Abstract
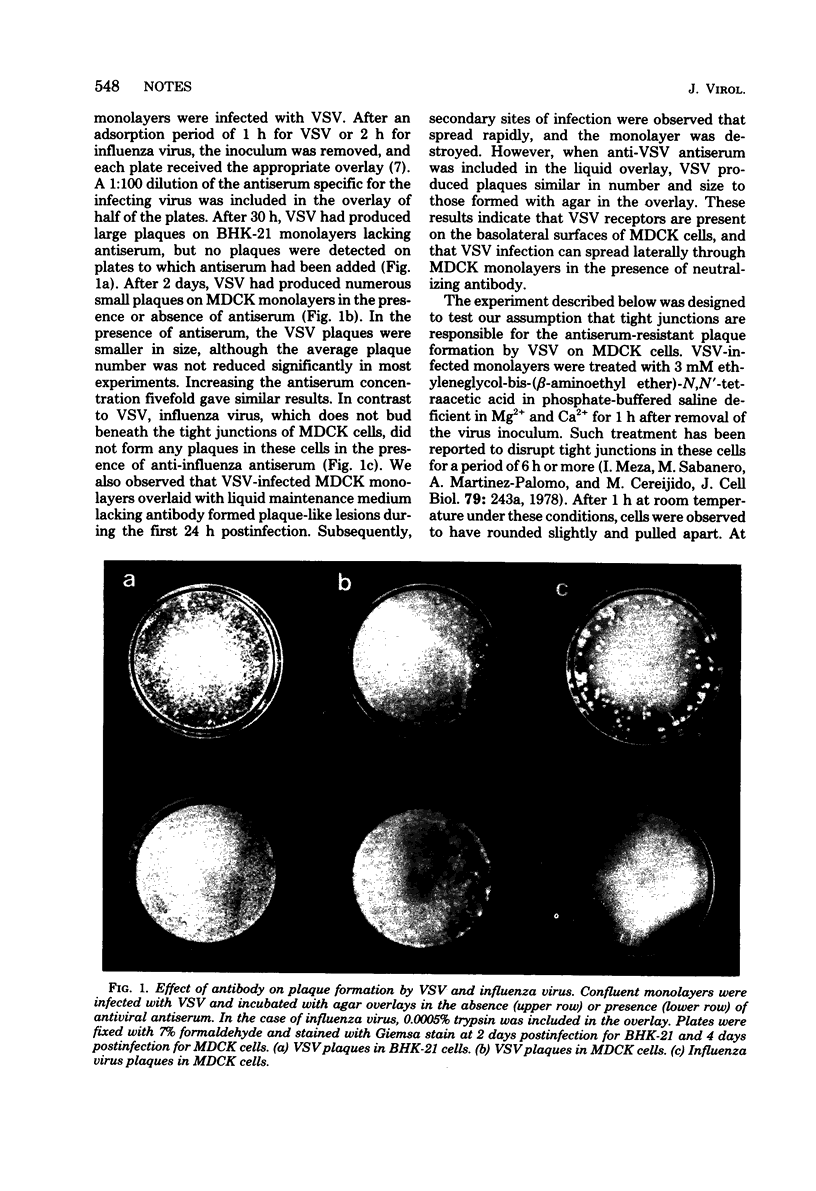
In MDCK cells, vesicular stomatitis virus (VSV) buds exclusively from the basolateral plasma membranes beneath tight junctions, whereas influenza virus forms only at the free apical surface. Anti-VSV antiserum did not prevent the formation of plaques on MDCK cell monolayers infected with VSV, whereas plaque formation in BHK-21 cells was completely inhibited by such antiserum. Under similar conditions, homologous antiserum completely prevented plaque formation by influenza virus on MDCK cells. In several other epithelioid cell lines, VSV also formed plaques in the presence of specific antiserum. These results suggest that VSV receptors are present on basolateral membranes in the cells studied and that junctional complexes present between cells may exclude antibody from intercellular spaces and thus permit the lateral spread of virus infection in the presence of neutralizing antibody.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J., Estes L. W., Mansukhani S., Brada Z. A cell line derived from normal dog kidney (MDCK) exhibiting qualities of papillary adenocarcinoma and of renal tubular epithelium. Cancer. 1970 Nov;26(5):1022–1028. doi: 10.1002/1097-0142(197011)26:5<1022::aid-cncr2820260509>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Inducers of mammalian cell differentiation stimulate dome formation in a differentiated kidney epithelial cell line (MDCK). Proc Natl Acad Sci U S A. 1979 Mar;76(3):1323–1327. doi: 10.1073/pnas.76.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler M. J., Chuman L. M., Shaffer L., Saier M. H., Jr Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J Cell Biol. 1979 Jun;81(3):635–648. doi: 10.1083/jcb.81.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Fitzpatrick J. P., Compans R. W. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]