. 1975 Dec 25;99(4):591–607. doi: 10.1016/s0022-2836(75)80174-4. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Bovre K., Szybalski W. Controls of rightward transcription in coliphage lambda. J Mol Biol. 1970 Dec 28;54(3):599–604. doi: 10.1016/0022-2836(70)90130-0. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Sakakibara Y., Tomizawa J. I. Regulation of transcription of the lambda bacteriophage genome. Virology. 1969 Dec;39(4):901–918. doi: 10.1016/0042-6822(69)90026-9. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Ratner D. Letter to the editor: Bacteriophage T4 transcriptional control gene 55 codes for a protein bound to Escherichia coli RNA polymerase. J Mol Biol. 1974 Nov 15;89(4):803–807. doi: 10.1016/0022-2836(74)90054-0. [DOI] [PubMed] [Google Scholar]
- Ratner D. The interaction bacterial and phage proteins with immobilized Escherichia coli RNA polymerase. J Mol Biol. 1974 Sep 15;88(2):373–383. doi: 10.1016/0022-2836(74)90488-4. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Uncoupling of late transcription from DNA replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):103–119. doi: 10.1016/0022-2836(70)90448-1. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Transcription termination and late control in phage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3300–3304. doi: 10.1073/pnas.72.9.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P., Howard-Flanders P. Initiation of recA+-dependent recombination in Escherichia coli (lambda). I. Undamaged covalent circular lambda DNA molecules in uvrA+ recA+ lysogenic host cells are cut following superinfection with psoralen-damaged lambda phages. J Mol Biol. 1977 Nov 25;117(1):137–158. doi: 10.1016/0022-2836(77)90028-6. [DOI] [PubMed] [Google Scholar]
- Runnels J., Snyder L. Isolation of a bacterial host selective for bacteriophage T4 containing cytosine in its DNA. J Virol. 1978 Sep;27(3):815–818. doi: 10.1128/jvi.27.3.815-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. D., Vetter D. Control of T4 endonuclease IV by the D2a region of bacteriophage T4. Virology. 1973 Aug;54(2):544–546. doi: 10.1016/0042-6822(73)90165-7. [DOI] [PubMed] [Google Scholar]
- Sirotkin K., Cooley W., Runnels J., Snyder L. R. A role in true-late gene expression for the T4 bacteriophage 5' polynucleotide kinase 3' phosphatase. J Mol Biol. 1978 Aug 5;123(2):221–233. doi: 10.1016/0022-2836(78)90322-4. [DOI] [PubMed] [Google Scholar]
- Sirotkin K., Wei J., Snyder L. T4 Bacteriophage-coded RNA polymerase subunit blocks host transcription and unfolds the host chromosome. Nature. 1977 Jan 6;265(5589):28–32. doi: 10.1038/265028a0. [DOI] [PubMed] [Google Scholar]
- Skalka A., Butler B., Echols H. Genetic control of transcription during development of phage gamma. Proc Natl Acad Sci U S A. 1967 Aug;58(2):576–583. doi: 10.1073/pnas.58.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Tigges M. A., Parson K. A., Bursch C. J., Caron F. M., Koerner J. F., Tutas D. J. Identification and preliminary characterization of a mutant defective in the bacteriophage T4-induced unfolding of the Escherichia coli nucleoid. J Virol. 1976 Feb;17(2):622–641. doi: 10.1128/jvi.17.2.622-641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L. R., Montgomery D. L. Inhibition of T4 growth by an RNA polymerase mutation of Escherichia coli: physiological and genetic analysis of the effects during phage development. Virology. 1974 Nov;62(1):184–196. doi: 10.1016/0042-6822(74)90314-6. [DOI] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Karlström O. H. Bacteriophage T4-induced shut-off of host-specific translation. J Virol. 1976 Feb;17(2):326–334. doi: 10.1128/jvi.17.2.326-334.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges M. A., Bursch C. J., Snustad D. P. Slow switchover from host RNA synthesis to bacteriophage RNA synthesis after infection of Escherichia coli with a T4 mutant defective in the bacteriophage T4-induced unfolding of the host nucleoid. J Virol. 1977 Dec;24(3):775–785. doi: 10.1128/jvi.24.3.775-785.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutas D. J., Wehner J. M., Koerner J. F. Unfolding of the host genome after infection of Escherichia coli with bacteriophage T4. J Virol. 1974 Feb;13(2):548–550. doi: 10.1128/jvi.13.2.548-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ghosh S., Echols H., Spiegelman W. G. Repression by the cI protein of phage lambda: in vitro inhibition of RNA synthesis. J Mol Biol. 1972 Jun 28;67(3):407–421. doi: 10.1016/0022-2836(72)90459-7. [DOI] [PubMed] [Google Scholar]
- Young E. T., 2nd, Sinsheimer R. L. Vegetative lambda DNA. 3. Pulse-labeled components. J Mol Biol. 1968 Apr 14;33(1):49–59. doi: 10.1016/0022-2836(68)90280-5. [DOI] [PubMed] [Google Scholar]



