Abstract
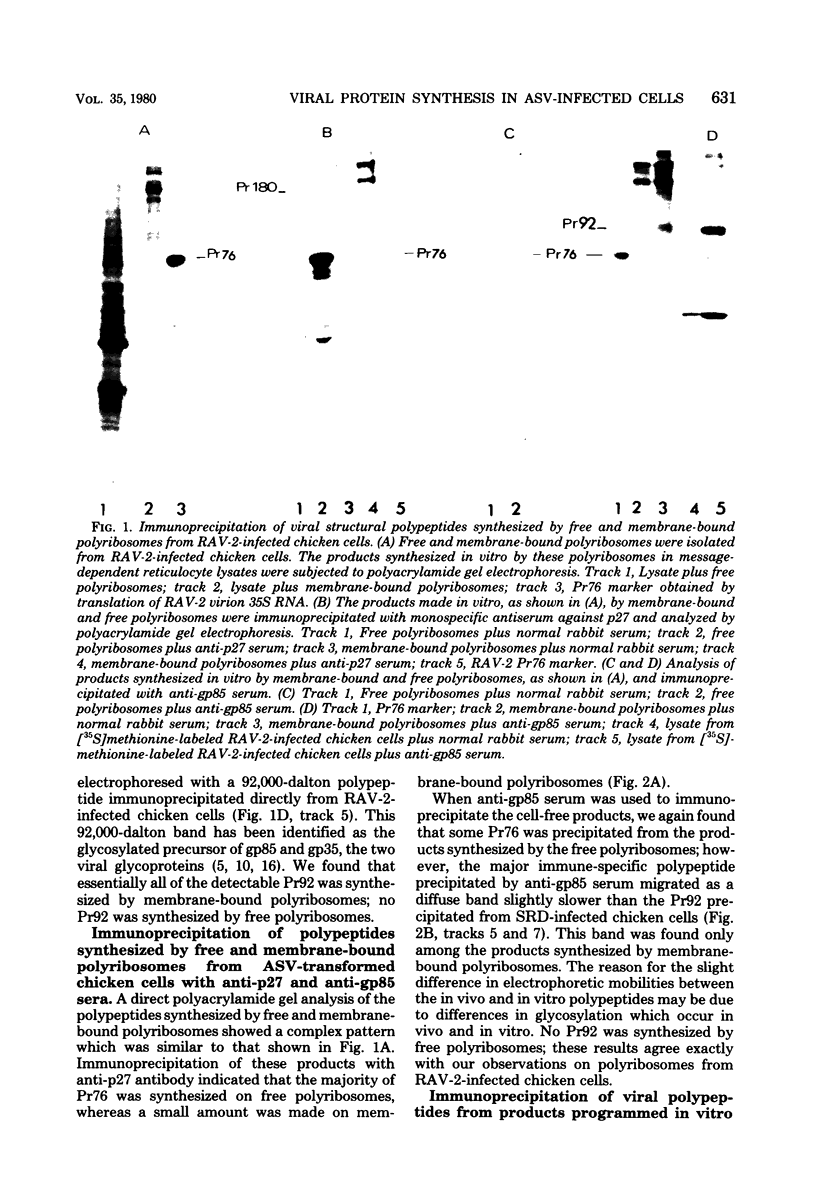
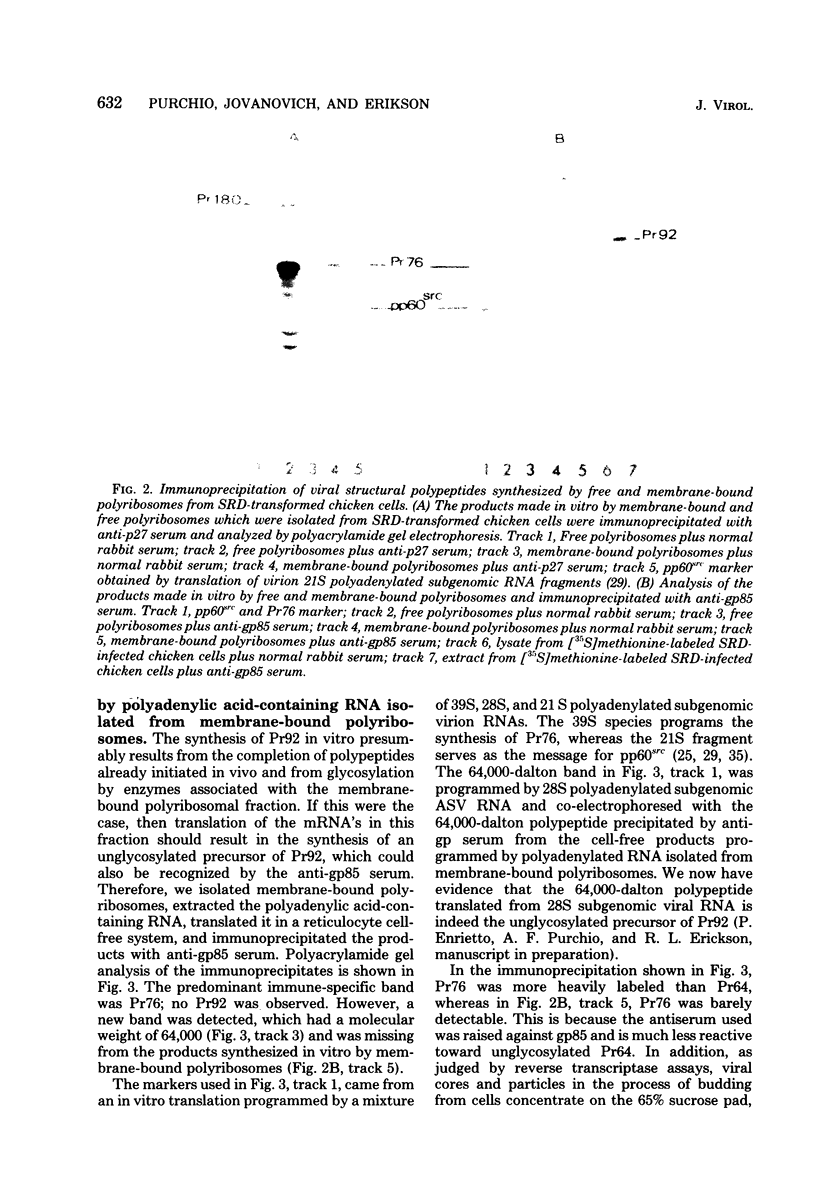
We determined the sites of synthesis of avian sarcoma virus-specific proteins in infected chicken cells by immunoprecipitation of the products synthesized in vitro by free and membrane-bound polyribosomes; 85% of Pr76, the precursor of the viral internal structural proteins (group-specific antigens), was synthesized on free polyribosomes, and 15% was synthesized on membrane-bound polyribosomes. Pr92, the lycosylated precursor of the viral glycoproteins (gp85 and gp35), was synthesized exclusively on membrane-bound polyribomes, which is consistent with its role as a membrane protein. When we investigated the site of synthesis of pp60src, the product of the avian sarcoma virus src gene, we found that 90% was synthesized on free polyribosomes, whereas 10% was detected on membrane-bound polyribosomes. The implications of these results with respect to the subcellular location of pp60src are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson E., Erikson R. L. Antibody to virion structural proteins in mammals bearing avian sarcoma virus-induced tumors. Virology. 1978 Feb;84(2):429–433. doi: 10.1016/0042-6822(78)90259-3. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Steinbaugh P. J., Erikson R. L. Characterization of the avian sarcoma virus protein p60src. Virology. 1978 Nov;91(1):130–140. doi: 10.1016/0042-6822(78)90361-6. [DOI] [PubMed] [Google Scholar]
- Buchhagen D. L., Hanafusa H. Intracellular precursors to the major glycoprotein of avian oncoviruses in chicken embryo fibroblasts. J Virol. 1978 Mar;25(3):845–851. doi: 10.1128/jvi.25.3.845-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggelmann H. Biosynthesis of an unglycosylated envelope glycoprotein of Rous sarcoma virus in the presence of tunicamycin. J Virol. 1979 Jun;30(3):799–804. doi: 10.1128/jvi.30.3.799-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- England J. M., Bolognesi D. P., Dietzschold B., Halpern M. S. Evidence that a precursor glycoprotein is cleaved to yield the major glycoprotein of avian tumor virus. J Virol. 1977 Feb;21(2):810–814. doi: 10.1128/jvi.21.2.810-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Collett M. S., Erikson R. L. In vitro synthesis of a functional avian sarcoma virus transforming-gene product. Nature. 1978 Aug 31;274(5674):919–921. doi: 10.1038/274919a0. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks S. J., Drysdale J. W., Munro H. N. Preferential synthesis of ferritin and albumin by different populations of liver polysomes. Science. 1969 May 2;164(3879):584–585. doi: 10.1126/science.164.3879.584. [DOI] [PubMed] [Google Scholar]
- Kamine J., Buchanan J. M. Processing of 60,000-dalton sarc gene protein synthesized by cell-free translation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4399–4403. doi: 10.1073/pnas.75.9.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. Extracellular cleavage of the glycoprotein precursor of Rous sarcoma virus. J Virol. 1979 Jan;29(1):285–292. doi: 10.1128/jvi.29.1.285-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Goldberg A. R. Evidence that the src gene product of Rous sarcoma virus is membrane associated. Virology. 1980 Feb;101(1):25–40. doi: 10.1016/0042-6822(80)90480-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Lodish H. F. Biosynthesis of reticulocyte membrane proteins by membrane-free polyribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1526–1530. doi: 10.1073/pnas.70.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Small B. Membrane proteins synthesized by rabbit reticulocytes. J Cell Biol. 1975 Apr;65(1):51–64. doi: 10.1083/jcb.65.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Bolognesi D. P. Structure and morphogenesis of type-C retroviruses. Adv Cancer Res. 1978;28:63–89. doi: 10.1016/s0065-230x(08)60646-6. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Rohrschneider L. R. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979 Jan;16(1):11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]