Abstract
In vascular smooth muscle cells (VSMC) Axl is a key receptor tyrosine kinase since it is up-regulated in injury, increases migration and neointima formation, and is activated by reactive oxygen species. Reaction of glutathione with cysteine residues (termed glutathiolation; GSSG) is an important post-translational redox modification that may alter protein activity and protein-protein interactions. To investigate the mechanisms by which reactive oxygen species (ROS) increase Axl-dependent VSMC function we assayed for glutathiolated proteins that associated with Axl in a redox-dependent manner. We identified glutathiolated non-muscle myosin heavy chain (MHC)-IIB as a novel Axl interacting protein. This interaction was specific in that other myosins did not interact with Axl. The endogenous ligand for Axl, Gas6, increased production of ROS in VSMC and also increased association of Axl with MHC-IIB. Antioxidants ebselen and N-acetylcysteine decreased association of Axl with MHC-IIB in response to both Gas6 and ROS. Blocking the Axl-MHC-IIB interaction with the specific myosin II inhibitor blebbistatin decreased phosphorylation of Axl and activation of ERK1/2 and Akt. Association of MHC-IIB with Axl was increased in balloon injured rat carotid vessels. Finally, expression of MHC-IIB was upregulated in the neointima of the carotid artery following balloon injury similar to upregulation of Axl protein expression as shown in our previous studies. These results demonstrate a novel interaction between Axl and MHC-IIB in response to ROS. This interaction provides a direct link between Axl and molecular motors crucial for directed cell migration, which may mediate increased migration in vascular dysfunction.
Keywords: vascular smooth muscle, receptor protein tyrosine kinase, myosin heavy chains, reactive oxygen species, vascular disease
Introduction
The receptor tyrosine kinase Axl is a 140 kDa protein expressed in many cell types including VSMC1 and is activated by Growth Arrest Gene 6 (Gas6), a homologue of Protein S2, 3. This leads to activation of downstream signaling cascades including the PI-3 kinase-Akt pathway, ERK1/2 and PLCγ. Gas6 activation of Axl in VSMC stimulates migration and inhibits apoptosis4, 5. Axl is a key VSMC receptor tyrosine kinase since it is upregulated by injury, activated by reactive oxygen species (ROS), and increases neointima formation1, 6, 7. This indicates a role for Axl in the pathogenesis of vascular diseases1, 5–9.
Recent evidence indicates that ROS act as signaling molecules by causing glutathiolation of redox sensitive proteins that contain cysteine thiols. On exposure to oxidants the cysteine can be reversibly oxidized to sulfenic acid, which can form a disulfide bond with glutathione. Since this process is rapidly reversible by glutaredoxin, it is a regulatory mechanism to prevent further oxidation, protecting proteins against irreversible oxidative damage10, 11. Glutathiolation can alter both enzyme activity and protein-protein interactions.
Since Axl is activated by ROS in VSMC, we hypothesized that ROS will modulate Axl signal transduction by altering interaction with unknown glutathiolated proteins. Therefore, in this study we used glutathiolation as a means to assay for novel redox sensitive Axl binding partners. We found a redox-induced interaction between Axl and glutathiolated non-muscle myosin heavy chain IIB (MHC-IIB) in VSMC. MHC-IIB is involved in directed cell migration 12–14. Expression of MHC-IIB is increased in atherosclerotic lesions, balloon-injured carotid vessels and in hypertensive arteries15–18. This strongly suggests that increased expression of MHC-IIB contributes to the increased migratory response in vascular pathology. The Axl-MHC-IIB interaction occurs on stimulation of VSMC with both ROS and Gas6. This interaction is important for Axl signaling in that inhibition of the interaction with the non muscle myosin II inhibitor blebbistatin decreases Axl phosphorylation and phosphorylation of downstream kinases ERK1/2 and Akt. Increased interaction between Axl and MHC-IIB in injured arteries suggests that this is important in response to vascular injury.
Methods and Materials
Antibodies to Axl and ERK1/2 were from Santa Cruz Biotechnology; antibodies to phospho-ERK, phospho-Akt (Ser-473) and Akt were from Cell Signaling; MHC-IIB antibody was from Covance, smooth muscle actin was from DAKO. LiCor fluorescent secondary antibodies were from Molecular Probes. Blebbistatin was from Calbiochem. LY83583 was from RBI. Gas6 and anti-Axl antibody for immunofluoresence were kindly provided by Brian Varnum (Amgen). Immunohistochemistry reagents were from Covance. All other reagents and chemicals were obtained from Sigma.
Cell Culture
Cultured VSMC were obtained from rat aorta as described19. VSMC were grown in Dulbecco’s modified Eagle Medium supplemented with 25 mM NaHCO3, 10 mM HEPES, pH 7.4, 50 IU/ml penicillin, 50 µg/ml streptomycin, 10 % fetal bovine serum (FBS) containing 5.5 mM glucose in a 5 % CO2/95% O2 incubator at 37 °C.
Biotinylated glutathione ethyl ester (BioGEE) labeling
Biotinylated glutathione ethyl ester (BioGEE), a membrane permeable analogue of glutathione, labeling was performed as described by Sullivan et al20. Briefly, biotinylated GSH ester was prepared by mixing 25 mM sulfo-NHS-biotin with 25 mM GSH ethyl ester in 50 mM NaHCO3 at pH 8.5 for 2 hrs followed by addition of 1.25 M Glycine at pH 8.5 for 5 mins. Cells were incubated in 250 µM BioGEE for 1 hr. Cells were then stimulated, rinsed in PBS and then immunoprecipitation was performed as described below. BioGEE labeled proteins were detected using HRP-streptavidin (Pierce).
Preparation of cell lysates and immunoprecipitations
Cell monolayers were rinsed with ice-cold phosphate-buffered saline (PBS; 150 mM NaCl, 20 mM Na2PO4, pH 7.4) and then scraped in 1 ml of PBS. After a brief centrifugation, the cells were solubilized in 1 ml of cell lysis buffer (10 mM HEPES, pH 7.4, 50 mM Na Pyrophosphate, 50 mM NaF, 50 mM NaCl, 5 mM EDTA, 5 mM EGTA, 1 mM Na3VO4, 0.5 % Triton plus 1:1000 protease inhibitor cocktail). Cells were sonicated for 20 s, agitated on a rotating rocker at 4 °C for 30 min and centrifuged at 12,000 g for 30 min to remove insoluble cellular debris.
For immunoprecipitation studies, lysates were precleared for 1 hr with protein G agarose (Invitrogen) followed by incubation with anti-Axl antibody for 3 hr, and protein G agarose for a further 1 hr. Immunoprecipitates were then washed 4 times with 1 ml cell lysis buffer before the addition of Laemmli sample buffer. After heating at 95 °C for 3 min, proteins were resolved on SDS-PAGE and transferred to nitrocellulose membranes for Western analysis. Immunoreactive bands were detected with LiCor fluorescent secondary antibodies and the LiCor Odyssey infrared Imaging system. Analysis of blots was performed using the LiCor densitometry software21.
Measurement of ROS production
Hydroethidium and 2',7'-dichlorodihydrofluorescein diacetate (DCF-DA) was used to measure ROS in VSMCs. VSMCs were loaded with DCF-DA (5 µmol/L for 30 minutes), medium was aspirated, and VSMCs were stimulated with Gas6 (100 ng/ml) or H2O2 (300 µM) for 3 min in a light-protected humidified chamber at 37°C. Cells were rinsed and images obtained for 1 min at 10 sec intervals with a Olympus BX51 epifluorescence microscope equipped with ×40 water immersion lens, excitation 485 nm and emission 535 nm.
Carotid balloon injury
Balloon injury of the rat left carotid was performed exactly as described7 using male Sprague-Dawley rats (300–400 g; Charles River Laboratories, Wilmington, MS). At the end of the experiment, the injured and uninjured contralateral vessels were removed and snap frozen in liquid nitrogen. In a separate experiment animals were perfusion fixed and carotid arteries were paraffin embedded as described7. All procedures were carried out in a specific pathogen-free animal care facility at the University of Rochester and were approved by the University's Committee on Animal Resource.
Immunohistochemistry
Following balloon injury carotid arteries were dissected and paraffin embedded. Cross sections of injured and contralateral uninjured carotid arteries were deparaffinized and incubated in 3% hydrogen peroxide for 10 minutes followed by antigen retrieval in citrate buffer pH 6.0 for 20 minutes. Sections were stained according to protocol proved by Covance. Polyclonal MHC-IIB antibody was diluted in DAKO antibody dilution buffer 1:5000. Negative control goat IgG was used at the corresponding dilution. Cross sections were counterstained with hemotoxylin.
Statistical analysis
All experiments were carried out at least 3 times. Differences were assessed by analysis of variance and p<0.05 was considered significant in all experiments.
Results
Redox sensitive interaction between Axl and MHC-IIB
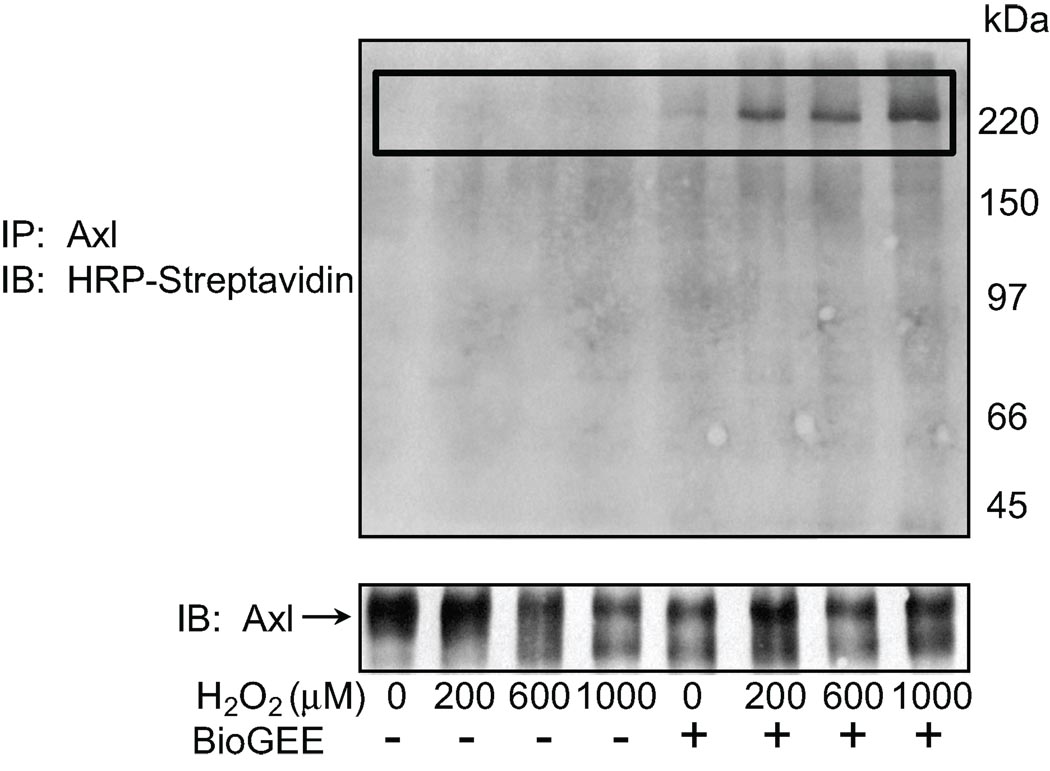
To detect proteins that associated with Axl in a redox dependent manner we used BioGEE labeling as described by Sullivan et al20. Rat aortic VSMC incubated with BioGEE were stimulated with H2O2 (0–1000 µM) and Axl was immunoprecipitated. Glutathiolated proteins that interacted with Axl were identified using Streptavidin-HRP after non-reducing SDS-PAGE. A 225 kDa protein co-immunoprecipitated with Axl (Fig. 1). This protein was no longer present after preabsorption of the H2O2 stimulated cell lysate with streptavidin agarose (data not shown). Additionally, samples were treated with DTT to disrupt disulfide bonds. This abolished the detection of the immunoprecipitated band upon H2O2 stimulation (Figure S1a, please see http://hyper.ahajournals.org). The 225 kDa protein was identified by mass spectrometry as MHC-IIB. To confirm that Axl and MHC-IIB do interact cells lysates were immunoprecipitated with Axl and MHC-IIB was immunoblotted. The interaction increased in response to H2O2 (Figure SIb).
Figure 1. Axl interacts with glutathiolated p225 in rat aortic VSMC.
Cells were loaded with 250 µM BioGEE for 1 hr, stimulated with H2O2 for 10 min, and Axl was immunoprecipitated. Non-reducing SDS-PAGE was performed and BioGEE labeled proteins were detected with streptavidin HRP (upper panel). Lower panel shows immunoprecipitation of Axl.
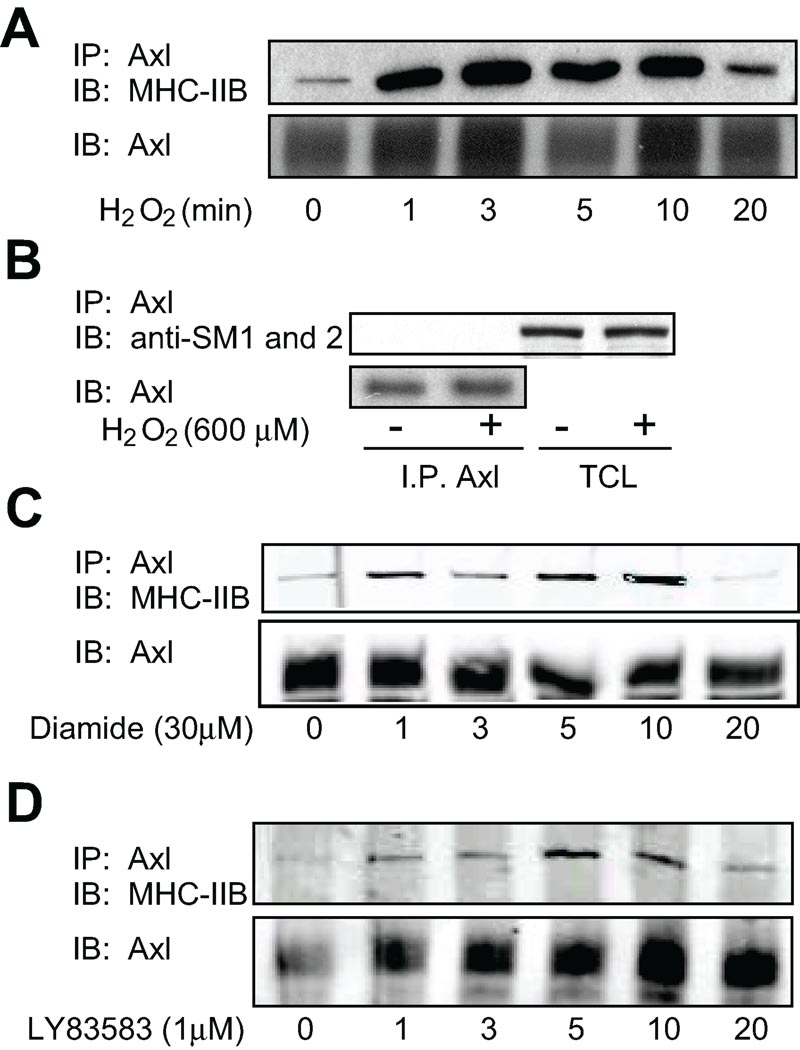
To demonstrate that MHC-IIB interacted with Axl in a redox sensitive manner, VSMC were stimulated with H2O2 (300 µM), for the indicated times (0–20 min) after which Axl was immunoprecipitated and MHC-IIB was immunoblotted (Fig. 2). MHC-IIB interaction with Axl increased upon stimulation with H2O2 in a time dependent manner peaking between 3 to 10 min. (Fig. 2A). To investigate the specificity of this interaction, the ability of smooth muscle MHCs (SM1 and 2) to associate with Axl was determined. Neither SM1 nor 2 co-immunoprecipitated with Axl after stimulation with H2O2 (Fig. 2B). Diamide (30 µM; a thiol oxidizer) and LY83583 (1 µM; a superoxide generator) also increased the interaction between Axl and MHC-IIB, further demonstrating the importance of glutathiolation and ROS in the association between the two proteins (Fig. 2C and 2D).
Figure 2. Redox dependent interaction between Axl and MHC-IIB in rat aortic VSMC.
A. Cells were stimulated with H2O2 (300 µM). Cell lysates were immunoprecipitated with anti-Axl antibody and interacting MHC-IIB detected by immunoblotting with MHC-IIB antibody. Equal loading was confirmed with anti-Axl antibody (lower). B. SM1 and 2 do not interact with Axl. Rat aortic VSMC were stimulated with H2O2 (300 µM). To examine interaction between Axl and SM1 and 2, cell lysates were immunoprecipitated with anti-Axl antibody and immunoblotted with SM1 and 2 antibody (upper panel). Lysates show expression of SM1 and 2 in VSMC (upper panel). Equal loading was confirmed using anti-Axl antibody (lower panel). C. Cells were stimulated with Diamide (30 µM) for the indicated times. Cell lysates were immunoprecipitated with anti-Axl antibody and interacting MHC-IIB detected by immunoblotting with MHC-IIB antibody. Equal loading was confirmed with anti-Axl antibody (lower). D. Cells were stimulated with LY83583 (1 µM) for the indicated times. Cell lysates were immunoprecipitated with anti-Axl antibody and interacting MHC-IIB detected by immunoblotting with MHC-IIB antibody. Equal loading was confirmed with anti-Axl antibody (lower).
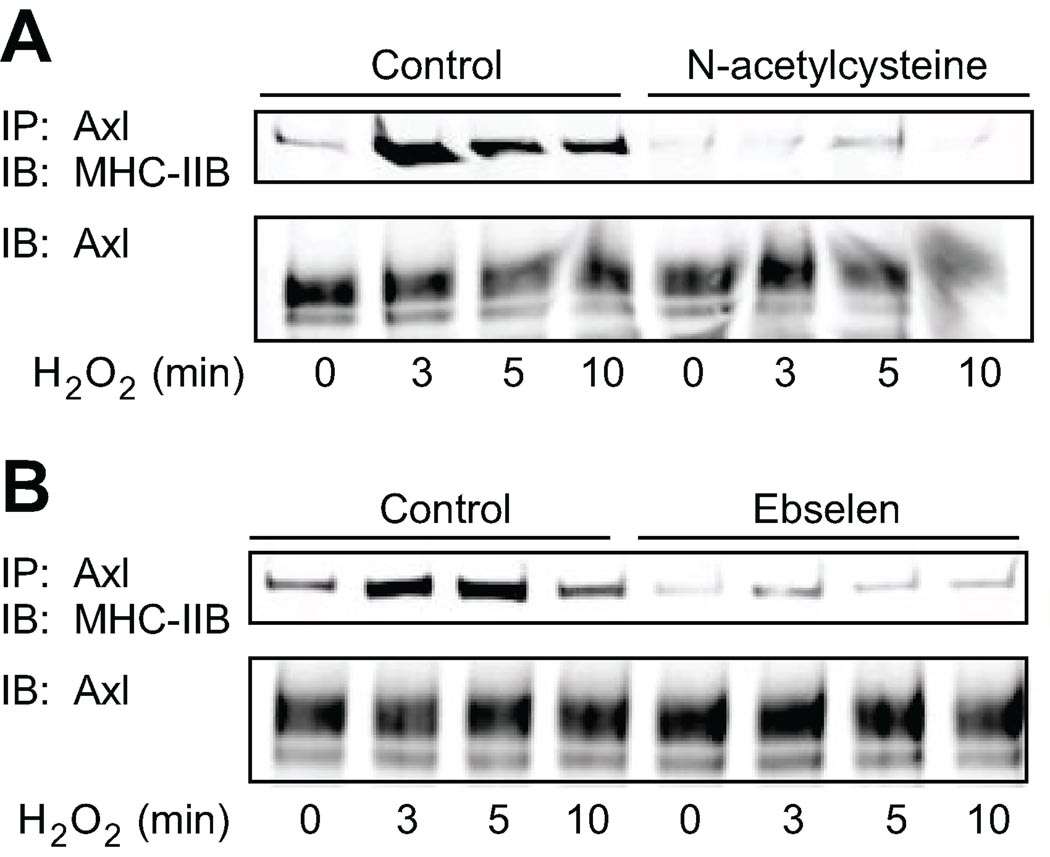
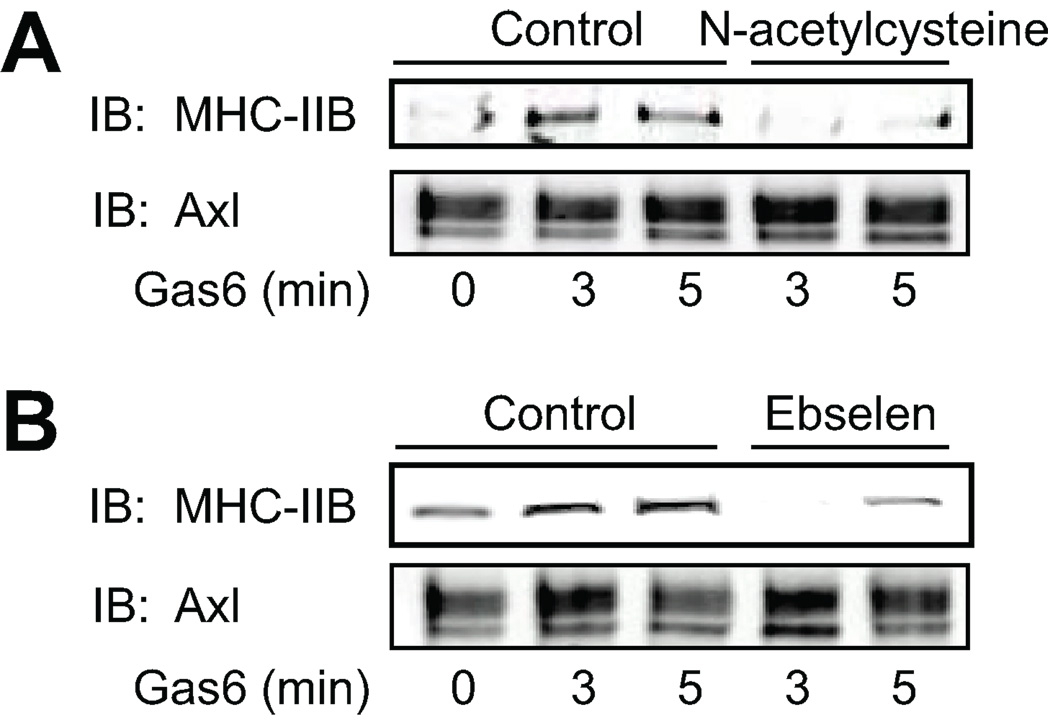
The effect of antioxidants on the interaction of MHC-IIB with Axl was studied by pretreatment of VSMC for 30 min with the antioxidants N-acetylcysteine (1 mM), which reduces protein thiols, and ebselen (40 µM), which is a glutathione peroxidase mimetic (Fig. 3A and 3B). Cells were then stimulated with H2O2 for the indicated times and Axl was immunoprecipitated (Fig. 3). Antioxidants completely abolished the interaction between Axl and MHC-IIB. Therefore ROS cause glutathiolation of MHC-IIB and induce association of MHC-IIB with Axl.
Figure 3. Redox dependent interaction between Axl and MHC-IIB is inhibited by antioxidants.
Cells were pretreated with 1 mM N-acetylcysteine (A) or 40 µM ebselen (B) for 30 min. Cells were then stimulated with 300 µM H2O2 for the indicated times. Cell lysates were immunoprecipitated with anti-Axl antibody and interacting MHC-IIB detected by immunoblotting with MHC-IIB antibody. Equal loading was confirmed with anti-Axl antibody (lower).
Interaction between Axl and MHC-IIB is induced by Gas6
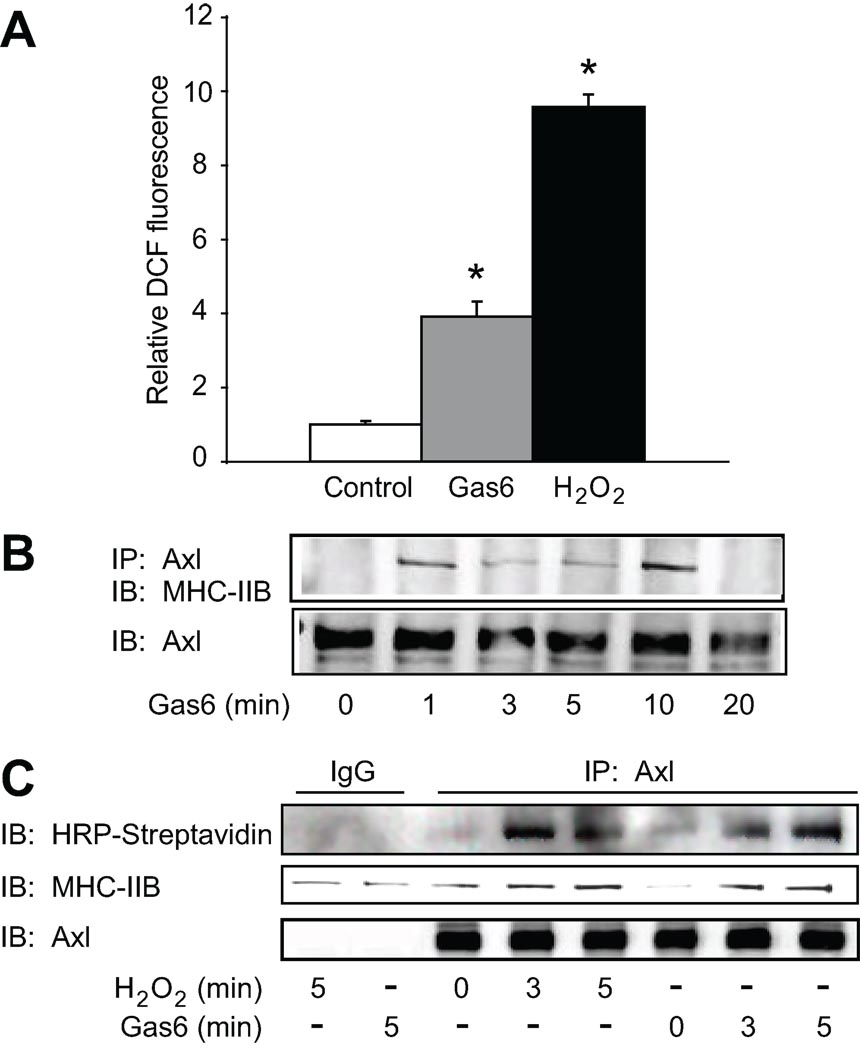
Gas6, the endogenous ligand for Axl, increases Rac activity, which is upstream of NADPH oxidase in neuronal cells22 suggesting that Gas6-Axl should increase ROS mediated effects in VSMC. Therefore we measured H2O2 generation in VSMC using the H2O2 sensitive fluorophore DCF-DA. Gas6 (100ng/ml) increased DCF fluorescence by 4-fold (approximately 50% of the increase with H2O2, Fig. 4A), demonstrating that Gas6 stimulates production of H2O2. Since Gas6 is a ligand for Axl and is upregulated in vascular injury we studied the ability of Gas6 to increase interaction between Axl and MHC-IIB as described for H2O2 above. Gas6 increased the Axl-MHC-IIB interaction with a similar time course to H2O2 (Fig. 4B). We further demonstrated that Gas6 increased association of glutathiolated MHC-IIB with Axl by labeling VSMC with BioGEE, and immunoprecipiating with Axl antibody as was done for Figure 1. Immunoblotting with HRP-streptavidin followed by MHC-IIB antibody demonstrated that glutathiolated MHC-IIB interacted with Axl after treatment with Gas6 at 3 and 5 minutes (Fig. 4C).
Figure 4. Gas6 enhances the interaction between glutathiolated MHC-IIB and Axl.
A. Production of H2O2 by Gas6. VSMC were loaded with DCF-DA for 30 min and stimulated with either Gas6 (100 ng/ml) or H2O2 (300 µM) for 3 min. Relative DCF fluorescence (RDF) was measured for 1 min at 10 sec intervals. Cells were viewed with an Olympus fluorescence microscope. Data are mean±SE for 2 separate experiments. *P<0.01 for increase in RDF versus control. B. VSMC were stimulated with Gas6 (100 ng/ml) for the indicated times. Cell lysates were immunoprecipitated with anti-Axl antibody and immunoblotted with MHC-IIB antibody. Equal loading was confirmed using anti-Axl antibody. C. Cells were loaded with 250 µM BioGEE for 1 hr, stimulated with H2O2 or Gas6 for 10 min, and Axl was immunoprecipitated. Non-reducing SDS-PAGE was performed and BioGEE labeled proteins were detected with streptavidin HRP (upper panel). The 225 kDa protein was verified to be MHC-IIB by probing the blot with MHC-IIB antibody, followed by detection using Licor infrared antibodies (middle panel). Lower panel shows immunoprecipitation of Axl.
The effect of antioxidants on the interaction of MHC-IIB with Axl mediated by Gas6 was studied by pre-treating VSMC for 30 min with N-acetylcysteine (1 mM), and ebselen (40 µM). Cells were then stimulated with Gas6 up to 5 minutes and Axl was immunoprecipitated. As shown in Fig. 5, antioxidants prevented the interaction between Axl and MHC-IIB as was also seen in response to H2O2 alone.
Figure 5. Gas6 dependent interaction between Axl and MHC-IIB is inhibited by antioxidants.
Cells were pretreated with 1 mM N-acetylcysteine (A) or 40 µM ebselen (B) for 30 min. Cells were then stimulated with Gas6 (100 ng/ml) for the indicated times. Cell lysates were immunoprecipitated with anti-Axl antibody and interacting MHC-IIB detected by immunoblotting with MHC-IIB antibody. Equal loading was confirmed with anti-Axl antibody (lower).
Inhibition of MHC-IIB activity inhibits MHC-IIB-Axl interaction and Axl signaling
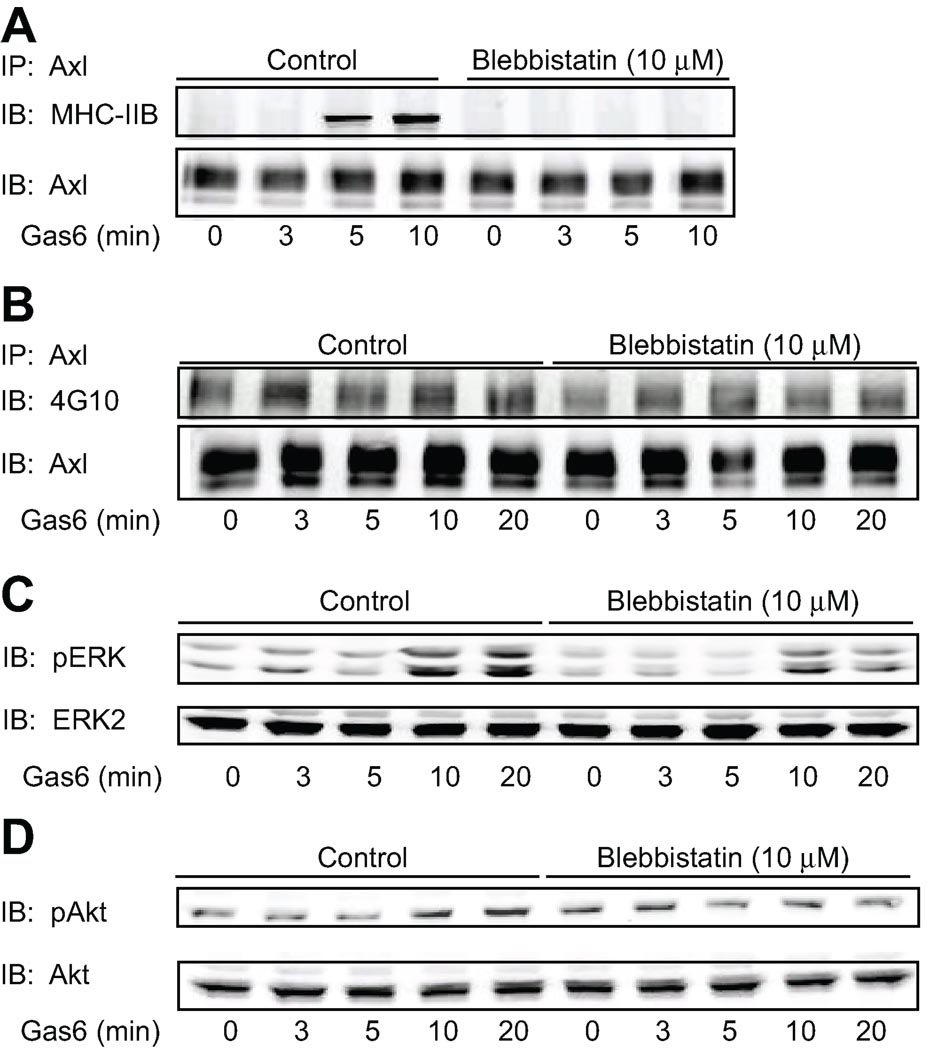
To investigate the role of the interaction between MHC-IIB and Axl on Axl dependent signaling we pre-treated VSMC with the myosin II inhibitor blebbistatin (10 µM) for 30 min followed by stimulation with Gas6 (100ng/ml) for varying times. Axl was then immunoprecipitated and interaction with MHC-IIB was determined by Western blotting. As shown in Figure 6A, treatment of cells with blebbistatin inhibited the interaction between Axl and MHC-IIB. We then determined the effect of blebbistatin on phosphorylation of Axl and Axl downstream targets. Blebbistatin inhibited tyrosine phosphorylation of Axl (and hence activation) by 50 % at 5, 10 and 20 min of Gas6 stimulation (Fig. 6B).
Figure 6. Disruption of the Axl-MHC-IIB interaction inhibits Axl signaling.
Cells were pretreated with blebbistatin (10 µM) for 30 min. Cells were then stimulated with Gas6 (100 ng/ml) for the indicated times. A. Cell lysates were immunoprecipitated with anti-Axl antibody and interacting MHC-IIB detected by immunoblotting with MHC-IIB antibody. Equal loading was confirmed with anti-Axl antibody (lower panel). B. Cell lysates were immunoprecipitated with anti-Axl antibody and immunoblotted with phospho-tyrosine 4G10 antibody. Equal loading was confirmed with anti-Axl antibody (lower panel). C. Lysates were immunoblotted with phosphospecific ERK1/2 antibody (above) and then reprobed with ERK1/2 antibody (below). (D) Lysates were immunoblotted with phosphospecific Akt antibody (above) and then reprobed with Akt antibody (below).
Activation of both ERK1/2 and Akt by Gas6 was inhibited by blebbistatin treatment in a time dependent manner (Fig.6 C and D). ERK phosphorylation in the presence of blebbistatin was decreased by approximately 2-fold compared to control at all time points, with maximal inhibition achieved at 20 min, when ERK phosphorylation was inhibited by 35% (p<0.05, n ≥ 3; Fig. 6C). Inhibition of Akt activation (approximately 1.3 fold) in the presence of blebbistatin compared to control was evident at 10 minutes and greatest following 20 min of Gas6 stimulation where Akt phosphorylation was inhibited by 20% (p<0.05, n ≥ 3; Fig. 6D). These data indicate that the interaction of MHC-IIB with Axl augments Gas6-Axl signaling in VSMC.
Association of Axl and MHC-IIB increases in injured vessels
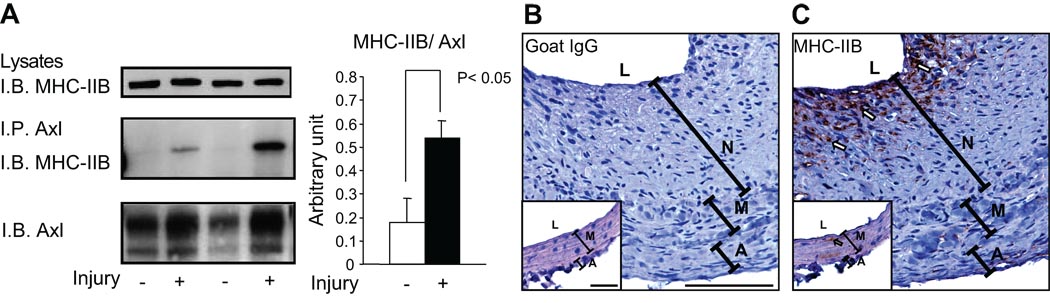
To determine whether the interaction between Axl and MHC-IIB was altered under pathologic conditions, the carotid arteries of Sprague-Dawley rats were balloon injured. At 7 days post injury, total protein was extracted from vessels and Axl was immunoprecipitated. Axl expression increased after injury (consistent with previously published findings7), while MHC-IIB total expression was unchanged (Fig. 7A). This is consistent with a previous report, in which MHC-IIB expression increased in the neointima and decreased in the media resulting in no overall change15. MHC-IIB association with Axl significantly increased even after normalization to Axl expression (Fig. 7A), suggesting that MHC-IIB association with Axl might be involved in the pathophysiology of remodeled vessels.
Figure 7. Association of Axl with MHC-IIB is increased after rat carotid balloon injury.
Rat carotid arteries were harvested 7 days after balloon injury. A. Lysates from left (injured; I) and right (uninjured; UI) carotids were immunoprecipitated with anti-Axl antibody. MHC-IIB expression (upper panel), MHC-IIB interacting with Axl (middle panel) and Axl expression (lower panel) were determined using anti-MHC-IIB antibody and anti-Axl antibody. Densitometry data of Axl and interacting MHC-IIB was quantified and MHC-IIB bound to Axl was normalized to Axl expression. Cross sections of injured rat left carotid arteries were stained with either negative control goat IgG (B) or MHC-IIB (C) antibody to detect localization of MHC-IIB 14 days following balloon injury. MHC-IIB was highly localized to neointimal cells lining the lumen (C). Images are 60× magnification, bar indicates 100µM. Insets are contralateral right carotid arteries demonstrating that there is a very low amount of MHC-IIB staining in uninjured arteries.
We previously demonstrated that Axl expression increased in neointima following balloon injury7. Specifically, Axl was highly expressed in the subluminal neointima. Similarly, MHC-IIB expression in the carotid artery is increased in the neointima 14 days following injury (Fig. 7C vs. 7B). The latter suggests that under pathophysiological conditions there is an increase in MHC-IIB expression in the neointima. Axl and MHC-IIB expression localize in the same region in the remodeled artery.
Discussion
The major finding of the present study is that ROS stimulate interaction between Axl and glutathiolated myosin heavy chain MHC-IIB, which augments Axl signaling. This interaction is induced by both ROS and Gas6, the endogenous ligand for Axl. Importantly, we show that Axl and MHC-IIB association regulates Axl signaling and occurs in the carotid artery after vascular injury. We propose that the interaction between MHC-IIB and Axl provides a direct link between receptor signaling and cytoskeletal molecular motors that are crucial for cell migration. This is the first study to demonstrate an interaction between receptor tyrosine kinases and non-muscle myosin II. Interestingly, a constitutive interaction between the G protein coupled receptor, CXCR4, and MHC-IIA has been shown to increase β-arrestin mediated receptor endocytosis, thus downregulating receptor signaling23. In contrast, we have found a ROS-induced interaction between MHC-IIB and Axl that increases Axl signaling.
The exact mechanism by which stimulation of Axl increases intracellular ROS is unclear although we propose it is mediated through NADPH oxidase. In our system ROS was generated intracellularly by two activators of Axl, H2O2 and Gas6. ROS can increase glutathioloation of redox sensitive proteins. We found that MHC-IIB undergoes glutathiolation upon exposure to ROS. Glutathiolation protects proteins from irreversible modification when exposed to ROS, and can also alter protein activity and ability to interact with other proteins. As examples, reversible glutathiolation of actin regulates its polymerization24, and glutathiolation of annexin A2 mediates its interaction with phospholipid liposomes and actin25. In VSMC, angiotensin II induces glutathiolation of Ras, which increases activity of Ras and may contribute to hypertrophy26. We hypothesize that glutathiolation of MHC-IIB causes a conformational change promoting dissociation from actin, thereby allowing interaction with Axl at the plasma membrane. Glutathiolation may also alter the intrinsic activity of MHC-IIB. Further studies will elucidate the role of glutathiolation in MHC-IIB function.
Disruption of the Axl-MHC-IIB interaction decreased Axl phosphorylation and downstream activation of ERK1/2 and Akt in a time dependent manner. MHC-IIB may regulate intracellular signaling of Axl by attenuating termination of receptor signaling, possibly by inhibiting an Axl protein tyrosine phosphatase and/or regulating receptor endocytosis and degradation. MHC-IIB may also participate as part of the mechanism that stabilizes Axl interacting proteins. For example, a previous study has shown that activation of the EGF receptor induces formation of a signaling complex that includes myosin, Shc, GRB2, Nck, PAK, caldesmon, and myosin light chain (MLC) kinase27.
Cell migration involves the coordination of signal transduction pathways and cytoskeletal modifications. Gas6 activation of Axl in VSMC has been shown to stimulate ERK1/2 and Akt which are required to promote migration and inhibit apoptosis, respectively4, 21. Several studies suggest that MHC-IIB has a role in directed migration of cells12–14,28–29. MHC-IIB is required for neuronal growth cone advancement28. Overexpression of dominant negative mutants of the MHC-IIB carboxyl-terminus result in abnormal cell shape, focal adhesions and chemotaxis29. Analysis of cell migration in MHC-IIB null fibroblasts demonstrated that MHC-IIB is required for directed cell movement by coordinating protrusive activities and stabilizing the cell periphery12. Phosphorylation of MHC-IIB regulates both its motor activity and its ability to assemble into filaments. In aortic smooth muscle angiotensin II stimulation results in a Rho kinase mediated phosphorylation of MHC-IIB yielding an increase in contractile force. This effect of angiontensin II in transgenic MHC-IIB knockout mice was diminished by 25%30.
We demonstrated that H2O2 significantly increases intracellular oxidative stress. In addition we have shown that oxidative stress can activate Axl signaling6 although the way by which this occurs is unclear. Our data suggest that one potential mechanism may be redox-dependent glutathiolation of MHC-IIB promoting its interaction with Axl. The finding that Axl stimulation leads to the activation of protein kinase C and Rac1 indicates that under conditions of oxidative stress, an interaction between MHC-IIB and Axl provides a direct link between receptor signaling and cytoskeletal molecular motors that are crucial for migration. Therefore, it is conceivable that Axl and MHC-IIB could regulate spatial location and localized activity of MHC-IIB thus providing a mechanism for localized and directed cell movement.
When grown in tissue culture VSMC undergo a phenotypic transition from the normal “contractile” phenotype observed in vivo to a synthetic type that resembles the cell type in atherosclerosis and restenosis. MHC-IIB is specifically expressed in VSMC of the synthetic type. Increased MHC-IIB expression is also apparent in atherosclerotic lesions, media of balloon-injured vessels, and in hypertensive arteries15–18. This strongly suggests that increased expression of MHC-IIB contributes to the increased migratory response in these pathological conditions.
Our data demonstrated that MHC-IIB is highly expressed in the subluminal neointima, a region consisting of highly proliferative cells, suggests that MHC-IIB may play a role in cell proliferation under pathological conditions. In support of our finding, Takeda et al31 showed that cardiac myocytes lacking MHC-IIB exhibited decreased proliferation as well as cell hypertophy. We have demonstrated that Axl expression is also increased in the subluminal neointima congruent with MHC-IIB expression. Specifically, Axl is highly upregulated in balloon injured carotid arteries with a time course paralleling that of neointima formation and Axl expression is increased in VSMC exposed to thrombin and angiotensin II7. In addition neointima formation is decreased in Axl knockout mice in response to cuff injury or low flow6, 32. Furthermore, genetic deletion of Axl was shown to prevent vascular dysfunction and remodeling in salt-induced hypertension8. Specifically, Axl knockout mice had reduced systolic blood pressure and improved vasorelaxation8. There is also evidence suggesting an important role for Axl in the vascular response to injury mediated by ROS. Importantly, Axl is activated by H2O2, which is increased in vascular injury7. Our results demonstrating an interaction between Axl and MHCIIB provide a plausible mechanism for how Axl regulates the vascular response in pathological conditions.
Perspectives.
We propose the following model (Figure S2, please see http://hyper.ahajournals.org): Ligand dependent (Gas6) and independent (H2O2) activation of Axl increases in intracellular ROS that promote glutathiolation of MHC-IIB. This results in Axl and MHC-IIB interacting and activating ERK and pro-migratory signaling. Given the importance of cell oxidative stress and cell migration in vascular pathologies it is highly likely that Axl-MHCIIB interaction increases VSMC migration relevant to the pathogenesis of vascular disease.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by an American Heart Association SDG grant (Award No. 05535197N, to M.E.C.) and a National Institutes of Health Grant HL68286 (to B.C.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Melaragno MG, Fridell YC, Berk BC. The Gas6/Axl system. A novel regulator of vascular cell function. Trends Cardiovasc Med. 1999;9:250–253. doi: 10.1016/s1050-1738(00)00027-x. [DOI] [PubMed] [Google Scholar]
- 2.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, Masiakowski P, Ryan TE, Tobkes NJ, Chen DH, DiStefano PS, Long GL, Basillico C, Goldfarb MP, Lemke G, Glass DJ, Yancopoulos GD. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 3.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ, Yanagihara D, Bennett L, Sylber M, Merewether LA, Tseng A, Escobar E, Liu T, Yamane HK. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–626. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- 4.Melaragno MG, Cavet ME, Yan C, Tai LK, Jin ZG, Haendeler J, Berk BC. Gas6 inhibits apoptosis in vascular smooth muscle: role of Axl kinase and Akt. J Mol Cell Cardiol. 2004;37:881–887. doi: 10.1016/j.yjmcc.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Fridell YW, Villa J, Jr, Attar EC, Liu ET. GAS6 induces Axl-mediated chemotaxis of vascular smooth muscle cells. J Biol Chem. 1998;273:7123–7126. doi: 10.1074/jbc.273.12.7123. [DOI] [PubMed] [Google Scholar]
- 6.Konishi A, Aizawa T, Mohan A, Korshunov VA, Berk BC. Hydrogen peroxide activates the gas6-axl pathway in vascular smooth muscle cells. J Biol Chem. 2004;279:28766–28770. doi: 10.1074/jbc.M401977200. [DOI] [PubMed] [Google Scholar]
- 7.Melaragno MG, Wuthrich DA, Poppa V, Gill D, Lindner V, Berk BC, Corson MA. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ Res. 1998;83:697–704. doi: 10.1161/01.res.83.7.697. [DOI] [PubMed] [Google Scholar]
- 8.Korshunov VA, Daul M, Massett MP, Berk BC. Axl mediates vascular remodeling induced by deoxycorticosterone acetate-salt hypertension. Hypertension. 2007;50:1057–1062. doi: 10.1161/HYPERTENSIONAHA.107.096289. [DOI] [PubMed] [Google Scholar]
- 9.Yagil C, Hubner N, Monti J, Schulz H, Sapojnikov M, Luft FC, Ganten D, Yagil Y. Identification of hypertension-related genes through an integrated genomic-transcriptomic approach. Circ Res. 2005;96:617–625. doi: 10.1161/01.RES.0000160556.52369.61. [DOI] [PubMed] [Google Scholar]
- 10.Klatt P, Pineda Molina E, Perez-Sala D, Lamas S. Novel application of S-nitrosoglutathione-Sepharose to identify proteins that are potential targets for S-nitrosoglutathione-induced mixed-disulphide formation. Biochem J. 2000;349:567–578. doi: 10.1042/0264-6021:3490567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Huang FL, Huang KP. Glutathiolation of proteins by glutathione disulfide S-oxide derived from S-nitrosoglutathione. Modifications of rat brain neurogranin/RC3 and neuromodulin/GAP-43. J Biol Chem. 2001;276:3098–3105. doi: 10.1074/jbc.M008260200. [DOI] [PubMed] [Google Scholar]
- 12.Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straussman R, Even L, Ravid S. Myosin II heavy chain isoforms are phosphorylated in an EGF-dependent manner: involvement of protein kinase C. J Cell Sci. 2001;114:3047–3057. doi: 10.1242/jcs.114.16.3047. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher PJ, Jin Y, Killough G, Blue EK, Lindner V. Alterations in expression of myosin and myosin light chain kinases in response to vascular injury. Am J Physiol Cell Physiol. 2000;279:C1078–C1087. doi: 10.1152/ajpcell.2000.279.4.C1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Packer CS, Roepke JE, Oberlies NH, Rhoades RA. Myosin isoform shifts and decreased reactivity in hypoxia-induced hypertensive pulmonary arterial muscle. Am J Physiol. 1998;274:L775–L785. doi: 10.1152/ajplung.1998.274.5.L775. [DOI] [PubMed] [Google Scholar]
- 17.Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F. cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. J Biol Chem. 1991;266:3768–3773. [PubMed] [Google Scholar]
- 18.Aikawa M, Sakomura Y, Ueda M, Kimura K, Manabe I, Ishiwata S, Komiyama N, Yamaguchi H, Yazaki Y, Nagai R. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. 1997;96:82–90. doi: 10.1161/01.cir.96.1.82. [DOI] [PubMed] [Google Scholar]
- 19.Ishida T, Ishida M, Suero J, Takahashi M, Berk BC. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J Clin Invest. 1999;103:789–797. doi: 10.1172/JCI4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan DM, Wehr NB, Fergusson MM, Levine RL, Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 21.Cavet ME, Smolock EM, Ozturk OH, World C, Pang J, Konishi A, Berk BC. Gas6-axl receptor signaling is regulated by glucose in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:886–891. doi: 10.1161/ATVBAHA.108.162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahajan NP, Earp HS. An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J Biol Chem. 2003;278:42596–42603. doi: 10.1074/jbc.M305817200. [DOI] [PubMed] [Google Scholar]
- 23.Rey M, Valenzuela-Fernandez A, Urzainqui A, Yanez-Mo M, Perez-Martinez M, Penela P, Mayor F, Jr, Sanchez-Madrid F. Myosin IIA is involved in the endocytosis of CXCR4 induced by SDF-1{alpha} J Cell Sci. 2007;120:1126–1133. doi: 10.1242/jcs.03415. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, McCullough KD, Wang XJ, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-g1 activation enhances cell survival. J Biol Chem. 2001;11:11. doi: 10.1074/jbc.M102693200. [DOI] [PubMed] [Google Scholar]
- 25.Caplan JF, Filipenko NR, Fitzpatrick SL, Waisman DM. Regulation of annexin A2 by reversible glutathionylation. J Biol Chem. 2004;279:7740–7750. doi: 10.1074/jbc.M313049200. [DOI] [PubMed] [Google Scholar]
- 26.Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 27.McManus MJ, Boerner JL, Danielsen AJ, Wang Z, Matsumura F, Maihle NJ. An oncogenic epidermal growth factor receptor signals via a p21-activated kinase-caldesmon-myosin phosphotyrosine complex. J Biol Chem. 2000;275:35328–35334. doi: 10.1074/jbc.M005399200. [DOI] [PubMed] [Google Scholar]
- 28.Wylie SR, Wu PJ, Patel H, Chantler PD. A conventional myosin motor drives neurite outgrowth. Proc Natl Acad Sci U S A. 1998;95:12967–12972. doi: 10.1073/pnas.95.22.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Ya'acov A, Ravid S. Epidermal growth factor-mediated transient phosphorylation and membrane localization of myosin II-B are required for efficient chemotaxis. J Biol Chem. 2003;278:40032–40040. doi: 10.1074/jbc.M306948200. [DOI] [PubMed] [Google Scholar]
- 30.Yuen SL, Ogut O, Brozovic FV. Nonmuscle myosin is regulated during smooth muscle contraction. Am J Physiol Heart Circ Physiol. 2009;297:H191–H199. doi: 10.1152/ajpheart.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ Res. 2003;93:330–337. doi: 10.1161/01.RES.0000089256.00309.CB. [DOI] [PubMed] [Google Scholar]
- 32.Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.