Abstract
Parkinson’s disease (PD) is a common neurodegenerative movement disorder characterized by extensive degeneration of dopaminergic neurons in the nigrostriatal system. Neurochemical and neuropathological analyses clearly indicate that oxidative stress, mitochondrial dysfunction, neuroinflammation and impairment of the ubiquitin-proteasome system (UPS) are major mechanisms of dopaminergic degeneration. Evidence from experimental models and postmortem PD brain tissues demonstrates that apoptotic cell death is the common final pathway responsible for selective and irreversible loss of nigral dopaminergic neurons. Epidemiological studies imply both environmental neurotoxicants and genetic predisposition are risk factors for PD, though the cellular mechanisms underlying selective dopaminergic degeneration remain unclear. Recent progress in signal transduction research is beginning to unravel the complex mechanisms governing dopaminergic degeneration. During 12th International Neurotoxicology meeting, discussion at one symposium focused on several key signaling pathways of dopaminergic degeneration. This review summarizes two novel signaling pathways of nigral dopaminergic degeneration that have been elucidated using neurotoxicity models of PD. Dr. Anumantha Kanthasamy described a cell death pathway involving the novel protein kinase C delta isoform (PKCδ) in oxidative stress-induced apoptotic cell death in experimental models of PD. Dr. Ajay Rana presented his recent work on the role of mixed lineage kinase-3 (MLK3) in neuroinflammatory processes in neurotoxic cell death. Collectively, PKCδ and MLK3 signaling pathways provide new understanding of neurodegenerative processes in PD, and further exploration of these pathways may translate into effective neuroprotective drugs for the treatment of PD.
Keywords: Protein kinase Cδ, MLK3, Parkinson’s disease, neurotoxicity, Dieldrin, Manganese, environmental factors, Translational research
1.0 Role of PKCδ Signaling in Neurotoxicity Models of Parkinson’s Disease (A.G.K)
Parkinson’s disease (PD) is a devastating neurodegenerative disorder affecting several million people worldwide. It inflicts a tremendous social and economic burden on modern society where the incidence of the disease increases with age. Currently, the mean age of onset is around 55 years. In all cases, the clinical features which characterize PD, including resting tremor, bradykinesia, and postural instability (Fahn and Sulzer, 2004), are progressive. Distinct among the pathological features of PD is the significant loss of dopaminergic neurons in the substantia nigra leading to a dramatic depletion of dopamine in the striatum. The current PD treatment approach employing levodopa, an intermediate molecule in the genesis of dopamine, is ranked among the most remarkable success stories in medical history. However, the drug confers only symptomatic relief of what remains an inexorably progressive and ultimately fatal neurodegenerative disorder As a result, the need for novel neuroprotective agents designed to interfere with the basic pathogenic mechanism of cell death in PD are clearly needed.
1.1 Environmental risk factors and Parkinson’s disease
The specific etiology of PD is still elusive, although results from extensive studies reveal that both accumulated environmental toxicant exposure and genetic mutations contribute to the onset of PD (Thomas, 2009)(Gerlach and Riederer, 1999). While sporadic cases of PD are largely attributed to genetic causes (approximately 90 percent), environmental toxin exposure triggering neuronal apoptosis is considered a dominant risk factor in the development of this disease. Most compelling among this evidence was the discovery of a synthetic heroin analog, 1,2,3,6-methyl -phenyl-tetrahydropyridine (MPTP), that was noted to produce a syndrome clinically and pathologically resembling PD in a group of narcotics addicts (Langston et al., 1983). The neurotoxicology of MPTP has subsequently been well characterized; MPP+, the active metabolite of MPTP, enters dopaminergic neurons via a dopamine transporter and inhibits the complex I of the mitochondrial respiratory chain, thereby selectively causing toxicity to dopaminergic neurons (Nicklas et al., 1985). In addition to MPTP, a variety of additional exogenous or endogenous toxic agents have been recognized to cause PD-like syndromes including dopamine and its metabolites, certain metals, and several agricultural chemicals (Kanthasamy et al., 2005). Many epidemiological, case-control and postmortem studies provide evidence for the involvement of heavy metals in PD pathogenesis (Aschner et al., 2009; Uversky et al., 2001). Of these metals, manganese (Mn) gains more attention than others because of its role in the human neurological condition known as manganism, which is characterized by clinical signs and morphological lesions similar to those seen in PD. Despite the similarities between manganism and PD, some distinct clinical and pathological differences are evident between the diseases. The cellular mechanisms underlying the neurotoxicology of manganese are still unclear although accumulating evidence indicates that neuronal apoptosis resulting from oxidative stress and mitochondrial dysfunction may play an important role. With regard to agricultural chemicals and PD etiology, dieldrin, an organochlorine pesticide, has been associated with PD based on postmortem and epidemiological studies (Kanthasamy et al., 2005; Richardson et al., 2006). Postmortem analysis revealed that dieldrin was present in PD brains, but not in control brains (Fleming et al., 1994). Moreover, a case-control study also demonstrated that dieldrin was a risk factor in PD (Semchuk et al., 1991).
1.2 Involvement of apoptosis in Parkinson’s disease
Apoptosis or programmed cell death is a genetically regulated death process of cells involving caspase activation and a lack of cell swelling (Williams and Smith, 1993). Apoptosis is recognized as a key fundamental and indispensable process in any normal cell function. Aberrant regulation of apoptosis is a common feature in many diseases including neurodegenerative diseases and cancer and is now widely accepted as a crucial cause of dopaminergic neuronal death in PD. This is based on extensive postmortem analysis of PD brains as well as experimental models (Anglade et al., 1997; Jellinger and Stadelmann, 2000; Kaul et al., 2003; Mochizuki et al., 1996; Tatton and Kish, 1997; Yuan and Yankner, 2000). Induction of apoptosis by both environmental insults and PD genetic predisposition suggests that biochemical events involved in the cell death process are highly conserved despite the differences in the nature of neurotoxic insults.
Impairment of mitochondrial function by neurotoxicants is known to result in the selective degeneration of dopaminergic neurons (Kanthasamy et al., 1994; Pallanck and Greenamyre, 2006; Schapira, 2008; Winkhofer and Haass, 2009). We and others have shown that neurotoxins such as MPP+ (Kaul et al., 2003), dieldrin (Kitazawa et al., 2004), manganese (Latchoumycandane et al., 2005), rotenone(Sherer et al., 2003; Testa et al., 2005), and paraquat (McCormack et al., 2005) activate major events of the apoptotic pathway, including cytochrome C release, caspase-3 activation, and DNA fragmentation. Thus, apoptosis is the primary cell death process of dopaminergic neurons and therefore, understanding the molecular mechanisms of apoptosis continues to be an important area of investigation in neurotoxicology.
1.3 Protein kinase C delta (PKCδ) and neuronal apoptosis
The detailed understanding of the molecular and cellular mechanisms in neuronal apoptosis offer more promising points of entry into the therapeutics of PD. Already, many signaling pathways have been identified in the neurodegenerative processes of dopaminergic neurons. During the past several years, our laboratory has focused on identifying key players involved in dopaminergic neuronal apoptosis and we found that protein kinase C delta (PKCδ) is an oxidative stress-sensitive kinase, which functions as a key mediator in apoptotic cell death in PD (Anantharam et al., 2002; Kanthasamy et al., 2003; Kaul et al., 2005; Kaul et al., 2003; Latchoumycandane et al., 2005; Yang et al., 2004). We also have developed translational PD therapeutic strategies targeting the proapoptotic PKCδ cell death signaling pathway (Kanthasamy et al., 2006; Zhang et al., 2007a). At least 12 isoforms have been identified and further divided into three groups: conventional PKCs (α, βI, βII, γ), novel PKCs (δ, ε, η, θ), and atypical PKCs (ζ, ι, λ), each based on its lipid requirement and dependency on Ca2+ for activation. PKCδ was first discovered by Gschwendt et al. (Gschwendt et al., 1986), and belongs to the protein kinase C serine/threonine kinase family. This family of kinase plays a role in the regulation of multiple cellular responses, including proliferation, cell cycle progression, differentiation, survival, and apoptosis (Dempsey et al., 2000; Kanthasamy et al., 2003). PKCδ, a Ca2+-independent isoform, is ubiquitously expressed in most tissues including brain, spleen, ovary, lung and uterus (Leibersperger et al., 1991). A survey of expression of PKC isoforms in the rat brain indicates that PKCδ is highly expressed in the thalamus, septal nuclei, and hippocampal CA1 pyramidal cell layer (Naik et al., 2000). Recently, we conducted double immunostaining analysis and confocal microscopy to evaluate the expression of PKCδ in nigral dopaminergic neurons (Zhang et al., 2007b). Our results revealed that PKCδ is highly expressed in mouse nigral tissues, and more importantly, PKCδ co-localizes with tyrosine hydroxylase (TH) in the substantia nigra. Also, we showed that PKCδ negatively regulates TH activity and dopamine synthesis, demonstrating an anatomical and functional relationship of the kinase in the nigrostriatal pathway (Zhang et al., 2007b).
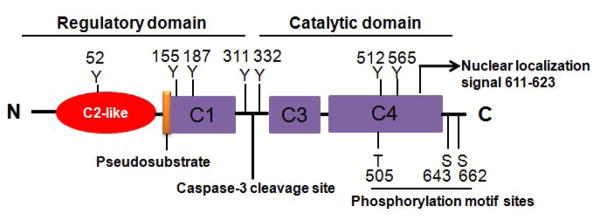
The domain structure of PKCδ is presented in Fig. 1. Consistent with other PKC isoforms, PKCδ consists of a regulatory domain (N-terminus) and a catalytic domain (C-terminus). Like other conventional and novel PKC isoforms, PKCδ is primarily activated by a lipid-mediated mechanism involving its translocation from cytosol to membrane. Two other pathways of PKCδ activation also have been elucidated: phosphorylation and proteolytic activation (Brodie and Blumberg, 2003; Kanthasamy et al., 2003; Kikkawa et al., 2002). Reportedly, phosphorylation of Thr-505, Ser-643, and Ser-662 in the activation loop can increase its kinase activity (Toker, 1998). In addition to the phosphorylation of Thr/Ser sites, tyrosine phosphorylation at tyrosine residues Tyr-52, Tyr-155, Tyr-187, Tyr-311, Tyr-332, and Tyr-565 has also been implicated in modulating activity (Gschwendt, 1999) in various cell types. Various types of stimulation reportedly induce the tyrosine phosphorylation of PKCδ (Kikkawa et al., 2002). For example, treatment with the known oxidative stress-inducing agent hydrogen peroxide (H2O2) reportedly caused Tyr-311 and Tyr-332 phosphorylation of PKCδ (Konishi et al., 2001). We have found that under certain stimulation, e.g., H2O2, the phosphorylation of Tyr-311 on PKCδ is particularly important for the proteolytic activation of PKCδ in dopaminergic neurons (Kaul et al., 2005). Because multiple tyrosine residues on PKCδ can be phosphorylated by upstream kinase, the effect of tyrosine phosphorylation may be different, depending on both the position of the phosphorylated tyrosine and the specific cellular context. We and others have identified an additional proteolytic activation mechanism of PKCδ that results in catalytic and regulatory fragments due to proteolysis. The cleavage of PKCδ is mediated by caspase-3, resulting in 41-kDa catalytically active and 38-kDa regulatory fragments. Compared to membrane translocation and serine/tyrosine phosphorylation, proteolytic cleavage of PKCδ causes a persistent activation of the kinase. Importantly, PKCδ proteolytic activation mediates apoptotic cell death.
Figure 1.
Schematic representation of the domain structure of PKCδ. Caspase-3 cleavage site, nuclear localization signal, and the phosphorylation sites including serine (S), threonine (T), and tyrosine (Y) residues are depicted.
1.4 PKCδ proteolytic activation in neurotoxicity models of dopaminergic degeneration
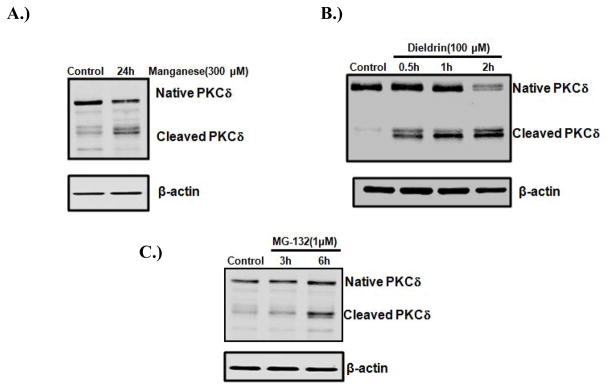
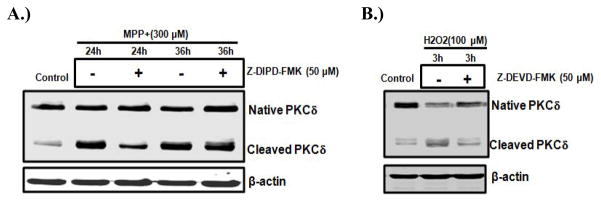
The proteolytic activation of PKCδ has been implicated in apoptosis in many cell types (Choi et al., 2006; D’Costa and Denning, 2005; Ryer et al., 2005). Our recent studies have characterized a critical role for the caspase-3-dependent proteolytic activation of PKCδ in oxidative stress-induced dopaminergic cell death in cell culture models of PD. In rat mesencephalic dopaminergic neuronal N27 cell models, a dose-dependent and time-dependent increase in the proteolytic activation of PKCδ was identified following exposure to dopaminergic neurotoxins such as inorganic manganese (Latchoumycandane et al., 2005), the organic manganese found in the gasoline additive methylcyclopentadienyl manganese tricarbonyl (MMT) (Anantharam et al., 2002), the agriculture chemical dieldrin (Kitazawa et al., 2003), MPP+ (Kaul et al., 2003; Yang et al., 2004), the proteasome inhibitor MG-132 (Sun et al., 2008), or the oxidative stress inducing agent H2O2 (Kaul et al., 2005). We also found that the active PKCδ isoform was not translocated to the cell membrane during neurotoxic insults, suggesting that the lipid-mediated activation mechanism is not involved in this process. Figure 2 shows PKCδ cleavage in N27 cells increased after manganese, dieldrin, or MG-132 treatment. Furthermore, using both pharmacological inhibitors (PKCδ specific inhibitor rottlerin; and caspase-3 inhibitors z-DEVD-fmk or z-DIPD-fmk) and genetic tools (PKCδ siRNA or a PKCδ cleavage-resistant mutant), we demonstrated that the caspase-3 dependent proteolytic activation of PKCδ plays an important role in neurotoxicant-induced apoptotic death (Kanthasamy et al., 2006; Sun et al., 2008; Yang et al., 2004). As shown in Figure 3, the caspase-3 inhibitor z-DEVD-fmk and the PKCδ cleavage site peptide inhibitor z-DIPD-fmk effectively blocked the Parkinsonian toxicant MPP+ (300μM) and the prooxidant H2O2 (100 μM) induced PKCδ cleavage. We demonstrated that the PKCδ cleavage site-specific peptide inhibitor z-DIPD-fmk was more potent than the general caspase-3 inhibitor z-DEVD-fmk in protecting dopaminergic neurons against apoptotic cell death, suggesting the possibility of a novel neuroprotective strategy targeting PKCδ proteolytic activation. We have noted that the proteolytically cleaved PKCδ catalytic fragment translocates into the nucleus. In the nucleus, PKCδ can induce phosphorylation of lamin B (Cross et al., 2000). Several other proteins also interact with PKCδ, including DNA-dependent protein kinase (DNA-PK) (Bharti et al., 1998) and p73 (Ren et al., 2002). Additionally, a positive feedback amplification loop between PKCδ and caspases-3 has been discovered by our laboratory. We found that the proteolytic activation of PKCδ regulates upstream caspase-3 activity, thus suggesting that PKCδ may function as both a mediator and signal amplifier during the neurotoxin-induced apoptotic pathway.
Figure 2.
PKCδ cleavage in neurotoxicity cell culture models of PD. N27 dopaminergic neuronal cells were treated with environmental neurotoxicants manganese, dieldrin, or the classic proteasome inhibitor MG-132. After treatment, cells were collected and subjected to Western blot analysis of PKCδ. Native PKCδ (~74KDa) and cleaved fragments (~42KDa) are shown in each panel.
Figure 3.
Effect of caspase inhibitors on oxidative stress induced PKCδ cleavage in cell culture models of PD. N27 dopaminergic cells were treated with prooxidant H2O2 or the Parkinsonian toxicant MPP+ in the presence or absence of either 50 μM Z-DIPD-FMK or 50 μM z-DEVD-FMK. After treatment, cells were collected and subjected to Western blot analysis of PKCδ.
2.0 Mixed Lineage kinase-3 Signaling: Relevance to Neuroinflammatory Processes in Neurotoxic Cell Death (AR)
2.1 Mixed Lineage Kinases (MLKs) and neuronal apoptosis
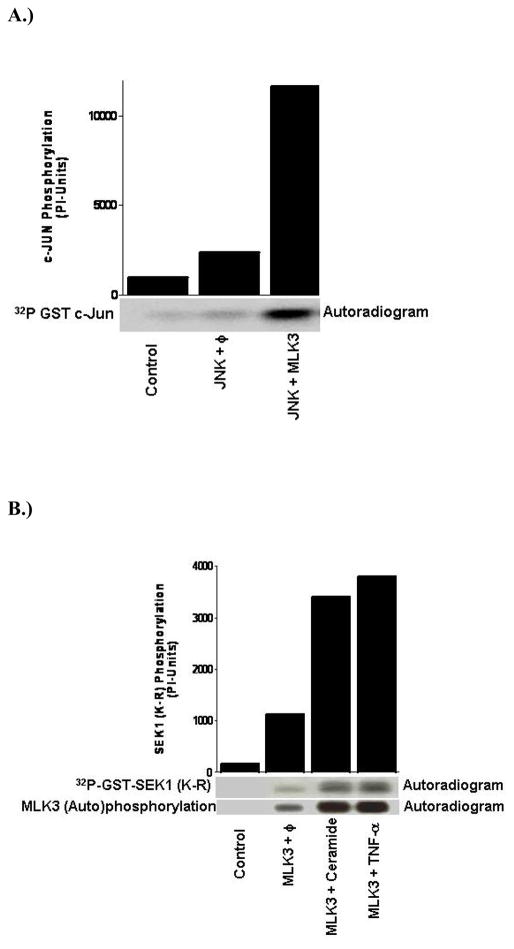
For the last several years our laboratory has focused on the dissection of the detailed cell signaling network mediated via a relatively novel and new family of kinases called Mixed Lineage Kinases (MLK). MLK are unique in that all family members contain signature sequences of Ser/Thr and Tyr kinases within their catalytic domains and thus they are called hybrid or mixed kinases. We have shown that MLK3, a member of MLK family, acts as MAPKKK and specifically activates the jun-N-terminal kinase (JNK) (Figure 4A) (Rana et al., 1996). In the JNK pathway, the MAP3K members, like mixed lineage kinases (MLKs), phosphorylate MAP2K members SEK1/MKK4 and MKK7 (Rana et al., 1996; Gallo et al., 2002). The activated MAP2K subsequently phosphorylate JNK at the tyrosine and threonine residues leading to phosphorylation and activation of transcription factors such as c-Jun, ELK-1 and ATF-2. The role of MLK in neuronal cell death pathways was unknown until recently when a specific inhibitor of MLK family, CEP-1347, and its analogue CEP-11004 were identified (Maroney et al., 2001). Subsequently, it was shown that a CEP compound appeared to prevent dopaminergic neuronal cell death in an MPTP model of Parakinson’s disease (PD) (Saporito et al., 1999; Teismann et al., 2003). Whether the mediated dopaminergic neuronal loss observed in this model of PD resulted from JNK activation or from an unknown pathway is still not established. However, it has been reported in studies of several cell culture models that the activation of JNK promotes cell death (Davis, 2000; Kyriakis et al., 2001) and therefore it is expected that activation of the MLK-JNK pathway also results in neuronal cell death. The CEP compounds capable of inhibiting MLK activation are not specific to a particular isoform and therefore in vivo studies with these inhibitors do not implicate specific MLK members in dopaminergic cell loss. The involvement of a specific MLK isoform can be identified only by generating genetic mouse models, where specific MLK members can be ablated. Interestingly, MLK3-and MLK1+MLK2-compound knockout mice have been generated recently (Brancho et al., 2005; Bisson et al., 2008). However, their role in dopaminergic neuronal loss has not been investigated. Currently investigation is underway in our lab to identify the specific MLK isoform capable of mediating dopaminergic neuronal loss in MPTP mouse models of PD.
Figure 4.
MLK3 Activation: (A) MLK3 is a potent activator of JNK: Mammalian cells were transfected either with JNK alone or along with MLK3 expression vectors. The ectotopically expressed JNK was immunoprecipitated and kinase assay was performed using GST-Jun as the substrate.(B) MLK3 is activated by TNFα and ceramide: Mammalian cells were transfected with MLK3 expression plasmid and 36 hours post-transfection, cells were starved in 0.2% serum containing medium for 12 hours. The starved cells were either treated with ceramide (10 μM) for 45 minutes or with TNFα (10 nM) for 30 minutes. MLK3 kinase activity was measured using GST-SEK1 protein as the substrate.
2.2 Neuro inflammation and MLKs-mediated cell death
Neuroinflammation has been reported to cause dopaminergic neuronal cell death in PD patients (Hald et al., 2005). Elevated levels of neuroinflammatory cytokines such as TNF-α, have been detected in animal model of PD and human postmortem brain samples from PD (Dufek et al., 2009). While specific agonists for each MLK member previously were not known, we recently identified TNF-α and ceramide as specific agonists of MLK3 (Figure 4B) (Sathyanarayana et al., 2002). In this study, we showed that MLK3 and its downstream target, JNK, were activated potently by TNF-α and ceramides (Sathyanarayana et al., 2002). The TNF-α is a proinflammatory cytokine implicated in the cell death pathway (Tansey et al., 2008). While it is possible that these proinflammatory pathways impinge on MLK3–JNK activation and promote dopaminergic neuronal cell death, elevated levels of circulatory ceramides have also been reported in PD patients (Arboleda et al., 2009). Similar to proinflammatory cytokine TNF-α, ceramides are also pleiotropic in nature having a range of physiological effects including the inducement of cell death (Villena et al., 2008). The premise that TNF-α and ceramides, both agonists of MLK3, might promote dopaminergic cell death via MLK3, is currently being examined in our lab via MLK3 knockout mice. Additionally, it is possible that TNF-α itself is regulated by the MLK3-JNK pathway because the TNF-α promoter contains AP-1 binding sites, which are ultimately regulated by JNK activation.
2. 3 Regulation of MLK by upstream kinases and its implication in neuronal cell death
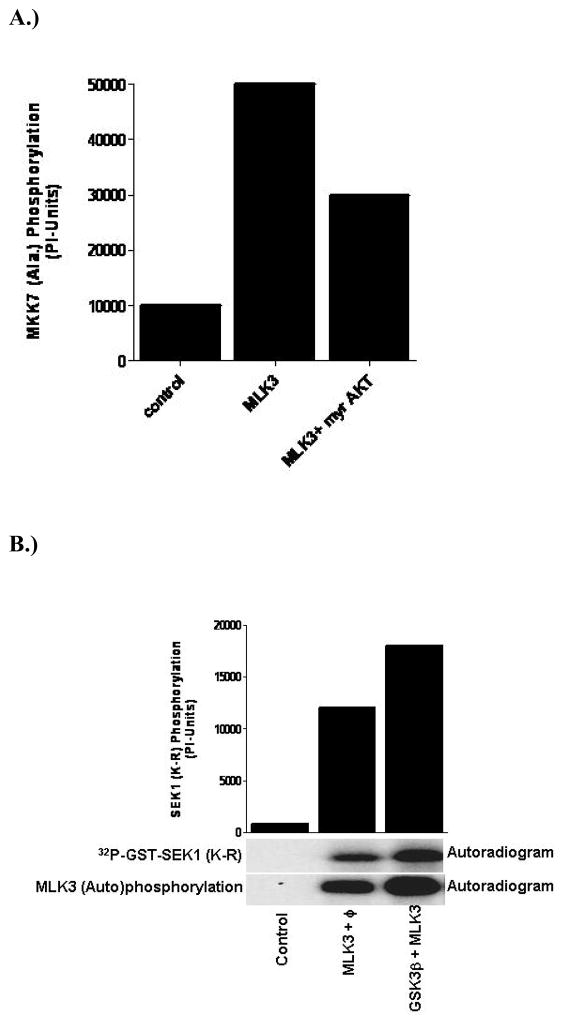
Dopaminergic neuronal cell death is a complex process where various upstream signaling pathways have been shown to either promote or prevent cell death (DeLegge et al., 2008). It has been reported that activation of AKT, a cell survival kinase, prevents dopaminergic neuronal cell death, while GSK3β, an AKT upstream kinase, negatively regulates AKT and thereby promotes dopaminergic neuronal cell death (Nair et al., 2008). More specifically, we have shown that AKT phosphorylates MLK3 on a specific residue, Ser674, and down regulates MLK3 kinase activity and associated cell death (Figure 5A) (Barthwal et al., 2003; Mishra et al., 2007). Interestingly, ceramide, which attenuates AKT kinase activity (Mora et al., 2002), is also an activator of MLK3 and therefore an elevated level of ceramide, as seen in PD patients might downregulate the cell survival pathway by inhibiting AKT and concurrently activating the cell death pathway mediated via MLK3 or other MLK. This also could explain how ceramide might cause cell death in dopaminergic neurons.
Figure 5.
Regulation of MLK3 activation by other kinases: (A) AKT negatively regulates MLK3 kinase activity: Mammalian cells were transfected with either MLK3 alone or along with active AKT (myr-AKT) expression vectors. The ectopically expressed MLK3 was immunoprecipitated and the kinase activity was measured using GST-MKK7 protein as the substrate (B)GSK3β activates MLK3 kinase activity: The mammalian cells were transfected with either MLK3 alone or along with GSK3β expression plasmids. The MLK3 kinase activity was measured as described in Figure 2.
Glycogen synthase kinase-3β(GSK-3β) has been identified primarily as a metabolic enzyme, regulating glycogen synthesis. However, GSK3β has been implicated recently in a neuronal cell death pathway (Sereno et al., 2009) and reported to phosphorylate Tau-protein and promote tangle formation (Baum et al., 1996), a hallmark of Alzheimer disease. In a neuronal cell death model system, we have seen that nerve growth factor (NGF) deprivation causes neuronal cell death via activation of GSK3β and MLK3 (Figure 5B) (Mishra et al., 2007). Further, investigation revealed that MLK3 was phosphorylated at two residues Ser789 and Ser793 by GSK3β (Mishra et al., 2007). This phosphorylation of MLK3 by GSK3β leads to its activation and concurrent activation of the MLK3-downstream kinase, JNK (Mishra et al., 2007). When, these two sites on MLK3 were mutated to non-phosphorable Ala, the activation of MLK3 by GSK3β was blocked, and neuronal cell death upon NGF withdrawal also prevented (Mishra et al., 2007). It is reported that PD patients lack many growth factors (Mogi et al., 1999) and therefore it is worthy to envision that lack of growth factors in PD patients might lead to activation of a GSK3β-MLK3-JNK pathway that ultimately can promote dopaminergic neuronal cell death. Our hypothesis was further confirmed recently when it was shown that GSK3β inhibition indeed promotes the dopaminergic neuronal cell survival in an MPTP mouse model of PD (Wang et al., 2007).
In conclusion, our results suggest that MLK3 could serve as a primary MLK member that regulates dopaminergic neuronal cell death via JNK activation in response to neuro-inflammatory triggers like TNF-α. Upstream regulators of MLK3, AKT and GSK3β, also might play central roles in regulating the cell death of dopaminergic neurons via MLK3 in PD patients. We believe that future investigation of MLK3 or GSK3β may prove beneficial in halting dopaminergic neuronal loss during PD pathogenesis.
3.0 Summary
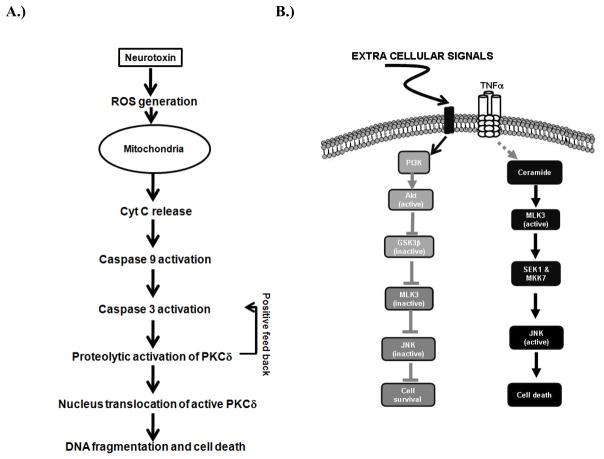
In this review, we describe the proapoptotic function of two kinases, PKCδ and MLK3, in neurotoxic models of Parkinson’s disease (PD). Figures 6A and 6B summarize the overall pathway involving the caspase-3-dependent proteolytic activation of PKCδ and MLK3-JNK in PD models. Based on these results, PKCδ and MLK3 may become valid pharmacological targets for the development of a neuroprotective strategy against oxidative stress-induced dopaminergic degeneration in PD. Further exploration of PKCδ and MLK3 signaling will also provide novel insights into the pathogenesis of PD.
Figure 6.
(A)Schematic model showing the role of PKCδ in neurotoxin-induced neuronal apoptosis. Exposure to neurotoxins, such as MPP+, dieldrin, manganese and H2O2, induces early events of apoptosis including generation of reactive oxygen species (ROS), mitochondrial dysfunction, and release of cytochrome C into cytosol. The released cytosolic cytochrome C activates caspase-9, which subsequently activates caspase-3. Activated caspase-3 mediates proteolytic cleavage of PKCδ to produce an active PKCδ fragment. Proteolytic activation of PKCδ eventually contributes to cell death. The proteolytic activation of PKCδ can also regulate upstream the mitochondrial dependent caspase cascade by a positive feedback loop. (B). Schematic model for cell death and cell survival signaling pathway regulated by MLK3: Under growth factor sufficient conditions, PI3 kinase-AKT pathway remains active that leads to inhibition of GSK3β. AKT also phosphorylates MLK3 at Ser674 site and attenuates MLK3 kinase activity. The inhibition of MLK3 kinase activity prevents the activation of JNK, finally leading to cell survival. On the contrary, the proinflammatory cytokines, TNFα activates MLK3 probably via ceramide generation or by some unknown mechanism. The activation of MLK3 by its agonists leads to activation of JNK and finally leading to cell death.
Acknowledgments
We thank the organizers of the 12th Meeting of the International Neurotoxicology Association for their tremendous efforts and support. This work was supported by National Institutes of Health (NIH) Grants ES10586 (AGK), NS38644(AGK), NS39958 (AGK), NS65167 (AK) and GM55835 (AR). The W. Eugene and Linda Lloyd Endowed Chair to AGK also is acknowledged. The authors acknowledge Ms. MaryAnn deVries for her assistance in the preparation of this manuscript.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histology and histopathology. 1997;12:25–31. [PubMed] [Google Scholar]
- Arboleda G, Morales LC, Benitez B, Arboleda H. Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev. 2009;59:333–346. doi: 10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Hernandez EH, Tjalkens R. Manganese and its Role in Parkinson’s Disease: From Transport to Neuropathology. Neuromolecular medicine. 2009 doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthwal MK, Sathyanarayana P, Kundu CN, Rana B, Pradeep A, Sharma C, Woodgett JR, Rana A. Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J Biol Chem. 2003;278:3897–3902. doi: 10.1074/jbc.M211598200. [DOI] [PubMed] [Google Scholar]
- Baum L, Hansen L, Masliah E, Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol Chem Neuropathol. 1996;29:253–261. doi: 10.1007/BF02815006. [DOI] [PubMed] [Google Scholar]
- Bharti A, Kraeft SK, Gounder M, Pandey P, Jin S, Yuan ZM, Lees-Miller SP, Weichselbaum R, Weaver D, Chen LB, et al. Inactivation of DNA-dependent protein kinase by protein kinase Cdelta: implications for apoptosis. Molecular and cellular biology. 1998;18:6719–6728. doi: 10.1128/mcb.18.11.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson N, Tremblay M, Robinson F, Kaplan DR, Trusko SP, Moss T. Mice lacking both mixed-lineage kinase genes Mlk1 and Mlk2 retain a wild type phenotype. Cell Cycle. 2008;7:909–916. doi: 10.4161/cc.7.7.5610. [DOI] [PubMed] [Google Scholar]
- Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol. 2005;25:3670–3681. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- Choi SH, Hyman T, Blumberg PM. Differential effect of bryostatin 1 and phorbol 12-myristate 13-acetate on HOP-92 cell proliferation is mediated by down-regulation of protein kinase Cdelta. Cancer research. 2006;66:7261–7269. doi: 10.1158/0008-5472.CAN-05-4177. [DOI] [PubMed] [Google Scholar]
- Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lord JM. PKC-delta is an apoptotic lamin kinase. Oncogene. 2000;19:2331–2337. doi: 10.1038/sj.onc.1203555. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- D’Costa AM, Denning MF. A caspase-resistant mutant of PKC-delta protects keratinocytes from UV-induced apoptosis. Cell death and differentiation. 2005;12:224–232. doi: 10.1038/sj.cdd.4401558. [DOI] [PubMed] [Google Scholar]
- DeLegge MH, Smoke A. Neurodegeneration and inflammation. Nutr Clin Pract. 2008;23:35–41. doi: 10.1177/011542650802300135. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. American journal of physiology. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- Dufek M, Hamanova M, Lokaj J, Goldemund D, Rektorova I, Michalkova Z, Sheardova K, Rektor I. Serum inflammatory biomarkers in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:318–320. doi: 10.1016/j.parkreldis.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Annals of neurology. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–672. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Riederer PF. Time sequences of dopaminergic cell death in Parkinson’s disease: indications for neuroprotective studies. Advances in neurology. 1999;80:219–225. [PubMed] [Google Scholar]
- Gschwendt M. Protein kinase C delta. European journal of biochemistry/FEBS. 1999;259:555–564. doi: 10.1046/j.1432-1327.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Kittstein W, Marks F. A novel type of phorbol ester-dependent protein phosphorylation in the particulate fraction of mouse epidermis. Biochemical and biophysical research communications. 1986;137:766–774. doi: 10.1016/0006-291x(86)91145-9. [DOI] [PubMed] [Google Scholar]
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Stadelmann C. Mechanisms of cell death in neurodegenerative disorders. Journal of neural transmission. 2000;59:95–114. doi: 10.1007/978-3-7091-6781-6_13. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Anantharam V, Zhang D, Latchoumycandane C, Jin H, Kaul S, Kanthasamy A. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase C delta (PKCdelta) protects against dopaminergic neuronal degeneration in Parkinson’s disease models. Free radical biology & medicine. 2006;41:1578–1589. doi: 10.1016/j.freeradbiomed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Borowitz JL, Pavlakovic G, Isom GE. Dopaminergic neurotoxicity of cyanide: neurochemical, histological, and behavioral characterization. Toxicology and applied pharmacology. 1994;126:156–163. doi: 10.1006/taap.1994.1102. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxidants & redox signaling. 2003;5:609–620. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. The Journal of biological chemistry. 2005;280:28721–28730. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. The European journal of neuroscience. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase C delta (PKC delta): activation mechanisms and functions. Journal of biochemistry. 2002:831–839. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy A, Kanthasamy AG. Dieldrin promotes proteolytic cleavage of poly(ADP-ribose) polymerase and apoptosis in dopaminergic cells: protective effect of mitochondrial anti-apoptotic protein Bcl-2. Neurotoxicology. 2004;25:589–598. doi: 10.1016/j.neuro.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, Ohmae K, Kikkawa U, Nishizuka Y. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science (New York, NY. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. The Journal of pharmacology and experimental therapeutics. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Leibersperger H, Gschwendt M, Gernold M, Marks F. Immunological demonstration of a calcium-unresponsive protein kinase C of the delta-type in different species and murine tissues. Predominance in epidermis. The Journal of biological chemistry. 1991;266:14778–14784. [PubMed] [Google Scholar]
- Maroney AC, Finn JP, Connors TJ, Durkin JT, Angeles T, Gessner G, Xu Z, Meyer SL, Savage MJ, Greene LA, Scott RW, Vaught JL. Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J Biol Chem. 2001;276:25302–25308. doi: 10.1074/jbc.M011601200. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. Journal of neurochemistry. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- Mishra R, Barthwal MK, Sondarva G, Rana B, Wong L, Chatterjee M, Woodgett JR, Rana A. Glycogen synthase kinase-3beta induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem. 2007;282:30393–30405. doi: 10.1074/jbc.M705895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Goto K, Mori H, Mizuno Y. Histochemical detection of apoptosis in Parkinson’s disease. Journal of the neurological sciences. 1996;137:120–123. doi: 10.1016/0022-510x(95)00336-z. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- Mora A, Sabio G, Risco AM, Cuenda A, Alonso JC, Soler G, Centeno F. Lithium blocks the PKB and GSK3 dephosphorylation induced by ceramide through protein phosphatase-2A. Cell Signal. 2002;14:557–562. doi: 10.1016/s0898-6568(01)00282-0. [DOI] [PubMed] [Google Scholar]
- Naik MU, Benedikz E, Hernandez I, Libien J, Hrabe J, Valsamis M, Dow-Edwards D, Osman M, Sacktor TC. Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain. The Journal of comparative neurology. 2000;426:243–258. doi: 10.1002/1096-9861(20001016)426:2<243::aid-cne6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3beta pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem. 2008;283:15469–15478. doi: 10.1074/jbc.M707238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life sciences. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Pallanck L, Greenamyre JT. Neurodegenerative disease: pink, parkin and the brain. Nature. 2006;441:1058. doi: 10.1038/4411058a. [DOI] [PubMed] [Google Scholar]
- Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, Kyriakis JM, Avruch J. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem. 1996;271:19025–19028. doi: 10.1074/jbc.271.32.19025. [DOI] [PubMed] [Google Scholar]
- Ren J, Datta R, Shioya H, Li Y, Oki E, Biedermann V, Bharti A, Kufe D. p73beta is regulated by protein kinase Cdelta catalytic fragment generated in the apoptotic response to DNA damage. The Journal of biological chemistry. 2002;277:33758–33765. doi: 10.1074/jbc.M110667200. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. Faseb J. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, Kent KC, Liu B. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. The Journal of biological chemistry. 2005;280:35310–35317. doi: 10.1074/jbc.M507187200. [DOI] [PubMed] [Google Scholar]
- Saporito MS, Brown EM, Miller MS, Carswell S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons In vivo. J Pharmacol Exp Ther. 1999;288:421–427. [PubMed] [Google Scholar]
- Sathyanarayana P, Barthwal MK, Kundu CN, Lane ME, Bergmann A, Tzivion G, Rana A. Activation of the Drosophila MLK by ceramide reveals TNF-alpha and ceramide as agonists of mammalian MLK3. Mol Cell. 2002;10:1527–1533. doi: 10.1016/s1097-2765(02)00734-7. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet neurology. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to rural environmental factors: a population based case-control study. The Canadian journal of neurological sciences. 1991;18:279–286. doi: 10.1017/s0317167100031826. [DOI] [PubMed] [Google Scholar]
- Sereno L, Coma M, Rodriguez M, Sanchez-Ferrer P, Sanchez MB, Gich I, Agullo JM, Perez M, Avila J, Guardia-Laguarta C, Clarimon J, Lleo A, Gomez-Isla T. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG. Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCdelta proteolytic activation and mitochondrial translocation. Journal of cellular and molecular medicine. 2008;12:2467–2481. doi: 10.1111/j.1582-4934.2008.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Frank-Cannon TC, McCoy MK, Lee JK, Martinez TN, McAlpine FE, Ruhn KA, Tran TA. Neuroinflammation in Parkinson’s disease: is there sufficient evidence for mechanism-based interventional therapy? Front Biosci. 2008;13:709–717. doi: 10.2741/2713. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain research. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Thomas B. Parkinson’s disease: from molecular pathways in disease to therapeutic approaches. Antioxidants & redox signaling. 2009;11:2077–2082. doi: 10.1089/ars.2009.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:D1134–1147. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. The Journal of biological chemistry. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Villena J, Henriquez M, Torres V, Moraga F, Diaz-Elizondo J, Arredondo C, Chiong M, Olea-Azar C, Stutzin A, Lavandero S, Quest AF. Ceramide-induced formation of ROS and ATP depletion trigger necrosis in lymphoid cells. Free Radic Biol Med. 2008;44:1146–1160. doi: 10.1016/j.freeradbiomed.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y, Li M. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Williams GT, Smith CA. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993;74:777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Winkhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochimica et biophysica acta. 2009 doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Molecular and cellular neurosciences. 2004;25:406–421. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhang D, Anantharam V, Kanthasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase C delta inhibitor rottlerin in cell culture and animal models of Parkinson’s disease. The Journal of pharmacology and experimental therapeutics. 2007a;322:913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Yang Y, Anantharam V, Kanthasamy A. Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci. 2007b;27:5349–5362. doi: 10.1523/JNEUROSCI.4107-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]