Abstract
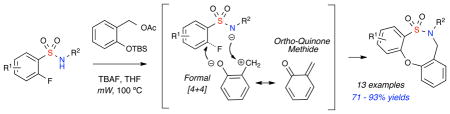
A formal, one-pot, [4+4] cyclization pathway for the generation of 8-member sultams via in-situ generation of an ortho-quinone methide (o-QM) is reported. The pairing of ambiphilic synthons in a complementary fashion is examined whereby o-fluorobenzenesulfonamides are merged with in-situ-generated o-QM in a formal [4+4] cyclization pathway to afford 5,2,1-dibenzooxathiazocine-2,2-dioxide scaffolds under microwave (mW) conditions. The method reported represents the first use of an o-QM in a formal hetero [4+4] cyclization.
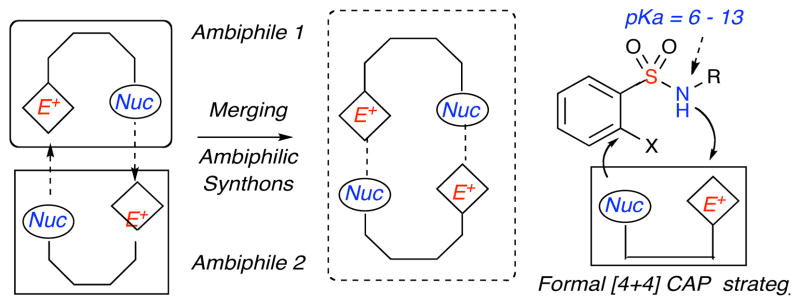
The development of chemical methodologies allowing ready access to novel heterocyclic scaffolds in a minimal number of steps is a key facet of drug discovery. Single pot, multi-component reaction (MCR) strategies in particular have gained high value in this regard.1 The great majority of these reactions involve the combination of reactive partners possessing single nucleophile-electrophile interactions, followed by subsequent merging in domino, cascade or tandem pathways to produce heterocyclic moieties. In comparison, synthons containing both electrophilic and nucleophilic sites (ambiphilic synthons)2,3,4 have great potential in developing new reaction pathways with high step economy,5 but remain underdeveloped. Interest in the development of chemical methodologies for the production of diverse sultam libraries has led to the exploration of complementary ambiphile pairing (CAP) pathways as part of a general strategy for the production of benzofused sultams in a facile manner (Figure 1).
Figure 1.
Pairing Strategies for Benzofused Sultam Synthesis.
At the heart of the titled method is the complementary pairing of two ambiphilic synthons, o-fluoro benzenesulfonamides and ortho-quinone methide (o-QM) in a formal one-pot [4+4] approach to afford the novel 5,2,1-dibenzooxathiazocine-2,2-dioxide ring system. This route augments a recently reported [4+3] epoxide cascade strategy developed in our laboratory and others using the ambiphilic character of o-fluorobenzenesulfonamides for the synthesis of benzofused sultams.6
Quinone methides are reactive intermediates that have been known for more than fifty years7 and are extensively utilized in biological processes.8 In addition to their use in nature, they also have great synthetic potential. In particular, o-QMs are highly versatile intermediates as they can serve as Michael acceptors or dienes in cycloaddition reactions. A number of natural products as well as biologically active heterocycles have been accessed utilizing in situ generated o-QMs.9 The over whelming majority of cases involve the utilization of o-QMs as dienes in hetero Diels-Alder reactions. Recently, elegant use of acyl-anion Michael additions into o-QMs has been reported for the generation of α-aryl ketones. 10 However, in spite of numerous reports of aza-Michael reactions of o-QMs in biological systems, the exploitation of these pathways in the synthesis of heterocycles is limited to reports of the dimerization of orthoquinone methides (o-QMs).11 In contrast, the utilization of o-QMs as ambiphiles in hetero [4+4] cyclizations has not been reported in the literature.
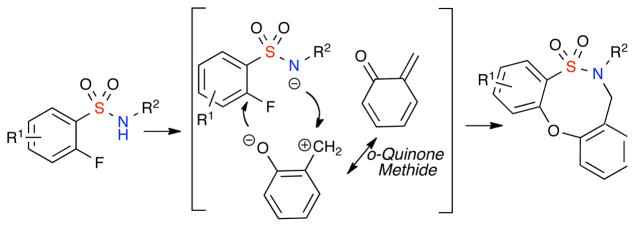
The aforementioned dibenzooxathiazocine ring system represents a new subclass within sultams that have not been reported to-date. Sultams are a class of non-natural heterocycles that have gained prominence in recent years due to their activity against a wide spectrum of biological targets.12,13 Their acyclic precursors, sulfonamides, are highly versatile synthons due to the tunability of the SO2NH pKa.14 In this regard, o-fluorobenzene sulfonamides are particularly attractive due to the highly electron withdrawing nature of the SO2 functionality, in conjunction with the o-fluoro substituent, which impart enhanced electrophilicity at the ortho-carbon as well as attenuated acidity/nucleophilicity of the sulfonamide NH (Scheme 1). The ability of o-fluorobenzenesulfonamides to undergo facile nucleophilic aromatic substitution (SNAr) reactions6,15 at the ortho-carbon, allow for their potential pairing with o-QMs in CAP strategies for production of benzofused sultams. This pairing entails an aza-Michael addition at the exo methylene o-QM carbon and subsequent interception of the nucleophilic phenoxy by o-fluoro benzenesulfonamides via an SNAr reaction in a formal [4+4] cyclization pathway (Scheme 1).
Scheme 1.
Pairing o-fluorobenzenesulfonamides and o-QM’s in a formal [4+4] reaction.
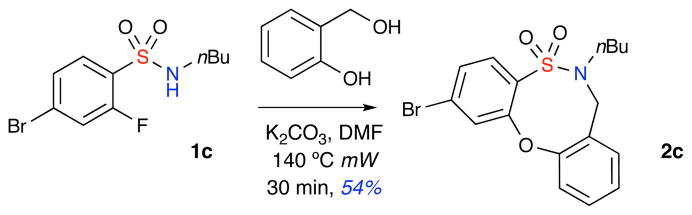
Investigations commenced with the production of an array of 2° o-fluorobenzenesulfonamides under modified Schotten-Baumann conditions. o-QMs can be formed in situ from 2-hydroxybenzyl alcohol derivatives under basic conditions.7 Accordingly, a mixture of 2-fluorobenzenesulfonamide 1, was mixed with 2-hydroxy benzyl alcohol in the presence of anhydrous K2CO3 (3.0 equiv.) in DMF and was subjected to microwave irradiation (mW) at 140 °C for 30 minutes (Scheme 2). The sulfonamide starting material was completely consumed to afford a compound that was highly visible on TLC under UV irradiation. Characterization of the product revealed the novel tricyclic sultam 2 containing the dibenzooxathiazocine ring system. However, the overall yield of the reaction was modest possibly due to inefficient generation of the o-QM intermediate as well as potential dimerzation/trimerization of the o-QM.16
Scheme 2.
Formal [4+4]-heterocyclization reaction utilizing o-QM under mW irradiation.
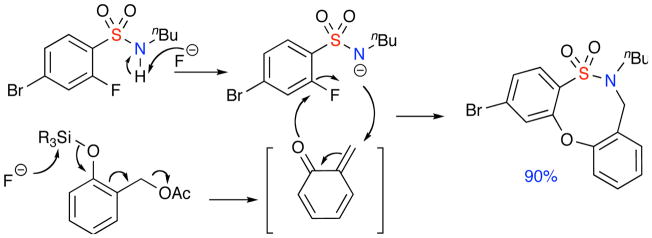
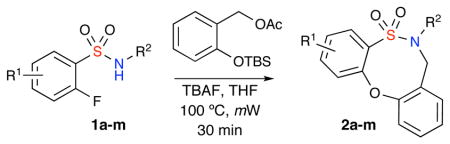
Investigations were then focused on alternate methods of o-QM generation. Interestingly, exposure of 1 to the o-acetoxy benzyl acetate of 2-hydroxy benzyl alcohol afforded the desired product in increased yields. Attention was then turned to the conditions reported by Rokita and co-workers.7a Accordingly, microwave irradiation of the sulfonamide in the presence of TBAF and o-silyloxy benzyl acetate in THF at 100 °C for 30 minutes furnished the desired product in 90% yield (Table 1, Entry 3). In this latter result, the fluoride anion serves to desilylate the silyl-protected phenol functionality thus generating the phenoxy anion, which further continues on the cascade pathway to produce the pivotal o-QM intermediate. In addition, it also serves as the base to produce the sulfonylamide intermediate required for the desired reaction to take place (Scheme 3).
Table 1.
Substrate scope of o-QM mediated [4+4] reaction
 | ||||
|---|---|---|---|---|
| entry | pdt | R1 | R2 | yield (%)a |
| 1 | 2a | H | 2-OMe Bn | 71 |
| 2 | 2b | H | 2,3-Cl CH2Ph | 73 |
| 3 | 2c | 4-Br | nBu | 90 |
| 4 | 2d | 4-Br | iBu | 91 |
| 5 | 2e | 4-Br | PMB | 82 |
| 6 | 2f | 4-Br | cyclopropyl | 81 |
| 7 | 2g | 4-Br | propargyl | 94 |
| 8 | 2h | 5-Cl | Allyl | 94 |
| 9 | 2i | 5-Cl | PMB | 71 |
| 10 | 2j | 5-Cl | 3-F Bn | 93 |
| 11 | 2k | 5-Cl | 2-OMe Bn | 87 |
| 12 | 2-l | 4-Br | (iPr)CHCO2Me | 93 |
| 13 | 2m | 4-Br | (iBu)CHCO2Me | 77 |
isolated yield after column chromatography
Scheme 3.
Mechanism for formal [4+4] CAP utilizing o-silyloxybenzyl acetate
The substrate scope was next investigated. A variety of sulfonamides were subjected to the above conditions and were found to proceed smoothly to afford the desired sultam in excellent yields (Table 1). The optimized reaction protocol was found to tolerate a range of substituents on the N atom including alkyl, propargyl and benzyl functionalities. Pleasingly, the reaction protocol was found to tolerate Cl and Br atoms on the aromatic ring. Halide substitution was important, as this would allow for functionalization of these scaffolds for library production via transition metal-catalyzed coupling reactions.
To the best of our knowledge, the ring system produced has not been reported in the literature. The structure of these unique heterocycles was confirmed via X-ray crystallography (Figure 2). The method reported herein represents the first instance of the use of an o-QM in a formal hetero [4+4] cyclization pathway to generate a heterocycle.
Figure 2.
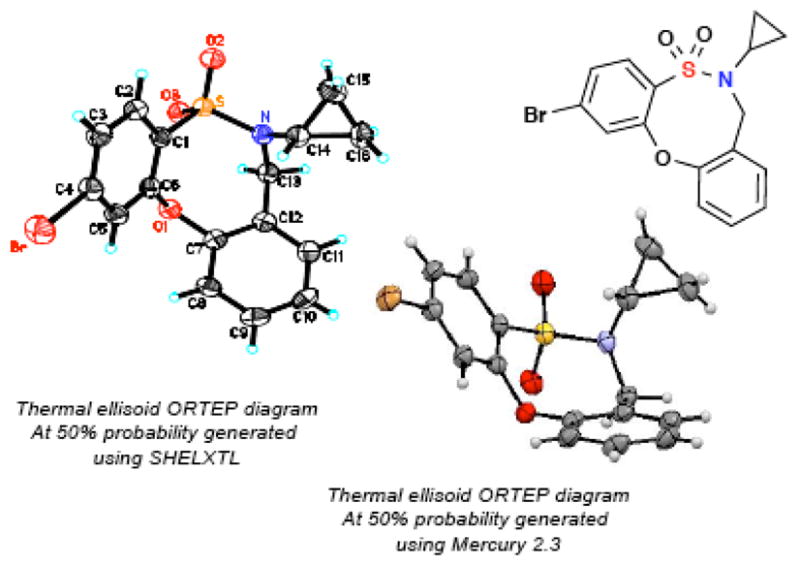
ORTEP diagram of 2-f.
In order to expand the method, application of the o-QM-mediated [4+4] CAP strategy was investigated with amino ester-derived o-fluorobenzene sulfonamides. Treatment of both leucine- and valine-derived substrates, 2-l and 2m, under above conditions gratifyingly afforded the desired product in excellent yields (Table 1, Entries 12–13). These results enhance the scope of the method and allow for further elaboration of the scaffolds in library efforts. X-ray data also confirmed the source of the unique upfield 1H-NMR shifts of the MeO-group in entries 12 and 13 which were found to be at 2.28 and 2.40 ppm, respectively, most likely due to shielding from the aromatic ring system in these conformationally rigid systems (Figure 3).
Figure 3.
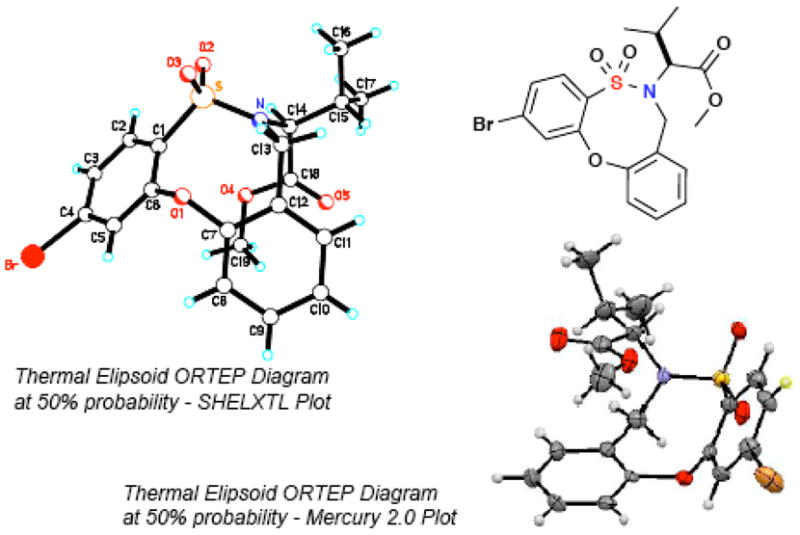
ORTEP diagram of 2-f.
In the last two entries (12–13), a 6:1 and 3.7:1 mixture of inseparable products is seen in the 1H NMR spectra, which have been tentatively assigned as N-C14 rotamers. Evidence of this lies in the fact that the 1H, 13C, 1H/1H COSY, 1H/13C HSQC, and DEPT all showed the existence of a set of peaks that was almost identical to that of the major product albeit with a change in chemical shift values, and matching the expected structure of the product. The 1H NMR shifts for the MeO-group in 2-l-minor and 2m-minor are 3.52 and 3.72 ppm, respectively (compared with 2.28 and 2.40 in the major isomers, respectively, as stated above). In contrast, the 1H NMR shifts for the diastereotopic gem-dimethyl groups in the 2-l-minor rotamer are at 0.75 and 0.02 ppm due to shielding by the aromatic ring system (as opposed to 1.10 and 0.98 ppm in the 2-l-major rotamer).
In conclusion, a one-pot, complementary ambiphile-pairing (CAP) reaction has been developed to produce 1,2-dibenzoxathiazocine-4,4-dioxides in a formal [4+4] cyclization pathway under MW conditions. This report represents the first instance of the generation of 5,2,1-dibenzooxathiazocine-2,2-dioxide scaffolds and the first reported method utilizing in-situ generated o-QM in a formal hetero [4+4] cyclization process. This method is suitable to library production and diversity-oriented synthesis. Efforts along these lines and additional application of CAP strategies are currently in order and will be reported in due course.
Supplementary Material
Acknowledgments
This investigation was generously supported by funds provided by the the National Institute of General Medical Sciences [Pilot-Scale Libraries Program (P41 GM076302) and The University of Kansas Center for Chemical Methodologies and Library Development (KU-CMLD) (P50 GM069663)].
Footnotes
Supporting Information Available: Experimental details and spectral charactization for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Zhu J, Bienayme H. Multicomponent Reactions. Wiley–VCH Verlag GmbH & Co; KGaA, Weinheim: 2005. [Google Scholar]; (b) Toure BB, Hall DG. Chem Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]; (c) Sunderhaus JD, Martin SF. Chem Eur J. 2009;15:1300–1308. doi: 10.1002/chem.200802140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Bontemps S, Gornitzka H, Bouhadir G, Miqueu K, Bourissou D. Angew Chem Int Ed. 2006;45:1611–1614. doi: 10.1002/anie.200503649. [DOI] [PubMed] [Google Scholar]; (b) Bontemps S, Bouhadir G, Miqueu K, Bourissou D. J Am Chem Soc. 2006;128:12056–12057. doi: 10.1021/ja0637494. [DOI] [PubMed] [Google Scholar]; (c) Bontemps S, Bouhadir G, Dyer PW, Miqueu K, Bourissou D. Inorg Chem. 2007;46:5149–5151. doi: 10.1021/ic7006556. [DOI] [PubMed] [Google Scholar]
- 3.Molecules containing both electrophilic and nucleophilic nodes have also been also defined as amphiphilic molecules, see: Nakamura H, Shim JG, Yamamoto Y. J Am Chem Soc. 1997;119:8113–8114.Nakamura H, Aoyagi K, Shim JG, Yamamoto Y. J Am Chem Soc. 2001;123:372–377.Kimura M, Tamaki T, Nakata M, Tohyama K, Tamaru Y. Angew Chem Int Ed. 2008;120:5887–5889. doi: 10.1002/anie.200801252.
- 4.Molecules containing both electrophilic and nucleophilic nodes have been also defined as amphoteric molecules by Yudin, see: Yudin A, Hili R. J Am Chem Soc. 2009;131:16404–16406. doi: 10.1021/ja9072194.Yudin A, Hili R. Angew Chem Int Ed. 2008;120:4188–4191. doi: 10.1002/anie.200705776.
- 5.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 6.(a) Rolfe A, Samarakoon TB, Hanson PR. Org Lett. 2010;12:1216–1219. doi: 10.1021/ol100035e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cleator E, Baxter CA, O’Hagan M, O’Riordan TJC, Sheen FJ, Stewart GW. Tetrahedron Lett. 2010:1079–1082. [Google Scholar]
- 7.For a comprehensive review on o-QM see: Rokita SE. Wiley Series of Reactive Intermediates in Chemistry and Biology. Vol. 1. John Wiley & Sons, Inc; Hoboken, NJ: 2009. Quinone Methides.Van De Water RW, Pettus TRR.Tetrahedron 2002585367–5404.19079773Pettus TRR, Selenski C. Sci Synth. 2006;28:831–872.
- 8.Wolkenberg SE, Boger DL. Chem Rev. 2002;102:2477–2495. doi: 10.1021/cr010046q. [DOI] [PubMed] [Google Scholar]
- 9.(a) Bender CF, Yoshimoto FK, Paradise CL, De Brabander JK. J Am Chem Soc. 2009;131:11350–11352. doi: 10.1021/ja905387r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lumb JP, Choong KC, Trauner D. J Am Chem Soc. 2008;130:9230–9231. doi: 10.1021/ja803498r. [DOI] [PubMed] [Google Scholar]; (d) Selenski C, Pettus TRR. J Org Chem. 2004;69:9196–9203. doi: 10.1021/jo048703c. [DOI] [PubMed] [Google Scholar]
- 10.Mattson AE, Scheidt KA. J Am Chem Soc. 2007;129:4508–4509. doi: 10.1021/ja068189n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Pisova N, Soucek M. Collect Czech Chem Commun. 1982;47:838–842. [Google Scholar]; (b) Wang P, Wang Y, Hu W, Liang X. Eur J Org Chem. 2009;13:2055–2058. [Google Scholar]; (c) Rosenau T, Bömdorfer S. Wiley Series of Reactive Intermediates in Chemistry and Biology. Vol. 1. John Wiley & Sons, Inc; Hoboken, NJ: 2009. Quinone Methides; p. 180. [Google Scholar]
- 12.(a) Drews J. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]; (b) Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Curr Med Chem. 2003;10:925–953. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Hopkins M, Hanson PR. Org Lett. 2008;10:2223–2226. doi: 10.1021/ol800649n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Rayabarappu DR, Zhou A, Jeon K, Samarakoon T, Rolfe A, Siddiqui H. Tetrahedron. 2009;65:3180–3188. doi: 10.1016/j.tet.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhou A, Hanson PR. Org Lett. 2009;11:531–534. doi: 10.1021/ol802467f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penso M, Albanese D, Landini D, Lupi V, Tagliabue A. J Org Chem. 2008;73:6686–6690. doi: 10.1021/jo800930g. [DOI] [PubMed] [Google Scholar]
- 16.Heating at 110 °C in DMF for 12 hours under reflux resulted in poor conversion and low product yields.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.