Abstract
Bovine adrenal zona fasciculata (AZF) cells express Cav3.2 T-type Ca2+ channels that function pivotally in adrenocorticotropic hormone (ACTH)-stimulated cortisol secretion. The regulation of Cav3.2 expression in AZF cells by ACTH, cAMP analogs, and their metabolites was studied using Northern blot and patch clamp recording. Exposing AZF cells to ACTH for 3–6 days markedly enhanced the expression of Cav3.2 current. The increase in Cav3.2 current was preceded by an increase in corresponding CACNA1H mRNA. O-Nitrophenyl,sulfenyl-adrenocorticotropin, which produces a minimal increase in cAMP, also enhanced Cav3.2 current. cAMP analogs, including 8-bromoadenosine cAMP (600 μm) and 6-benzoyladenosine cAMP (300 μm) induced CACNA1H mRNA, but not Cav3.2 current. In contrast, 8-(4-chlorophenylthio) (8CPT)-cAMP (10–50 μm) enhanced CACNA1H mRNA and Cav3.2 current, whereas nonhydrolyzable Sp-8CPT-cAMP failed to increase either Cav3.2 current or mRNA. Metabolites of 8CPT-cAMP, including 8CPT-adenosine and 8CPT-adenine, increased Cav3.2 current and mRNA with a potency and effectiveness similar to the parent compound. The Epac activator 8CPT-2′-O-methyl-cAMP and its metabolites 8CPT-2′-OMe-5′-AMP and 8CPT-2′-O-methyl-adenosine increased CACNA1H mRNA and Cav3.2 current; Sp-8CPT-2′-O-methyl-cAMP increased neither Cav3.2 current nor mRNA. These results reveal an interesting dichotomy between ACTH and cAMP with regard to regulation of CACNA1H mRNA and Ca2+ current. Specifically, ACTH induces expression of CACNA1H mRNA and Cav3.2 current in AZF cells by mechanisms that depend at most only partly on cAMP. In contrast, cAMP enhances expression of CACNA1H mRNA but not the corresponding Ca2+ current. Surprisingly, chlorophenylthio-cAMP analogs stimulate the expression of Cav3.2 current indirectly through metabolites. ACTH and the metabolites may induce Cav3.2 expression by the same, unidentified mechanism.
Keywords: Calcium Channels, Cyclic AMP (cAMP), Cyclic Nucleotide Analogs, Ion Channels, mRNA, ACTH, Adrenal
Introduction
Bovine adrenal zona fasciculata (AZF)2 cells express low voltage-activated T-type Ca2+ channels and bTREK-1 K+ channels that function pivotally in ACTH-stimulated cortisol secretion (1–4). bTREK-1 K+ channels set the resting potential of these AZF cells. ACTH receptor activation is coupled to bTREK-1 inhibition, membrane depolarization, T-type Ca2+ channel activation, and cortisol secretion (2, 3, 5).
ACTH-stimulated cortisol production includes both rapid and delayed components (6, 7). The rapid effects of ACTH on cortisol secretion occur within minutes and do not require the synthesis of new proteins. In contrast, delayed increases occur only after a period of hours and involve transcriptional activation of genes coding for steroid hydroxylases and other steroidogenic proteins (6–8).
Parallel rapid and delayed responses to ACTH occur in the regulation of ion channel function and gene expression in AZF cells. The rapid actions of ACTH leading to depolarization-dependent Ca2+ entry occur in seconds or minutes and are mediated by the inhibition of pre-existing bTREK-1 channels (3, 9). Treating AZF cells with ACTH for prolonged periods also regulates bTREK-1 gene expression (10).
Three types of low voltage-activated T-type Ca2+ channels have been identified with molecular cloning and patch clamp techniques (11). One of these, Cav3.2, is coded for by the CACNA1H gene and is distinctive in its sensitivity to Ni2+ (12). The T-type Ca2+ channel in bovine AZF cells is blocked by Ni2+ with an IC50 of 20 μm, characteristic of Cav3.2 (1). In whole cell patch clamp recordings, ACTH has no rapid effect on the activity of pre-existing Cav3.2 channels (supplemental Fig. S1). It is not known whether ACTH exerts long term control over CACNA1H gene expression in these cells.
The signaling mechanisms by which ACTH produces rapid and delayed effects in the adrenal cortex are complex. Early studies identified cAMP as the principal intracellular messenger for ACTH in AZF cells (13–16). Accordingly, bovine AZF cells express a high affinity MC2R melanocortin receptor coupled to adenylate cyclase through Gs (17, 18). Until recently, nearly all of the actions of cAMP in eukaryotic cells were believed to be mediated through the activation of PKA. However, two cAMP-activated guanine nucleotide exchange factors (Epac1 and Epac2 also known as cAMP-GEFI and cAMP-GEFII) that activate Rap1 and Rap2 GTPases have been identified (19, 20). Epac proteins regulate a variety of cellular processes by mechanisms that, in some cases, include regulation of gene expression (21–23). Although Epac1 is widely expressed, Epac2 is robustly expressed mainly in selected areas of the brain and the adrenal glands of rats and humans (19, 20).
Elucidating the signaling pathways by which ACTH and cAMP function in the adrenal cortex has been facilitated by the discovery of cAMP derivatives that, at appropriate concentrations, selectively activate Epacs or PKA (24–26). These derivatives have been used to independently study the role of these cAMP-activated proteins in the regulation of cell function. In this regard, we previously found that cAMP inhibits the activity of bTREK-1 K+ channels by a mechanism that is independent of PKA but mimicked by Epac-specific cAMP analogs (ESCAs) (27, 28).
Recently, we discovered that the most widely used ESCA 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8CPT-2′-OMe-cAMP), after a delay of many hours, stimulated large increases in the expression of steroid hydroxylase mRNAs and cortisol synthesis by a cAMP-, PKA-, and Epac-independent mechanism. These effects were mediated by one or more metabolites of the parent compound through an unknown signaling pathway (29).
In addition to its cAMP-dependent actions, ACTH produces effects in AZF cells by mechanisms that may be completely independent of cAMP. At concentrations that produce little or no measurable increase in cAMP synthesis, ACTH and its O-nitrophenyl,sulfenyl derivative NPS-ACTH stimulate large increases in corticosteroid secretion by rat and bovine cells (30, 31).
Currently, few reports exist describing the signaling mechanisms that regulate T-type Ca2+ channel expression in specific cells. Two patch clamp studies on rat adrenal chromaffin and AZF cells have provided seemingly contradictory results. In chromaffin cells, it was reported that exposing cells for 3–5 days to 8CPT-cAMP or the ESCA 8CPT-2′-OMe-cAMP enhanced the expression of a Ni2+-sensitive T-type Ca2+ current through activation of Epac proteins (32). In contrast, in rat AZF cells, it was reported that prolonged treatment with a different 8-substituted cAMP analog, the PKA and Epac activator 8-Br-cAMP, or forskolin failed to enhance the expression of the T-type Ca2+ current (33). Neither of these studies measured the effect of these specific cAMP analogs on CACNA1H mRNA.
In this study, we examined the regulation of CACNA1H mRNA and corresponding Cav3.2 Ca2+ currents in AZF cells by ACTH, cAMP analogs, and their metabolites using Northern blot and patch clamp techniques. It was discovered that ACTH and metabolites of chlorophenylthio-cAMP analogs induce the expression of CACNA1H mRNA and Ca2+ current. In contrast, other cAMP analogs enhanced the expression of CACNA1H mRNA but not the associated current.
EXPERIMENTAL PROCEDURES
DMEM/F12, antibiotics, and fetal bovine serum were obtained from Invitrogen. Phosphate-buffered saline, bovine plasma fibronectin, tocopherol, selenite, ascorbic acid, 8CPT-cAMP, adenosine, and ACTH (1–24) were obtained from Sigma. O-Nitrophenyl,sulfenyl-adrenocorticotropin (NPS-ACTH) was custom synthesized by Celtek Peptides (Nashville, TN). 8-Bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP; Biolog B007), N6-benzoyladenosine 3′,5′-cyclic monophosphate (6-Bnz-cAMP; Biolog B009), 8CPT-2′-OMe-cAMP (Biolog C041), hydrolysis-resistant 8-(4-chlorophenylthio)-2′-O-methyl-cAMP, Sp-isomer (Sp-8CPT-2′-OMe-cAMP; Biolog C052), 8-(4-chlorophenylthio)-2′-O-methyladenosine 5′-O-monophosphate (8CPT-2′-OMe-5′-AMP; Biolog C078), 8-(4-chlorophenylthio)-2′-O-methyladenosine (8CPT-2′-OMe-Ado; Biolog C070), 8-(4-chlorophenylthio)-cAMP (8CPT-cAMP; Biolog C010), hydrolysis-resistant 8-(4-chlorophenylthio)-cAMP (Sp-8CPT-cAMP; Biolog C012), 8-(4-chlorophenylthio)adenosine (8CPT-Ado; Biolog C086), and 8-(4-chlorophenylthio)adenine (8CPT-Ade; Biolog C069) were purchased from Axxora, LLC (San Diego, CA).
Isolation and Culture of AZF Cells
Bovine adrenal glands were obtained from steers (age, 2–3 years) at a local slaughterhouse. Isolated AZF cells were obtained and prepared as previously described (34). After isolation, the cells either were resuspended in DMEM/F12 (1:1) with 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin, 0.1 mg/ml streptomycin, and the antioxidants 1 μm tocopherol, 20 nm selenite and 100 μm ascorbic acid (DMEM/F12+) and plated for immediate use or were resuspended in fetal bovine serum, 5% Me2SO, divided into 1-ml aliquots, and stored in liquid nitrogen for future use. To ensure cell attachment, the dishes were treated with fibronectin (10 μg/ml) for 30 min at 37 °C and then rinsed with warm, sterile phosphate-buffered saline immediately before adding cells. The cells were maintained at 37 °C in a humidified atmosphere of 95% air, 5% CO2.
Northern Blot Hybridization and Measurement of mRNA
Total RNA isolation and Northern blot procedures have been described previously (10). Briefly, 5–7 × 106 AZF cells were plated on 60-mm fibronectin-treated dishes in DMEM/F12+. After 24 h, the media were replaced with either control media (DMEM/F12+) or the same media containing ACTH (1–24), 6-Bnz-cAMP, 8-Br-cAMP, 8CPT-cAMP, Sp-8CPT-cAMP, or other agents as required. At the end of the incubation period, total RNA was extracted using RNeasy columns (Qiagen), electrophoresed on a denaturing gel, and transferred to a nylon transfer membrane (GeneScreen Plus; PerkinElmer Life Sciences). The probes were generated by reverse transcription-PCR using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI), specific primers for bovine CACNA1H, and total RNA isolated from bovine AZF cells as described above. CACNA1H primers (GGGGAGAGCCTGGCGGGAAGA and GGGTCTCCTGAGGGCACTGTGGACT) were selected from GenBankTM accession number XM_581124.4 (Predicted: Bos taurus calcium channel, voltage-dependent, T-type, α1H subunit (CACNA1H)) mRNA. This ∼800-bp probe is specific for bovine CACNA1H mRNA and has low homology to either CACNA1G or CACNA1I. The CACNA1H probe was labeled with [α-32P]dCTP by random primer labeling (Prime-It II; Stratagene, La Jolla, CA). Northern autoradiograms were imaged using a Typhoon 9200 variable mode imager and quantitated using ImageQuant TL v2003.3 software (GE Healthcare). The mRNA values are presented as the means ± S.E. of at least three independent experiments normalized to densitometric values for the corresponding 18 S rRNA bands.
Ca2+ Current Recording
Patch clamp recordings of voltage-gated Ca2+ currents from bovine AZF cells were made in the whole cell configuration as previously described (1). The standard pipette solution was 120 mm CsCl, 1 mm CaCl2, 2 mm MgCl2, 11 mm 1,2,-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid, 10 mm HEPES, and 1 mm MgATP with pH titrated to 7.2 with CsOH. The external solution consisted of 117 mm tetra-ethylammonium chloride, 5 mm CsCl, 20 mm CaCl2, 2 mm MgCl2, 5 mm HEPES, and 5 mm glucose, with pH adjusted to 7.4 with tetraethylammonium hydroxide. All of the solutions were filtered through 0.22-μm cellulose acetate filters.
Recording Conditions and Electronics
AZF cells were used for patch clamp experiments 24–144 h after plating. Typically, cells with diameters of <15 μm and capacitances of 10–15 pF were selected. Coverslips upon which cells were plated (Bellco, Vineland NJ) were transferred from 35-mm culture dishes to a recording chamber (volume, 1.5 ml) that was continuously perfused by gravity at a rate of 3–5 ml/min. For whole cell recordings, patch electrodes with resistances of 1.0–2.0 MΩ were fabricated from Corning 0010 glass (World Precision Instruments, Sarasota, FL). These electrodes routinely yielded access resistances of 1.5–4.0 MΩ and voltage-clamp time constants of <100 μs. Ca2+ currents were recorded at room temperature (22–25 °C) according to the procedure of Hamill et al. (35) using a List EPC-7 patch clamp amplifier. Pulse generation and data acquisition were done using a personal computer and PCLAMP software with Digidata 1200 interface (Axon Instruments, Inc., Burlingame, CA). The currents were digitized at 2–10 KHz after filtering with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA). Linear leak and capacity currents were subtracted from current records using summed scaled hyperpolarizing steps of ¼ pulse amplitude. The data were analyzed using PCLAMP (CLAMPFIT 9.2) and SigmaPlot (version 11.0) software. For selected experiments (supplemental Fig. S1), ACTH (200 pm) was applied by bath perfusion, controlled manually by a six-way rotary valve.
RESULTS
ACTH Enhances Expression of Cav3.2 Ca2+ Current in Bovine AZF Cells
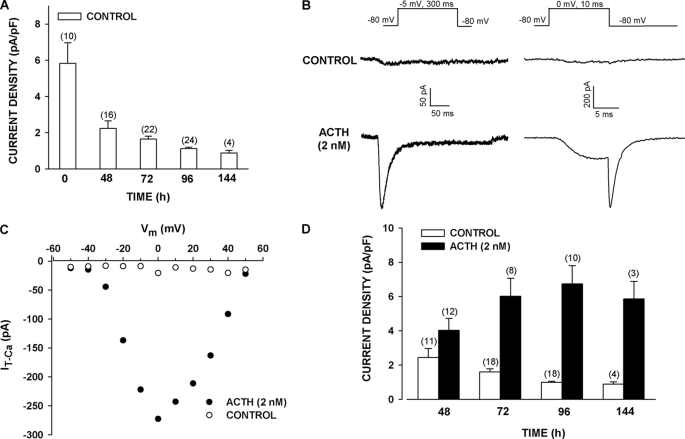
When bovine AZF cells were cultured in serum-supplemented media, the Cav3.2 current disappeared over a period of days, as determined from whole cell patch clamp recordings (Fig. 1A). This rapidly inactivating and slowly deactivating Ca2+ current was recorded in response to long (300 ms) or short (10 ms) voltage steps (Fig. 1B). After 96 h, Cav3.2 current density diminished from a control value of 5.83 ± 1.14 pA/pF (n = 10) to 1.12 ± 0.08 pA/pF (n = 24) (Fig. 1A). Prolonged exposure of AZF cells to ACTH (2 nm) reversed the time-dependent decrease in the Ca2+ current that occurred in the absence of the peptide. By 48 h, the Cav3.2 current began to increase, reaching a maximum by 96 h (Fig. 1, B–D). After 96 h, Cav3.2 current density was 6.74 ± 1.06 pA/pF (n = 10) in ACTH-treated cells, compared with 0.99 ± 0.06 pA/pF (n = 18) in the time-matched controls (Fig. 1D). The T-type Ca2+ current induced by ACTH was blocked by Ni2+ with a potency that identified it as Cav3.2 (supplemental Fig. S2).
FIGURE 1.
Long term effect of ACTH on expression of Cav3.2 T-type Ca2+ current in bovine AZF cells. AZF cells were cultured up to 144 h in media containing no further addition (control) (A–D) or 2 nm ACTH (B–D). Whole cell Ca2+ currents were activated by either long (300 ms) or short (10 ms) voltage steps applied at 30-s intervals from a holding potential of −80 mV. A, effect of time in culture on Cav3.2 T-type Ca2+ current. Summary of experiments in which AZF cells were cultured and T-type Ca2+ current was measured after the indicated times. The values are the means ± S.E. of the indicated number of determinations. B, representative Ca2+ current traces recorded from AZF cells that were cultured for 96 h either without (control) or with ACTH (2 nm). Ca2+ current traces were activated by long (left panel) and short (right panel) depolarizing steps. C, current-voltage relationship. Peak current amplitudes from cells incubated for 48 h either without (control) or with ACTH (2 nm) are plotted against test potential. D, summary of experiments in which AZF cells were either left untreated (control) or treated with ACTH (2 nm) for times from 48 to 144 h. The bars represent Cav3.2 T-type Ca2+ current density expressed as the means ± S.E. of the indicated number of determinations.
NPS-ACTH but Not 8-Br-cAMP Induces Expression of Cav3.2 Current in AZF Cells
cAMP has long been regarded as the principal intracellular messenger for ACTH. Experiments were done to determine whether ACTH-stimulated expression of Cav3.2 current was mediated by cAMP. 8-Br-cAMP activates PKA and Epac proteins at concentrations above 100 μm and stimulates rapid and delayed increases in cortisol secretion by bovine AZF cells (25, 26, 36). However, as illustrated in Fig. 2 (A and B), exposing cells to 8-Br-cAMP (300 or 600 μm) for times from 48 to 96 h failed to increase the expression of T-type Ca2+ current, compared with time-matched controls. In contrast, ACTH (2 nm) increased the expression of T-type Ca2+ current 2–3-fold (Fig. 2, A and B).
FIGURE 2.
Long term effect of 8-Br-cAMP and NPS-ACTH on expression of Cav3.2 T-type Ca2+ current in bovine AZF cells. AZF cells were cultured up to 96 h in media containing no further addition (control), ACTH (2 nm), 8-Br-cAMP (300 or 600 μm), or NPS-ACTH (50 or 100 nm). Whole cell Ca2+ currents were recorded after activation by either long (300 ms) or short (10 ms) voltage steps applied at 30-s intervals from a holding potential of −80 mV. A, representative Ca2+ current traces recorded from AZF cells that were cultured for 96 h with ACTH (2 nm), 8-Br-cAMP (600 μm), or NPS-ACTH (50 nm). Ca2+ current traces were activated by long (left) and short (right) depolarizing steps. B, summary of experiments in which AZF cells were either left untreated (control) or treated with ACTH (2 nm) or 8-Br-cAMP (300 or 600 μm) for 48–96 h. The bars represent Cav3.2 T-type Ca2+ current density expressed as the means ± S.E. of the indicated number of determinations. C, summary of experiments in which AZF cells were either left untreated (control) or treated with ACTH (2 nm) or NPS-ACTH (50 or 100 nm) for 48 or 72 h. The bars represent Cav3.2 T-type Ca2+ current density expressed as the means ± S.E. of the indicated number of determinations.
NPS-ACTH stimulates large increases in [Ca2+]i and corticosteroid secretion in both rat and bovine AZF cells at concentrations that produce little or no increase in cAMP synthesis (30, 31). Although less potent than ACTH, at maximally effective concentrations NPS-ACTH produces increases in cortisol secretion comparable with those produced by ACTH (30). We compared ACTH to NPS-ACTH with respect to their effectiveness at enhancing the expression of Cav3.2 current. At concentrations of 50 and 100 nm, NPS-ACTH increased Cav3.2 current density by an amount similar to that produced by ACTH (10 nm) at 48 and 72 h (Fig. 2, A and C).
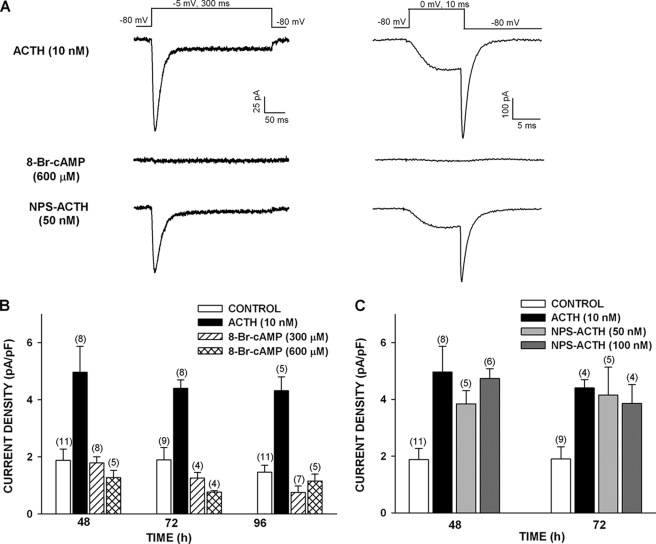
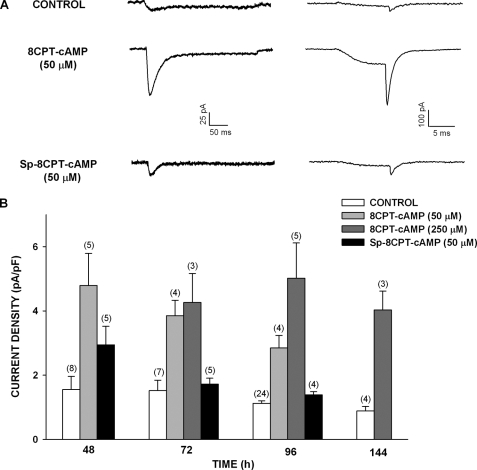
8CPT-cAMP but Not Sp-8CPT-cAMP Enhances Expression of Cav3.2 Current
Experiments with 8-Br-cAMP suggested that cAMP alone cannot induce or maintain the expression of functional Cav3.2 channels in bovine AZF cells. However, in other experiments, we found that a second 8-substituted, membrane-permeable cAMP analog, 8CPT-cAMP, at relatively low concentrations (50 and 250 μm) enhanced the expression of T-type Ca2+ current upon treating the cells for 48–144 h. Surprisingly, the poorly hydrolyzable analog Sp-8CPT-cAMP (50 μm) was much less effective and failed to prevent the time-dependent disappearance of the Cav3.2 current (Fig. 3).
FIGURE 3.
8CPT-cAMP but not Sp-8CPT-cAMP enhances expression of Cav3.2 T-type Ca2+ current in bovine AZF cells. AZF cells were cultured up to 144 h in media containing no further addition (control), 8CPT-cAMP (50 or 250 μm), or Sp-8CPT-cAMP (50 μm). Whole cell Ca2+ currents were recorded after activation by either long (300 ms) or short (10 ms) voltage steps applied at 30-s intervals from a holding potential of −80 mV. A, representative Ca2+ current traces recorded from AZF cells that were cultured for 48 h with 8CPT-cAMP (50 μm) or Sp-8CPT-cAMP (50 μm). Ca2+ current traces were activated by long (left) and short (right) depolarizing steps. B, summary of experiments in which AZF cells were either left untreated (control) or treated with 8CPT-cAMP (50 or 250 μm) or Sp-8CPT-cAMP (50 μm) for 48–144 h. The bars represent Cav3.2 T-type Ca2+ current density expressed as the means ± S.E. of the indicated number of determinations.
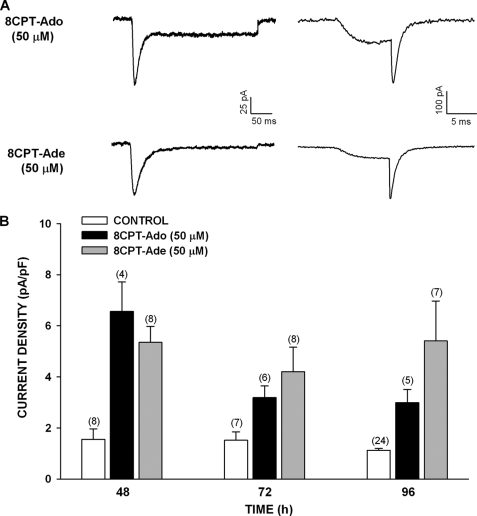
Metabolites of 8CPT-cAMP Induce Expression of Cav3.2 Current
The failure of Sp-8CPT-cAMP to enhance the expression of the Cav3.2 current suggested that 8CPT-cAMP acted as a pro-drug and increased the current indirectly after conversion to one or more active metabolites. 8CPT-cAMP can be sequentially converted to 8CPT-5′-AMP, 8CPT-Ado, and 8CPT-Ade by the cellular enzymes cyclic nucleotide phosphodiesterase, 5′ nucleotidases, and purine nucleotide phosphorylase, respectively (36, 37).
We found that treating AZF cells with 8CPT-Ado (50 μm) or 8CPT-Ade (50 μm) enhanced the expression of Cav3.2 current measured at times from 48 to 96 h (Fig. 4). The 2–4-fold increases in current density were similar in magnitude to those induced by 8CPT-cAMP.
FIGURE 4.
Long term effect of metabolites of 8CPT-cAMP on the expression of Cav3.2 T-type Ca2+ current in bovine AZF cells. AZF cells were cultured up to 96 h in media containing no further addition (control), 8CPT-Ado (50 μm), or 8CPT-Ade (50 μm). Whole cell Ca2+ currents were activated by either long (300 ms) or short (10 ms) voltage steps applied at 30-s intervals from a holding potential of −80 mV. A, representative Ca2+ current traces recorded from AZF cells that were cultured for 48 h with 8CPT-Ado (50 μm) or 8CPT-Ade (50 μm). Ca2+ current traces were activated by long (left panel) and short (right panel) depolarizing steps. B, summary of experiments in which AZF cells were either left untreated (control, white bars) or treated with 8CPT-Ado (50 μm, black bars) or 8CPT-Ade (50 μm, gray bars) for 48–96 h. The bars represent Cav3.2 T-type Ca2+ current density expressed as the means ± S.E. of the indicated number of determinations.
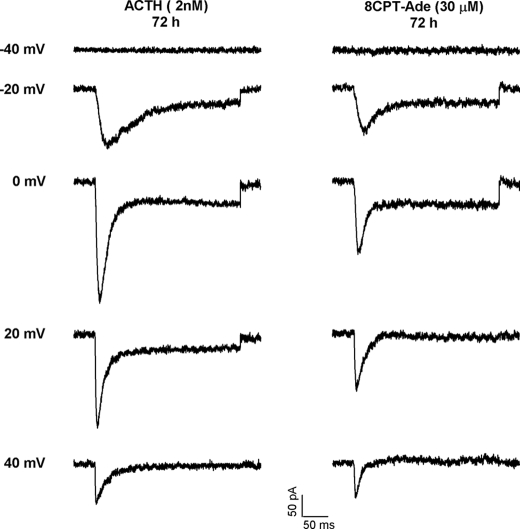
In addition to inducing the expression of the rapidly inactivating T-type Ca2+ current, prolonged exposures to ACTH, 8CPT-cAMP, or the associated metabolites induced the expression of a much smaller, noninactivating component of Ca2+ current in ∼20% of cells tested. The traces in Fig. 5 show currents recorded from two cells at test potentials from −40 to +40 mV, after 72 h in the presence of either ACTH (2 nm) or 8CPT-Ade (30 μm). The cells treated with ACTH or 8CPT-Ade expressed both rapidly inactivating and noninactivating Ca2+ currents. Although a noninactivating component of Ca2+ current was induced by ACTH and 8CPT-Ade, this current was typically smaller than that expressed in the two cells shown here. As previously reported, the noninactivating current was absent in nearly every freshly plated cell (1). It was also absent in untreated cells after 72 h. The noninactivating Ca2+ current was blocked by the L-type Ca2+ channel antagonist nimodipine (1 μm) (data not shown). This L-type Ca2+ current was not studied further.
FIGURE 5.
Effect of ACTH and 8CPT-Ade on expression of inactivating and noninactivating Ca2+ currents. AZF cells were incubated with either ACTH (2 nm) or 8CPT-Ade (30 μm) for 72 h, after which Cav3.2 T-type Ca2+ currents were recorded. The currents were activated from −80 mV by 300-ms voltage steps applied at 0.1 Hz to various test potentials between −40 and +40 mV. The current traces were recorded at the indicated test potentials.
Effect of PKA and Epac2 Activators on Cav3.2 Expression
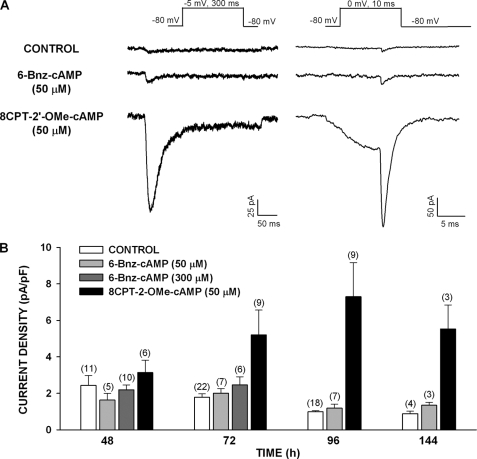
Experiments with the cAMP analog 8-Br-cAMP indicated that the activation of both PKA and Epac2 in AZF cells was not sufficient to induce the expression of functional Cav3.2 channels. cAMP derivatives with substitutions at the 6-position of the adenine ring potently activate PKA but not Epac proteins (38, 39). At concentrations above 100 μm, 6-Bnz-cAMP markedly enhances cortisol secretion (36). Treating bovine AZF cells with 6-Bnz-cAMP for prolonged periods failed to induce the expression of Cav3.2 current. In the experiments illustrated in Fig. 6, the cells were treated with 6-Bnz-cAMP (50 and 300 μm) for periods of 48–144 h. At no time did 6-Bnz-cAMP significantly increase Cav3.2 current density over the control value.
FIGURE 6.
Effect of 8CPT-2′-OMe-cAMP and 6-Bnz-cAMP on the expression of Cav3.2 T-type Ca2+ current in bovine AZF cells. AZF cells were cultured up to 144 h in media containing no further addition (control), 8CPT-2′-OMe-cAMP (50 μm), or 6-Bnz-cAMP (50 or 300 μm). Whole cell Ca2+ currents were activated by either long (300 ms) or short (10 ms) voltage steps applied at 30-s intervals from a holding potential of −80 mV. A, representative Ca2+ current traces recorded from AZF cells cultured for 96 h without (control) or with 8CPT-2′-OMe-cAMP (50 μm) or 6-Bnz-cAMP (50 μm). B, summary of experiments in which AZF cells were either left untreated (control, white bars) or treated with 8CPT-2′-OMe-cAMP (50 μm, black bars) or 6-Bnz-cAMP (50 μm, light gray bars; 300 μm, dark gray bars) for 48–144 h. The bars represent Cav3.2 T-type Ca2+ current density expressed as the means ± S.E. of the indicated number of determinations.
8CPT-2′-OMe-cAMP binds to Epac with an affinity greater than 100-fold that of its affinity for the cyclic nucleotide binding domain of the PKA regulatory subunit Iα (39). In contrast to 6-Bnz-cAMP, 8CPT-2′-OMe-cAMP (50 μm) prevented the time-dependent down-regulation of Cav3.2 expression and instead markedly enhanced the expression of this current at times from 72 to 144 h (Fig. 6). After exposing AZF cells to this Epac activator for 96 h, Cav3.2 expression was increased from the control value of 0.99 ± 0.06 pA/pF (n = 18) to 7.30 ± 1.86 pA/pF (n = 9) (Fig. 6).
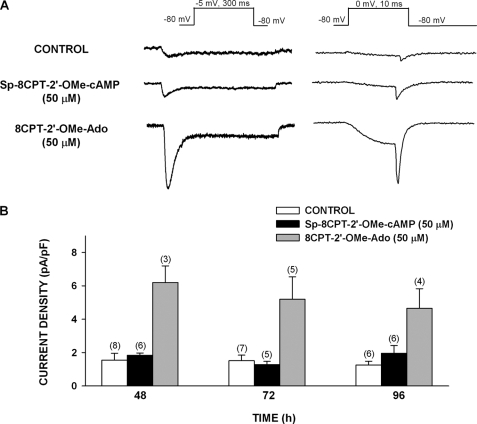
These results suggested that cAMP could induce the expression of Cav3.2 by selectively activating Epac2 but not PKA. However, like 8CPT-cAMP, 8CPT-2′-OMe-cAMP can be hydrolyzed by cellular enzymes to produce similar metabolites (29, 40). The nonhydrolyzable ESCA Sp-8CPT-2′-OMe-cAMP (50 μm) failed to enhance the expression of Cav3.2 current in AZF cells (Fig. 7).
FIGURE 7.
8CPT-2′-OMe-Ado but not Sp-8CPT-2′-OMe-cAMP enhances expression of Cav3.2 T-type Ca2+ current in bovine AZF cells. AZF cells were cultured up to 96 h in media containing no further addition (control), Sp-8CPT-2′-OMe-cAMP (50 μm), or 8CPT-2′-OMe-Ado (50 μm). Whole cell Ca2+ currents were recorded after activation by either long (300 ms) or short (10 ms) voltage steps applied at 30-s intervals from a holding potential of −80 mV. A, representative Ca2+ current traces recorded from AZF cells that were cultured for 48 h with control, Sp-8CPT-2′-OMe-cAMP (50 μm), or 8CPT-2′-OMe-Ado (50 μm). B, summary of experiments in which AZF cells were either left untreated (control) or treated with Sp-8CPT-2′-OMe-cAMP (50 μm) or 8CPT-2′-OMe-Ado (50 μm) for 48–96 h. The bars represent Cav3.2 T-type Ca2+ current density expressed as the means ± S.E. of the indicated number of determinations.
The failure of Sp-8CPT-2′-OMe-cAMP to increase the expression of Cav3.2 suggested that activation of Epac2 alone did not enhance the expression of this current. This result further suggested that 8CPT-2′-OMe-cAMP induced the expression of Cav3.2 indirectly through a metabolite. Accordingly, 8CPT-2′-OMe-adenosine (8CPT-2′-OMe-Ado), a metabolite of the hydrolyzable Epac activator, markedly increased the expression of Cav3.2 measured at 48, 72, and 96 h. At each of these times, current density was 3.5–4-fold greater than that of the time-matched control (Fig. 7).
ACTH and cAMP Derivatives and Metabolites Induce CACNA1H mRNA Expression
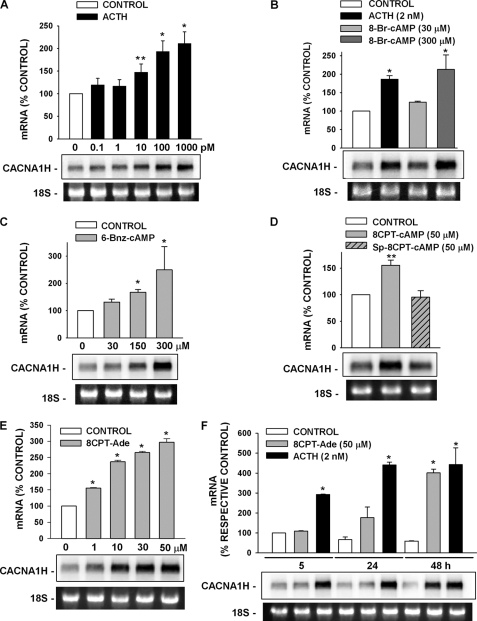
In bovine AZF cells, ACTH enhanced the expression of a Ni2+-sensitive T-type Ca2+ current presumed to be Cav3.2. If these increases in current occurred through activation of gene transcription or enhanced stability of the specific mRNA, then they would be accompanied by a corresponding increase in CACNA1H mRNA. We found that, at concentrations from 10 to 1000 pm, ACTH induced a concentration-dependent increase of a ∼8-kb CACNA1H transcript (Fig. 8A). ACTH-induced increases in CACNA1H mRNA were present by 5 h and reached a maximum by 24–48 h (Fig. 8F).
FIGURE 8.
Effects of ACTH and cAMP analogs on expression of the CACNA1H gene. AZF cells were incubated either without (control) or with ACTH, 8-Br-cAMP, 6-Bnz-cAMP, 8CPT-cAMP, Sp-8CPT-cAMP, or 8CPT-Ade as indicated. Total RNA was isolated at the indicated times. Each lane contained 10 μg of total RNA. The membranes were hybridized with specific probe for bovine CACNA1H as described under “Experimental Procedures.” mRNA values are presented as the means ± S.E. of at least three independent experiments normalized to densitometric values for the corresponding 18 S rRNA bands. **, p < 0.01 versus control; *, p < 0.001 versus control using Student's t test. Representative Northern blot is shown. 18 S rRNA bands from representative gels are shown as evidence of even loading. A, concentration-dependent effect of ACTH on CACNA1H mRNA. AZF cells were either left untreated (control, white bar) or treated with 10–1000 pm ACTH (black bars) for 24 h before isolating total RNA. B, effect of 8-Br-cAMP on CACNA1H mRNA. AZF cells were incubated without (control, white bar) or with ACTH (2 nm, black bar), 8Br-cAMP (30 μm, light gray bar; 300 μm, dark gray bar) as indicated for 48 h, after which total RNA was isolated. C, concentration-dependent effect of PKA activator 6-Bnz-cAMP on CACNA1H mRNA. AZF cells were incubated either without (control, white bar) or with 6-Bnz-cAMP (30–300 μm, gray bar) for 48 h, after which total RNA was isolated. D, effect of 8CPT-cAMP and nonhydrolyzable Sp-8CPT-cAMP on CACNA1H mRNA. AZF cells were incubated either without (control, white bar) or with 8CPT-cAMP (50 μm, gray bar) or Sp-8CPT-cAMP (50 μm, gray striped bar) for 48 h before isolating total RNA. E, concentration-dependent effect of 8CPT-cAMP metabolite 8CPT-Ade on CACNA1H mRNA. AZF cells were incubated either without (control, white bar) or with 8CPT-Ade (1–50 μm, gray bar) for 48 h, after which total RNA was isolated. F, time-dependent effect of 8CPT-Ade and ACTH on CACNA1H mRNA. AZF cells were incubated without (control, white bar) or with 8CPT-Ade (50 μm, gray bars) or ACTH (2 nm, black bars) for the times indicated, after which total RNA was isolated. 8CPT-Ade and ACTH values are presented as percentages of respective control values for each time shown.
Patch clamp experiments showed that the cAMP analogs 8-Br-cAMP and 6-Bnz-cAMP at concentrations up to 600 μm failed to stimulate an increase in functional Cav3.2 Ca2+ channels. However, both of these analogs did induce concentration-dependent increases in CACNA1H mRNA. Significant increases were observed only at concentrations above 100 μm (Fig. 8, B and C).
At low concentrations, 8CPT-cAMP and its nonhydrolyzable analog Sp-8CPT-cAMP had effects on CACNA1H mRNA similar to their effects on the associated Ca2+ current. At a concentration of 50 μm, 8CPT-cAMP increased expression of the CACNA1H transcript, whereas 50 μm Sp-8CPT-cAMP did not (Fig. 8D).
These results suggested that 8CPT-cAMP (50 μm) enhanced the expression of CACNA1H mRNA indirectly, after conversion to an active metabolite. Accordingly, at concentrations from 1 to 50 μm, its putative metabolite 8CPT-Ade stimulated a concentration-dependent increase in CACNA1H mRNA (Fig. 8E).
With respect to temporal pattern, the 8CPT-Ade-induced increases in CACNA1H mRNA lagged behind that induced by ACTH. Although no 8CPT-Ade-mediated increase in the CACNA1H transcript could be observed after 5 h, by 48 h the increase was similar in magnitude to those stimulated by ACTH (Fig. 8F).
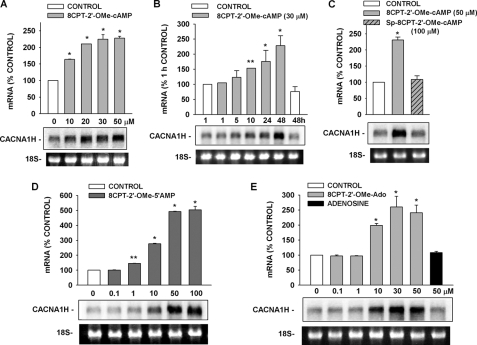
Enhancement of CACNA1H mRNA Expression by ESCAs and Metabolites
The hydrolyzable and nonhydrolyzable ESCAs 8CPT-2′-OMe-cAMP and Sp-8CPT-2′-OMe-cAMP produced effects on CACNA1H mRNA similar to their effects on the associated membrane current. Measured over a 48-h period, 8CPT-2′-OMe-cAMP (10–50 μm) induced a delayed, concentration-dependent increase in the CACNA1H mRNA (Fig. 9, A and B). In contrast, nonhydrolyzable Sp-8CPT-2′-OMe-cAMP (100 μm) failed to enhance CACNA1H mRNA expression, just as it failed to increase Cav3.2 current (Fig. 9C).
FIGURE 9.
Effects of Sp-8CPT-2′-OMe-cAMP, 8CPT-2′-OMe-cAMP, and metabolites on CACNA1H mRNA. AZF cells were incubated either without (control) or with 8CPT-2′-OMe-cAMP, Sp-8CPT-2′-OMe-cAMP, 8CPT-2′-OMe-5′-AMP, 8CPT-2′-OMe-Ado, or adenosine, as indicated. Each lane contained 10 μg of total RNA. The membranes were hybridized with specific probe for bovine CACNA1H as described under “Experimental Procedures.” mRNA values are presented as the means ± S.E. of at least three independent experiments normalized to densitometric values for the corresponding 18 S rRNA bands. **, p < 0.01 versus control; *, p < 0.001 versus control using Student's t test. Representative Northern blot is shown for each set of experiments. 18 S rRNA bands from representative gels are shown as evidence of even loading. A, concentration-dependent effect of 8CPT-2′-OMe-cAMP on CACNA1H mRNA. AZF cells were either left untreated (control, white bar) or treated with 10–50 μm 8CPT-2′-OMe-cAMP (gray bars) for 48 h before isolating total RNA. B, time-dependent effect of 8CPT-2′-OMe-cAMP on CACNA1H mRNA. AZF cells were incubated without (control, white bar) or with 8CPT-2′-OMe-cAMP (30 μm, gray bars) for the times indicated, after which total RNA was isolated. C, effect of 8CPT-2′-OMe-cAMP and nonhydrolyzable Sp-8CPT-2′-OMe-cAMP on CACNA1H mRNA. AZF cells were incubated either without (control, white bar) or with 8CPT-2′-OMe-cAMP (50 μm, gray bar) or Sp-8CPT-2′-OMe-cAMP (50 μm, gray striped bar) for 48 h before isolating total RNA. D, concentration-dependent effect of 8CPT-2′-OMe-cAMP metabolite 8CPT-2′-OMe-5′-AMP on CACNA1H mRNA. AZF cells were incubated either without (control, white bar) or with 8CPT-2′-OMe-5′-AMP (0.1–50 μm, dark gray bar) for 48 h, after which total RNA was isolated. E, concentration-dependent effect of 8CPT-2′-OMe-Ado and adenosine on CACNA1H mRNA. AZF cells were incubated without (control, white bar) or with 8CPT-2′-OMe-Ado (0.1–50 μm, gray bars) or adenosine (50 μm, black bar) for 48 h, after which total RNA was isolated.
These results again indicated that one or more metabolites of 8CPT-2′-OMe-cAMP enhanced CACNA1H mRNA expression by an Epac2-independent mechanism. Accordingly, we discovered that both 8CPT-2′-OMe-5′-AMP and 8CPT-2′-OMe-Ado stimulated concentration-dependent increases in the expression of CACNA1H mRNA with potencies similar to the parent compound (Fig. 9, D and E). In contrast, adenosine (50 μm) did not increase the expression of the CACNA1H transcript (Fig. 9E).
DISCUSSION
In this study, we have reported several novel findings regarding the physiological mechanisms that regulate Cav3.2 expression in bovine AZF cells. First, ACTH induces the expression of CACNA1H mRNA and Ca2+ current through mechanisms that are, at most, only partially dependent on cAMP. cAMP analogs that activate PKA and Epac, as well as those that activate only PKA, increase the expression of CACNA1H mRNA, but not the associated Ca2+ current. Epac activation alone is not sufficient to induce the expression of either mRNA or current. Chlorophenylthio-cAMP analogs potently induce the expression of CACNA1H mRNA and ionic current after conversion to one or more active metabolites. It is possible that ACTH and the cAMP metabolites stimulate the expression of CACNA1H mRNA and current through a common cAMP-independent mechanism.
ACTH and Cav3.2 Expression
ACTH reversed the spontaneous time-dependent decrease in Cav3.2 current typically observed in cultured bovine AZF cells and induced a delayed increase in expression of functional channels that lasted for at least 6 days. With respect to temporal pattern, maximum increases in Cav3.2 current were similar to those observed for ACTH-induced increases in steroid hydroxylase proteins and bTREK-1 current in these same cells (6, 7, 10, 41). ACTH also induced CACNA1H mRNA with a temporal pattern and potency similar to that previously observed for steroid hydroxylase messages (6, 7, 41). ACTH increases the steroid hydroxylases by increasing the rate of transcription of each of these genes (6–8, 42). It is likely that ACTH-induced increases in Cav3.2 expression involve increases in transcription of the CACNA1H gene. However, we have not eliminated the possibility that ACTH increases CACNA1H mRNA expression by stabilizing this transcript. The signaling pathway(s) by which ACTH regulates CACNA1H mRNA and Cav3.2 ionic current expression appear to be complex and may involve transcriptional and post-transcriptional mechanisms activated through cAMP-dependent and -independent pathways.
In this regard, the contrasting effects of ACTH compared with the cyclic AMP analogs 8-Br-cAMP and 6-Bnz-cAMP on CACNA1H mRNA and ionic currents are particularly interesting. Specifically, at concentrations above 100 μm, both of these cAMP analogs stimulate the expression of CACNA1H mRNA, but not that of functional Ca2+ channels. Therefore, ACTH-stimulated increases in cAMP alone are not sufficient to enhance Cav3.2 expression, even though they may contribute to this response. ACTH-induced effects may be mediated through additional or entirely separate signaling mechanisms that function at the transcriptional or post-transcriptional level. ACTH and NPS-ACTH increase intracellular Ca2+ and stimulate cortisol secretion from bovine AZF cells at concentrations that trigger little or no increase in cAMP synthesis (30, 31, 43). Perhaps in bovine AZF cells, Ca2+ and cAMP function cooperatively to regulate CACNA1H transcription and Cav3.2 translation and trafficking.
These results show that the mechanisms by which ACTH regulates the expression of Cav3.2 differ fundamentally from those that control the expression of steroid hydroxylases and bTREK-1 K+ channels. Specifically, ACTH-stimulated increases in cAMP alone appear to be sufficient to induce the expression of steroid hydroxylase and bTREK-1 mRNAs and their corresponding functional proteins (6, 7, 36).
cAMP, PKA, Epac2, and CACNA1H mRNA Expression
Experiments with 8-Br-cAMP showed that increasing cAMP in AZF cells was sufficient to increase CACNA1H mRNA, but not the corresponding Ca2+ current. Further, the robust concentration-dependent increase in CACNA1H mRNA induced by 6-Bnz-cAMP suggests that the increase is mediated through activation of PKA. However, it is possible that 6-Bnz-cAMP functions through a third, unidentified cAMP receptor. In patch clamp experiments, we found that, when included in the pipette solution at very low concentrations (i.e. <10 μm), 6-Bnz-cAMP completely blocks the activity of bTREK-1 channels, even in the presence of several PKA antagonists (36).
Experiments with the nonhydrolyzable ESCA Sp-8CPT-2′-OMe-cAMP clearly demonstrated that cAMP does not enhance the expression of CACNA1H mRNA by activation of Epac2. Specifically, at concentrations that activate Rap1 in these cells, Sp-8CPT-2′-OMe-cAMP failed to increase the quantity of CACNA1H mRNA (29). Thus, cAMP-stimulated expression of CACNA1H mRNA may be mediated entirely or in part by PKA but is independent of Epac2. Furthermore, a third cAMP-activated protein may be involved. In a number of cells, cAMP synthesized in response to activation of G-protein-coupled receptors produces effects that are independent of Epac and PKA proteins (44–48).
8-Chlorophenylthio-cAMP Analogs and CACNA1H Expression
In contrast to 8-Br-cAMP and 6-Bnz-cAMP, 8-chlorophenylthio-cAMP analogs, including 8CPT-cAMP and the ESCA 8CPT-2′-OMe-cAMP (each at 50 μm), induced CACNA1H mRNA and Ca2+ current expression. It is not likely that these effects were mediated through cAMP, because at identical concentrations, the nonhydrolyzable monophosphothioate derivatives of these two compounds failed to increase either CACNA1H mRNA or Cav3.2 current.
Accordingly, we found that four potential metabolites of these 8-chlorophenylthio-cAMP analogs induced the expression of CACNA1H mRNA transcripts and Cav3.2 ion current with effectiveness and potency similar to the parent compounds. Enzymes that catalyze the conversion of these two cAMP derivatives to each of these putative metabolites are expressed in mammalian cells (37). The active metabolite(s), receptors, and signaling pathways that mediate the increases in CACNA1H mRNA and the corresponding current have not been identified. In this regard, it is interesting that both 8CPT-cAMP and the ESCA 8CPT-2′-OMe-cAMP can be converted to 8CPT-adenine, which may be the common active metabolite.
Regardless of their identity, it is clear that the active metabolite(s) do not function in the AZF cell through cAMP. First, we have previously shown that neither 8CPT-2′-OMe-5′-AMP, 8CPT-2′-OMe-adenosine, nor 8CPT-adenine activates PKA when applied to bovine AZF cells (29). PKA activation is an extremely sensitive measure of cAMP synthesis in AZF cells (49). In addition, 8-Br-cAMP failed to increase Cav3.2 current in AZF cells, even at concentrations that have been shown to activate PKA and Epac2, stimulate large increases in cortisol synthesis and steroid hydroxylase mRNAs, and increase both bTREK-1 mRNA and K+ current (36).
With respect to temporal pattern and potency, 8CPT-adenine and other metabolites in this study induced the expression of CACNA1H mRNA and Ca2+ current with characteristics similar to those previously observed for bTREK-1 mRNA and K+ current, as well as that for steroid hydroxylase mRNAs and cortisol secretion (29, 36). These increases in gene expression were selective; total RNA synthesis was not significantly increased in metabolite-treated cells. Expression of Kv1.4 K+ channel mRNA and current were also not altered by these metabolites.3
The mechanism by which a small molecule such as 8CPT-adenine could activate specific genes in AZF cells is unclear. Regardless, our findings are consistent with the hypothesis that 8CPT-adenine and perhaps other similar metabolites induce the expression of CACNA1H mRNA and Ca2+ channels by a cAMP-independent pathway that is also activated by ACTH and NPS-ACTH.
Regulation of Cav3.2 Expression in Other Adrenal Cells
The finding that in bovine AZF cells ACTH can induce the expression of CACNA1H mRNA and Cav3.2 current, whereas cAMP analogs like 8-Br-cAMP increase only the mRNA was unexpected. However, it is in agreement with a previous patch clamp study in which a 3-day exposure of rat AZF cells to ACTH was found to increase T-type Ca2+ current in these cells, whereas 8-Br-cAMP failed (33). Apparently, in both bovine and rat AZF cells, ACTH stimulates the expression of functional Cav3.2 channels by a signaling pathway that includes a cAMP-independent component. Because 8-Br-cAMP increases CACNA1H mRNA but not Ca2+ current, the cAMP-independent action of ACTH likely occurs at the level of translation or protein trafficking. Regardless, the results of the present study reveal that the control of CACNA1H expression is complex and involves multiple signaling pathways operating at transcriptional and post-transcriptional levels.
Our results also may clarify some potentially confusing findings regarding the control of T-type Ca2+ channel expression in adrenal cells. In a patch clamp study on rat adrenal chromaffin cells, it was shown that prolonged treatment with 8CPT-cAMP or 8CPT-2′-OMe-cAMP induced the expression of a low voltage-activated Ni2+-sensitive Ca2+ current. Because the effects of these cAMP analogs on Ca2+ channel expression were not blocked by the PKA inhibitor H-89, it was concluded that the Ca2+ channel “recruitment” was mediated through cAMP by activation of an Epac protein (32). In view of our findings and those of Barbara and Takeda (33), it is quite possible that the effects of the chlorophenylthio-cAMP analogs on the Ni2+-sensitive T-type current in rat adrenal chromaffin cells were mediated indirectly through the metabolites of these compounds.
Physiological Significance
The results of this and previous studies strongly suggest that ACTH exerts long term control over the electrical properties of AZF cells by regulating the expression of genes that code for ion channels (10, 36, 50). Cav3.2 Ca2+ channels function as the primary pathway for depolarization-dependent Ca2+ entry, leading to cortisol secretion by these cells (2). Prolonged exposure of AZF cells to ACTH, as occurs in chronic stress, may enhance cortisol production by increasing the number of Cav3.2 channels.
Cav3.2 channels are expressed in other tissues, including the brain, where they regulate the initiation of action potentials in neurons. Overexpression of this specific T-type Ca2+ channel in neurons has been shown to play a significant role in various forms of epilepsy, as well as neuropathic pain (51–53). To understand the molecular basis of the pathophysiology of diseases such as these, it will be essential to identify the signaling mechanisms that regulate Cav3.2 expression. Our study indicates that ACTH likely induces the expression of CACNA1H mRNA by activating PKA. However, other post-transcriptional mechanisms must be activated by cAMP-independent pathways to produce functional Cav3.2 channels. It will be important to identify this other ACTH activated pathway and to determine whether it is also activated by metabolites of 8-chlorophenylthio-cAMP analogs that increase CACNA1H mRNA and current.
The extent to which metabolites of the chlorophenylthio-derivatives of cAMP regulate gene expression in other cells is unknown. Hydrolysis products of selected cAMP analogs transform the morphology of the protozoa Trypanosoma brucei (40). It is not known whether these effects observed in distantly related eukaryotes are mediated by a common signaling pathway (36, 40).
Finally, 8CPT-cAMP and 8CPT-2′-OMe-cAMP have been used in many hundreds of studies to identify and characterize the roles of cAMP, PKA, and Epac proteins in cell signaling. The findings of this and several other recent studies indicate that the results of many earlier studies may require re-evaluation (29, 36, 40).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01-DK47875 (to J. J. E.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
H. Liu, J. A. Enyeart, and J. J. Enyeart, unpublished observations.
- AZF
- adrenal zona fasciculata
- ACTH
- adrenocorticotropic hormone
- NPS-ACTH
- O-nitrophenyl,sulfenyl-adrenocorticotropin
- PKA
- cAMP-dependent protein kinase
- ESCA
- Epac-specific cAMP analog
- DMEM
- Dulbecco's modified Eagle's medium
- 8-Br-cAMP
- 8-bromoadenosine 3′,5′-cyclic monophosphate
- 6-Bnz-cAMP
- N6-benzoyladenosine 3′,5′-cyclic monophosphate
- 8CPT-2′-OMe-cAMP
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- Sp-8CPT-2′-OMe-cAMP
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP, Sp-isomer
- 8CPT-2′-OMe-5′-AMP
- 8-(4-chlorophenylthio)-2′-O-methyladenosine 5′-O-monophosphate
- 8CPT-2′-OMe-Ado
- 8-(4-chlorophenylthio)-2′-O-methyladenosine
- 8CPT-cAMP
- 8-(4-chlorophenylthio)-cAMP
- Sp-8CPT-cAMP
- 8-(4-chlorophenylthio)-cAMP
- 8CPT-Ado
- 8-(4-chlorophenylthio)adenosine
- 8CPT-Ade
- 8-(4-chlorophenylthio)adenine.
REFERENCES
- 1.Mlinar B., Biagi B. A., Enyeart J. J. (1993) J. Gen. Physiol. 102, 217–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enyeart J. J., Mlinar B., Enyeart J. A. (1993) Mol. Endocrinol. 7, 1031–1040 [DOI] [PubMed] [Google Scholar]
- 3.Mlinar B., Biagi B. A., Enyeart J. J. (1993) J. Biol. Chem. 268, 8640–8644 [PubMed] [Google Scholar]
- 4.Enyeart J. J., Xu L., Danthi S., Enyeart J. A. (2002) J. Biol. Chem. 277, 49186–49199 [DOI] [PubMed] [Google Scholar]
- 5.Quinton P. M., Reddy M. M. (1992) Nature 360, 79–81 [DOI] [PubMed] [Google Scholar]
- 6.Simpson E. R., Waterman M. R. (1988) Annu. Rev. Physiol. 50, 427–440 [DOI] [PubMed] [Google Scholar]
- 7.Waterman M. R. (1994) J. Biol. Chem. 269, 27783–27786 [PubMed] [Google Scholar]
- 8.Parker K. L., Schimmer B. P. (1995) Vitam. Horm. 51, 339–370 [DOI] [PubMed] [Google Scholar]
- 9.Lederer W. J., Nichols C. G. (1989) J. Physiol. 419, 193–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enyeart J. A., Danthi S., Enyeart J. J. (2003) Mol. Pharmacol. 64, 132–142 [DOI] [PubMed] [Google Scholar]
- 11.Perez-Reyes E. (2003) Physiol. Rev. 83, 117–161 [DOI] [PubMed] [Google Scholar]
- 12.Lee J. H., Gomora J. C., Cribbs L. L., Perez-Reyes E. (1999) Biophys. J. 77, 3034–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes R. C., Jr., Berthet L. (1957) J. Biol. Chem. 225, 115–124 [PubMed] [Google Scholar]
- 14.Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. (1967) J. Biol. Chem. 242, 5535–5541 [PubMed] [Google Scholar]
- 15.Richardson M. C., Schulster D. (1973) Biochem. J. 136, 993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sala G. B., Hayashi K., Catt K. J., Dufau M. L. (1979) J. Biol. Chem. 254, 3861–3865 [PubMed] [Google Scholar]
- 17.Penhoat A., Jaillard C., Saez J. M. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 4978–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raikhinstein M., Zohar M., Hanukoglu I. (1994) Biochim. Biophys. Acta 1220, 329–332 [DOI] [PubMed] [Google Scholar]
- 19.de Rooij J., Zwartkruis F. J., Verheijen M. H., Cool R. H., Nijman S. M., Wittinghofer A., Bos J. L. (1998) Nature 396, 474–477 [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki H., Springett G. M., Mochizuki N., Toki S., Nakaya M., Matsuda M., Housman D. E., Graybiel A. M. (1998) Science 282, 2275–2279 [DOI] [PubMed] [Google Scholar]
- 21.Borland G., Smith B. O., Yarwood S. J. (2009) Br. J. Pharmacol. 158, 70–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bos J. L. (2006) Trends Biochem. Sci. 31, 680–686 [DOI] [PubMed] [Google Scholar]
- 23.Holz G. G., Kang G., Harbeck M., Roe M. W., Chepurny O. G. (2006) J. Physiol. 577, 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002) Nat. Cell Biol. 4, 901–906 [DOI] [PubMed] [Google Scholar]
- 25.Holz G. G., Chepurny O. G., Schwede F. (2008) Cell. Signal. 20, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehmann H., Schwede F., Døskeland S. O., Wittinghofer A., Bos J. L. (2003) J. Biol. Chem. 278, 38548–38556 [DOI] [PubMed] [Google Scholar]
- 27.Enyeart J. J., Mlinar B., Enyeart J. A. (1996) J. Gen. Physiol. 108, 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H., Enyeart J. A., Enyeart J. J. (2008) J. Gen. Physiol. 132, 279–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enyeart J. A., Enyeart J. J. (2009) PLoS One 4, e6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyle W. R., Kong Y. C., Ramachandran J. (1973) J. Biol. Chem. 248, 2409–2417 [PubMed] [Google Scholar]
- 31.Yamazaki T., Kimoto T., Higuchi K., Ohta Y., Kawato S., Kominami S. (1998) Endocrinology 139, 4765–4771 [DOI] [PubMed] [Google Scholar]
- 32.Novara M., Baldelli P., Cavallari D., Carabelli V., Giancippoli A., Carbone E. (2004) J. Physiol. 558, 433–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbara J. G., Takeda K. (1995) J. Physiol. 488, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enyeart J. J., Gomora J. C., Xu L., Enyeart J. A. (1997) J. Gen. Physiol. 110, 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. (1981) Pflügers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 36.Enyeart J. A., Liu H., Enyeart J. J. (2010) Mol. Pharmacol. 77, 469–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price N. C., Stevens L. (1999) Fundamentals of Enzymology: The Cell and Molecular Biology of Catalytic Proteins, 3rd Ed., pp. 450–470, Oxford University Press, New York [Google Scholar]
- 38.Zazopoulos E., Lalli E., Stocco D. M., Sassone-Corsi P. (1997) Nature 390, 311–315 [DOI] [PubMed] [Google Scholar]
- 39.Christensen A. E., Selheim F., de Rooij J., Dremier S., Schwede F., Dao K. K., Martinez A., Maenhaut C., Bos J. L., Genieser H. G., Døskeland S. O. (2003) J. Biol. Chem. 278, 35394–35402 [DOI] [PubMed] [Google Scholar]
- 40.Laxman S., Riechers A., Sadilek M., Schwede F., Beavo J. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19194–19199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John M. E., John M. C., Boggaram V., Simpson E. R., Waterman M. R. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4715–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waterman M. R., Simpson E. R. (1989) Recent Prog. Horm. Res. 45, 533–566 [DOI] [PubMed] [Google Scholar]
- 43.Omura M., Suematsu S., Nishikawa T. (2007) Endocr. J. 54, 585–592 [DOI] [PubMed] [Google Scholar]
- 44.Fujita T., Meguro T., Fukuyama R., Nakamuta H., Koida M. (2002) J. Biol. Chem. 277, 22191–22200 [DOI] [PubMed] [Google Scholar]
- 45.Ivins J. K., Parry M. K., Long D. A. (2004) J. Neurosci. 24, 1212–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iacovelli L., Capobianco L., Salvatore L., Sallese M., D'Ancona G. M., De Blasi A. (2001) Mol. Pharmacol. 60, 924–933 [DOI] [PubMed] [Google Scholar]
- 47.Stork P. J., Schmitt J. M. (2002) Trends Cell Biol. 12, 258–266 [DOI] [PubMed] [Google Scholar]
- 48.Busca R., Abbe P., Mantoux R., Aberdam E., Peyssonnaux C., Eychene A., Ortonne J. P., Ballotti R. (2000) EMBO J. 19, 2900–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enyeart J. J., Enyeart J. A. (1998) Endocr. Res. 24, 325–334 [DOI] [PubMed] [Google Scholar]
- 50.Enyeart J. A., Xu L., Enyeart J. J. (2000) J. Biol. Chem. 275, 34640–34649 [DOI] [PubMed] [Google Scholar]
- 51.Becker A. J., Pitsch J., Sochivko D., Opitz T., Staniek M., Chen C. C., Campbell K. P., Schoch S., Yaari Y., Beck H. (2008) J. Neurosci. 28, 13341–13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su H., Sochivko D., Becker A., Chen J., Jiang Y., Yaari Y., Beck H. (2002) J. Neurosci. 22, 3645–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jagodic M. M., Pathirathna S., Nelson M. T., Mancuso S., Joksovic P. M., Rosenberg E. R., Bayliss D. A., Jevtovic-Todorovic V., Todorovic S. M. (2007) J. Neurosci. 27, 3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.