Abstract
Theiler’s murine encephalomyelitis virus (TMEV) infection of mice is an experimental model for multiple sclerosis (MS). TMEV induces a biphasic disease in susceptible mouse strains. During the acute phase, 1 week after infection, TMEV causes polioencephalomyelitis characterized by infection and apoptosis of neurons in the gray matter of the brain. During the chronic phase, about 1 month after infection, virus infects glial cells and macrophages, and induces inflammatory demyelination with oligodendrocyte apoptosis and axonal degeneration in the white matter of the spinal cord. Although antibody, CD4+, and CD8+ T cell responses against TMEV capsid proteins play important roles in neuropathogenesis, infectious virus with persistence is necessary to induce demyelination; in general, adoptive transfer of antibody or T cells alone did not induce central nervous system (CNS) disease. The TMEV model can be useful for testing new therapeutic strategies specifically as a viral model for MS. Therapies targeting adhesion molecules, axonal degeneration, and immunosuppression can be beneficial for pure autoimmune CNS demyelinating diseases, such as experimental autoimmune encephalomyelitis, but could be detrimental in virus-induced demyelinating diseases, such as progressive multifocal leukoencephalopathy.
Keywords: adhesion molecules, apoptosis, axonal damage, central nervous system, experimental autoimmune encephalomyelitis, inside–out model
Introduction
The two most commonly studied animal models for multiple sclerosis (MS) are a neurotropic viral infection and experimental autoimmune (or allergic) encephalomyelitis (EAE; Owens 2006). Theiler’s murine encephalomyelitis virus (TMEV) infection of mice is one of the neurotropic viral infection models for MS (Libbey and Fujinami 2003). Max Theiler isolated TMEV from the central nervous system (CNS) of mice with spontaneous flaccid paralysis of the hind legs in 1934 (Theiler 1934, 1937). In 1952, Daniels et al. (1952) first described demyelination in the CNS of mice infected with the Daniels (DA) strain of TMEV. Lipton (1975) reawakened interest in the demyelinating disease, and since then TMEV infection of mice has been used as an animal model for MS.
Viral strains and clinical disease
TMEV belongs to the genus Cardiovirus, family Picornaviridae. This family consists of various positive-strand RNA viruses, such as poliovirus, foot-and-mouth disease virus, rhinovirus, and coxsackievirus, which induce diseases in humans and other animals (Tracy et al. 2006). TMEV is divided into two subgroups, GDVII and TO, based on the ability to cause disease in the CNS. The GDVII subgroup (strains GDVII and FA) is highly neurovirulent for mice, resulting in death within 1 to 2 weeks [reviewed in Tsunoda and Fujinami (1996, 1999)]. The DA and BeAn8386 (BeAn) strains of the TO subgroup induce acute polioencephalomyelitis, 1 week after infection (acute phase), and an inflammatory demyelinating disease, 1 month after infection (chronic phase) [reviewed in Tsunoda and Fujinami (1996, 1999)]. The disease caused by DA virus differs in some ways, including infected cell types and pathology, from that induced by the closely related BeAn virus (Ure and Rodriguez 2005). Nevertheless, the clinical course of demyelinating disease induced by both DA and BeAn viruses is, in general, chronic progressive. This is in contrast to EAE models, most of which develop monophasic or relapsing–remitting (RR) disease courses; only a few models for primary progressive and secondary progressive (SP)-MS have been established (Tsunoda et al. 2000, 2005b). Unlike EAE, which is inducible in several different species, such as rodents and primates, TMEV can induce inflammatory demyelinating disease only in mice (Owens 2006).
The WW strain, also a member of the TO subgroup, was isolated during the course of inoculating brain homogenate from human MS into mice (Wroblewska et al. 1977). WW virus causes acute encephalitis with inclusion bodies in neurons in ICR mice, and mice that survive have cellular infiltrates in the anterior horns and dystrophic axons with spongy state in the dorsolateral region with lymphocytic perivascular cuffing (Wroblewska et al. 1977). WW virus is presumed to be unrelated to the original tissue inoculum from MS, since: (1) asymptomatic mice can harbor TMEV in tissues, including intestine (Olitsky 1939) and (2) TMEV was also isolated by serial mouse-brain passage of a tissue inoculum from a human with Hodgkin’s disease (Siegel 1961). Unlike the DA and BeAn strains, the WW strain has been reported to produce a RR disease course in outbred CD-1 mice (Dal Canto and Barbano 1984). Remission is followed by remyelination, mainly due to the influx of Schwann cells, in association with a lack of gliosis (Dal Canto and Lipton 1980; Dal Canto and Barbano 1984). In contrast, there is no Schwann cell remyelination in DA infection of SJL/J mice and extensive gliosis is present (Dal Canto and Lipton 1975). By 7 weeks post-infection with a tissue culture adapted WW virus, ICR mice, infected as weanlings, had a severe necrotizing and demyelinative myelitis (Stroop et al. 1982).
Neuropathology
Viral infection and demyelination
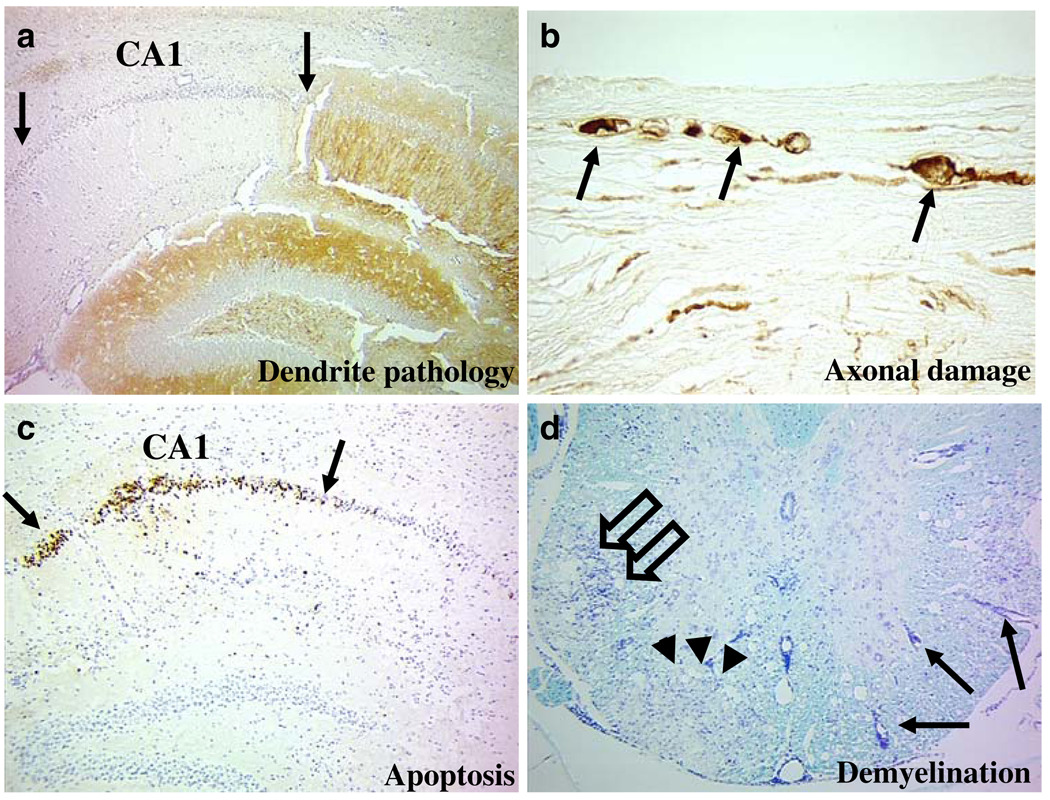
GDVII virus predominantly infects neurons (Liu et al. 1967; Stroop et al. 1981; Tsunoda et al. 1996). Viral antigen-positive neurons are seen in the gray matter, including the cerebral cortex, the hippocampus, and the anterior horns of the spinal cord. Dying neurons have chromatin condensation and apoptotic (fragmented) nuclei (karyorrhexis) (Tsunoda et al. 1997). A lack of microtubule-associated protein (MAP)-2 immunostain in the lesions indicates pathology of the dendrites (Fig. 1a; Zhu et al. 2003), and axons staining positive for nonphosphorylated neurofilaments is indicative of axonal damage (Fig. 1b). An inflammatory mononuclear cell (MNC) reaction is not prominent, but microglia activation is present in the parenchyma. The paucity of inflammatory cells in the areas in which apoptotic neurons are present is consistent with the general observation that apoptosis per se does not induce an inflammatory response (Kerr et al. 1972; Tsunoda et al. 2007a).
Fig. 1.
Neuropathology of TMEV infection. a–c During the acute phase, TMEV induces pathology in dendrites (a), axons (b), and neurons (c). a A lack of immunoreactivity against MAP-2 (clone HM-2, Sigma-Aldrich, St. Louis, MO, USA; between arrows) indicates dendrite pathology in the CA1 area of the pyramidal cell layer of the hippocampus. b Degenerated axons (arrows) were positive for nonphosphorylated neurofilaments [SMI 311 (a cocktail of monoclonal antibodies (SMI 32, 33, 37, 38, and 39)), Sternberger Monoclonal, Inc., Baltimore, MD, USA] in the spinal cord white matter. c Apoptotic neurons (arrows) were TUNEL+ in the CA1 area of the pyramidal layer of the hippocampus. d During the chronic phase, Luxol fast blue stain visualized inflammation (open arrows), demyelination (arrowheads), and perivascular cuffs (arrows) in the white matter of the spinal cord. a and b GDVII infection. c and d DA infection. Magnification: a, c and d ×47, b ×277
During the acute phase of DA infection (1 week post-infection), the distribution of viral antigen-positive neurons and apoptotic neurons (Fig. 1c) is similar to GDVII infection, but the number of infected neurons and apoptotic neurons is smaller. In contrast to GDVII infection, parenchymal as well as perivascular and subarachnoidal MNC infiltrates, including CD3+ T cells, are present in the gray matter of the brain (Tsunoda et al. 2007a). During the chronic phase of DA infection (a month or more after infection), the inflammation in the gray matter of the CNS subsides. Demyelination with perivascular and subarachnoidal MNC inflammation is seen in the ventral and lateral funiculi, particularly at the ventral root exit zone, of the spinal cord; the dorsal funiculus is relatively preserved (Fig. 1d; Ure and Rodriguez 2005). Involvement of the fasciculus gracilis of the dorsal funiculus of the spinal cord can be detected in some mice during the late chronic phase, but the brain as well as the fasciculus cuneatus and the pyramidal tract of the dorsal funiculus are preserved (Tsunoda et al. 1999, 2007b).
During the chronic phase, viral antigen and viral genome have been demonstrated by immunohistochemistry and in situ hybridization, respectively, in oligodendrocytes, microglia/macrophages, and astrocytes, but not in neurons. Infectious virus and viral genome were also quantified by plaque assays and reverse transcription polymerase chain reaction (PCR), respectively. In other picornaviral infections, one of the main mechanisms by which virus persists in vivo is a restriction of viral RNA replication, as a consequence of inhibition of positive-strand RNA synthesis (Girard et al. 2002). However, using in situ hybridization, Cash et al. (1988) showed that CNS cells infected with DA virus contained 100 to 500 copies of viral RNA, and that, by quantifying positive- and negative-strand RNAs in infected CNS cells, RNA replication was blocked at the level of negative-strand RNA synthesis. Using Northern hybridization, Trottier et al. (2001) detected a large number of viral genomes (mean of 3.0 × 109 per spinal cord) in the spinal cords of mice infected with BeAn virus. The ratio of BeAn virus positive- to negative-strand RNA indicated that viral RNA replication was unperturbed in the spinal cord. Differences between DA versus BeAn viral infection of mice could explain the reported differences. Infection with DA virus results in a higher incidence of demyelinating disease, more viral RNA and more antigen-positive cells in the spinal cord than infection with BeAn virus (Zoecklein et al. 2003).
During the chronic phase of BeAn infection, the ratio of viral RNA copies to plaque-forming units (PFU) of infectious virus isolated from infected mouse CNS was on the order of 106:1, compared with a ratio of 103:1 during the acute phase of BeAn infection (Trottier et al. 2001). In vitro infection studies showed that total RNA per cell as well as the viral RNA-to-PFU ratio differed between baby hamster kidney cells, an oligodendrocyte cell line and macrophage cell lines. Thus, the discrepancy during the acute versus chronic phase can be explained by the difference in cell types infected: neurons during the acute phase versus macrophages and glial cells during the chronic phase, although contamination by serum and tissue-bound neutralizing antibody could also contribute to the higher viral RNA-to-PFU ratio during the chronic phase.
Axonal damage
Although axonal damage is observed in MS and its animal model, EAE (Shriver and Dittel 2006), it is believed that axonal damage occurs secondarily to severe inflammatory demyelination, where myelin and/or oligodendrocytes are damaged primarily. Here, lesions develop from the outside (myelin) to the inside (axon; outside-in model; Tsunoda and Fujinami 2002; Tsunoda et al. 2007b; Geurts et al. 2009). However, evidence suggests that not all axonal degeneration in MS can be explained by this outside-in model, since: (1) acute inflammatory demyelination did not correlate with axonal injury in SP-MS; (2) axonal injury in MS did not correlate with the number of CD3+ T cells (Bitsch et al. 2000); (3) damaged axons are present not only in active demyelinating lesions, but also in remyelinating lesions and in normal-appearing white matter (NAWM), suggesting that axonal injury is independent of demyelinating activity; (4) significant reduction of axonal density has been seen in SP-MS, where there was no significant difference in axonal density in the MS plaque versus contralateral NAWM (Lovas et al. 2000); and (5) axonal damage occurred early during the course of MS, and damaged axons were found in the periplaque white matter without any overt signs of demyelination (Kuhlmann et al. 2002). Magnetic resonance spectroscopy and magnetization transfer ratio analyses have found gray matter lesions (Sharma et al. 2001) and axonal injury in NAWM as well as normal-appearing gray matter in MS (Matthews et al. 1998; Lovas et al. 2000; Bjartmar et al. 2001; Ramió-Torrentà et al. 2006).
In TMEV infection, axonal damage precedes demyelination (Tsunoda et al. 2003). Here, lesions develop from the inside (axon) to the outside (myelin; inside–out model; Tsunoda and Fujinami 2002; Tsunoda et al. 2003). One week after DA infection, damaged axons were detected in the NAWM of the spinal cord by immunohistochemistry against nonphosphorylated neurofilaments. The number of damaged axons increased over time. By 2 and 3 weeks after infection, damaged axons were accompanied by activated microglia/macrophages, but were never associated with perivascular T cell infiltration or demyelination until the chronic phase (1 month after infection). Oligodendrocyte apoptosis was also detected in the NAWM. The distribution of damaged axons observed during the early phase corresponded to regions where subsequent demyelination occurs during the chronic phase. Axonal degeneration (Dal Canto and Lipton 1975) and loss (Ure et al. 2001) have also been demonstrated during the late chronic phase, 6 to 9 months after TMEV infection. Interestingly, GDVII infection induces more severe axonal damage than DA infection (Fig. 1b; Tsunoda et al. 2003). However, axonal degeneration in GDVII infection is not accompanied by macrophages, T cells, or viral antigen-positive cells.
The precise mechanism of axonal damage in the early stage of TMEV infection is not clear. The relative preservation of the dorsal funiculus suggests that axonal degeneration in the white matter of the spinal cord could result from Wallerian degeneration induced by neuronal cell death in the gray matter. The dorsal funiculus is mainly comprised of ascending tracts, whereas the ventral and lateral funiculi contain both ascending and descending tracts. During the acute phase, TMEV infects neurons and induces apoptosis, which could lead to Wallerian degeneration of the descending tracts. One could hypothesize that early axonal damage in the ventral and lateral funiculi in TMEV infection recruits inflammatory cells to sites of Wallerian degeneration, leading to demyelination. Indeed, when axonal degeneration was experimentally induced in the dorsal funiculus during the early stage of TMEV infection, inflammatory demyelination could be detected in the dorsal funiculus during the chronic phase of infection (Tsunoda et al. 2007b). Thus, axonal degeneration itself can contribute to recruitment of inflammatory cells into the CNS, targeting demyelinating lesion development.
Although preservation of axons seems to be beneficial to hosts, a lack of or delay in axonal degeneration can be detrimental in some instances. Since TMEV can spread in the CNS via axonal transport, preservation of axons in TMEV infection may favor virus spread in the CNS. Tsunoda et al. [(2007c); reviewed in Penberthy and Tsunoda (2009)] tested whether a delay in axonal degeneration could affect the disease severity in TMEV infection by comparing wild-type C57BL/6 (B6) mice to C57BL/WldS (Wld) mice that carry a mutation that delays axonal degeneration (Lyon et al. 1993). While resistant B6 mice had no clinical disease, 30% of Wld mice were paralyzed during the acute phase and 50% during the chronic phase. Wld mice had increased inflammation, viral antigen-positive cells, and TMEV-specific lymphoproliferative responses, compared to B6 mice. Since TMEV can use axons to disseminate in the CNS, axonal degeneration in B6 mice could be a beneficial self-destructive mechanism that limits the virus spread, while slow axonal degeneration in Wld mice can favor virus spread. In contrast, when EAE was induced in B6 and Wld mice, Wld mice had less severe disease, compared to B6 mice (Tsunoda et al. 2007c). Thus, axonal degeneration can play contrasting roles (beneficial versus detrimental) depending on the type of disease.
Apoptosis
Apoptosis has been suggested to play a role in the pathogenesis of MS and its animal models (Satoh et al. 2005). Several picornaviruses have been reported to induce apoptosis, including poliovirus, hepatitis A virus, enteroviruses 70 and 71, coxsackieviruses B3 and B4, encephalomyocarditis virus, and foot-and-mouth disease virus (Agol 2002; Chen et al. 2006; Romanova et al. 2009). Induction of apoptosis by TMEV infection has been demonstrated in vivo by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL; Tsunoda et al. 1997, 2007a; Buenz et al. 2009). During the acute phase, apoptosis was induced in neurons in both GDVII and DA viral infections (Fig. 1c). Some TUNEL+ apoptotic cells were co-labeled with viral antigen, but others were negative for viral antigen. The number of apoptotic neurons was much greater in GDVII infection than in DA infection, suggesting that this difference contributes to the TMEV subgroup-specific neurovirulence. During the chronic phase of DA infection, apoptotic cells were detected only in the white matter of the spinal cord (Tsunoda et al. 1997; Rose et al. 1998; Carlson et al. 2006). Some of these TUNEL+ cells were labeled with the oligodendrocyte-specific marker, carbonic anhydrase II, or with the microglia/macrophage marker, Ricinus Communis agglutinin I, but not with the astrocyte marker, glial fibrillary acidic protein. Apoptosis was virtually absent in perivascular infiltrates during the chronic phase of DA infection, while 8% of infiltrates were TUNEL+ in acute EAE lesions (Tsunoda et al. 1997). Apoptotic death of encephalitogenic T cells has been suggested to play an important role in remission in EAE. Thus, a failure in encephalitogenic T cell elimination by apoptosis may contribute to the chronic progressive course of DA infection.
In contrast, in BeAn infection, induction of apoptosis was reported in T cells, macrophages, and astrocytes (Palma et al. 1999; Schlitt et al. 2003) and in vitro in macrophages (Jelachich and Lipton 2005). The early apoptosis of infected neuronal cells in the CNS may be a protective mechanism against CNS viral infection in the absence of humoral and cellular immune responses or prior to the generation of immune responses. Elimination of virus-infected host cells by apoptosis prior to the assembly of infectious virion could inhibit viral replication in the CNS (Tsunoda 2008). Since dendritic cells have been shown to present antigen derived from apoptotic cells and stimulate major histocompatibility complex (MHC) class I-restricted CD8+ cytotoxic T lymphocytes (CTLs; Albert et al. 1998), induction of apoptosis in TMEV infection may contribute to induction of virus-specific CTLs. The mechanism for induction of apoptosis in macrophages by the BeAn virus is related to the activation of p53 that in turn upregulates puma and noxa and is a potential mechanism for viral persistence (Son et al. 2009).
Role of immune responses
Toll-like receptors
Toll-like receptors (TLR) are a family of pattern recognition receptors expressed on cells that allow for the recognition of conserved structural motifs found on a wide array of pathogens, referred to as pathogen-associated molecular patterns, as well as endogenous molecules (Kielian 2006; Akira et al. 2006). TMEV carries a positive single-stranded (ss) RNA genome and can form double-stranded (ds) RNA in the replication complex. ssRNA is recognized by murine TLR7 and human TLR8, while dsRNA is recognized by TLR3 (CD283; Crozat and Beutler 2004; O’Neill 2004). Stimulation of both TLRs 3 and 7 causes induction of a type I interferon (IFN), which is important in controlling viral replication. Microglia infected with TMEV in vitro increased expression of TLRs 2, 3, 5, and 9 (Olson and Miller 2004), while microglia isolated from neonatal mice express mRNAs for TLRs 1–9. Another in vitro study reported that TLR3, but not TLR7, mediates induction of chemokine and cytokine genes in astrocytic cell lines during TMEV infection (So et al. 2006). Since TMEV infects microglia and astrocytes during the chronic phase, these studies suggest that TLRs may play a role in viral persistence. We do not know whether TLRs play a role during the acute phase of infection (real “innate” stage of infection), where TMEV predominantly infects neurons.
During the chronic phase of TMEV infection in vivo, Turrin (2008) showed significant upregulation of TLRs 2, 3, 6, 7, 8, and 9 in the CNS of SJL/J mice infected with DA virus, while TLR4 showed visual but insignificant increases in expression. More recently, using a combined microarray and immunohistological approach, an upregulated TLR4-induced pathway was found to be associated with demyelination in SJL/J mice infected with BeAn virus (Ulrich et al. 2009).
TLR9 (CD289) recognizes bacterial and viral DNAs that contain a high number of unmethylated CpG motifs. Although these sequences also occur in mammalian DNA, they are typically methylated and thus do not trigger TLR9-mediated signaling. Tsunoda et al. (1999) demonstrated that bacterial DNA that contained multiple CpG motifs exacerbated TMEV-induced demyelinating disease, as well as EAE. Although immunization with naked plasmid DNA encoding microbial immune epitopes is a novel vaccination strategy that can induce both humoral and cellular immune responses against pathogens, CpG motifs in the plasmid DNA backbone can induce proinflammatory responses, which potentially exacerbate autoimmune diseases, such as MS. In addition, bacterial DNA encoding different antigens administered following TMEV infection could mimic a polymicrobial infection, where modulation of anti-microbial immune responses by cross-reacting epitopes has been reported (Welsh and Selin 2002).
Antibody
Serum anti-TMEV neutralizing antibody responses are detectable within 1 week after infection and high neutralizing antibody titers are seen in mice with persistent TMEV infection (Tsunoda et al. 1996). Adoptive transfer of neutralizing antibody into TMEV-infected nude mice results in viral clearance (Fujinami et al. 1989). Thus, virus-specific antibody can play a role in viral protection and clearance in vivo.
Antigenic variation of the immunodominant epitope that is critical for neutralization provides a means of evading virus-specific antibody responses. Using neutralizing antibody, two antibody-resistant variants (escape mutants) of TMEV have been selected and mutations were found at the amino acid position 268 of viral protein (VP)1 or 101 of VP1 (Ohara et al. 1988; Zurbriggen et al. 1989). However, intracerebral infection with these two escape mutants caused little or no demyelination and virus did not persist. Thus, it is unlikely that the mutants are important for escape from immune surveillance leading to viral persistence.
Although serum antibody to myelin basic protein (MBP) can be found in TMEV-infected mice, sera from TMEV-infected mice did not demyelinate or prevent myelination in organotypic cultures (Barbano and Dal Canto 1984). However, Fujinami et al. (1988) raised a monoclonal antibody, H8, from TMEV-infected mice, which reacts with TMEV and galactocerebroside (GC; molecular mimicry between TMEVand GC). GC is a major lipid component of myelin. Although in vivo administration of H8 into naïve mice did not cause demyelination, H8 administration into mice with EAE exacerbated demyelination (Yamada et al. 1990). Serum antibodies to GC were also found in TMEV-infected mice, 10 days after infection.
CD4+ T cells
CD4+ T cells are divided into T helper (Th) 1 and Th2 subtypes, depending on their cytokine secretion profile [reviewed in (Coffman 2006)]. Th1 cells function as mediators of the delayed type hypersensitivity (DTH) response and secrete interleukin (IL)-2, IFN-γ, and lymphotoxin (tumor necrosis factor-β). Th2 cells help in humoral immunity and secrete IL-4, IL-5, IL-6, IL-10, and IL-13. Th1 cells help in the immunoglobulin (Ig) isotype switch from IgM to IgG2a, whereas Th2 cells help in the switch to IgG1 and IgE (Coffman et al. 1986). These two types of T cells reciprocally regulate each other through the cytokines that they secrete.
In an autoimmune model for MS, EAE, CD4+ Th1 cells initiate inflammatory demyelinating disease. This has been proven by the evidence that adoptive transfer of myelin-specific CD4+ T cells can induce EAE in recipient animals [reviewed in Tsunoda and Fujinami (1996)]. In contrast, in TMEV infection, adoptive transfer of T cells alone cannot induce demyelinating disease in recipient mice. Although there are many similarities between EAE and TMEV infection, one should keep in mind that there are distinct differences between the two models, including cytokine and chemokine profiles (Tsunoda and Fujinami 1996; Tsunoda et al. 2004).
TMEV-specific CD4+ T cells play an important role in demyelination since: (1) CD4+ T cells infiltrate into demyelinating lesions; (2) in vivo depletion of CD4+ T cells diminished the severity of demyelination; and (3) TMEV-specific DTH and lymphoproliferative responses, which are mediated by MHC class II-restricted CD4+ T cells, are associated with clinical signs to some extent (Tsunoda and Fujinami 1999). One intriguing report is that after adoptive transfer of VP270–86-specific Th1 cells into SJL/J mice infected with a suboptimal dose of TMEV, recipient mice had exacerbated demyelinating disease (Gerety et al. 1994b). However, in this experiment, recruitment of TMEV-specific CD4+ T cells required intracerebral infection with TMEV. Intravenous transfer of TMEV-specific CD4+ T cells resulted in demyelinating disease only if cells were transferred into mice infected with a suboptimal dose of TMEV; transfer into uninfected mice caused no disease. This suggests that TMEV-specific CD4+ Th1 cells alone cannot induce demyelinating disease and that homing of virus-specific T cells into the CNS requires virus infection in the CNS. This has been observed in other virus infections, including lymphocytic choriomeningitis virus (LCMV) and Borna disease virus (Richt et al. 1989; Rall et al. 1995).
Although myelin-specific T cell responses appear not to be involved in disease initiation in TMEV infection, they may play an important role during the very late chronic phase of BeAn infection (Miller et al. 1997). Two to 3 months after BeAn infection, T cell responses against myelin proteolipid protein (PLP) or myelin oligodendrocyte glycoprotein have been detected (epitope spreading). Although epitope spreading could be the cause of exacerbation of demyelination, it can also be the simple result of the release of myelin antigens due to the destruction of myelin sheaths via other mechanisms. Epitope spreading was first demonstrated in the EAE model as a plausible mechanism that causes remission of demyelinating disease and it may not be involved in all TMEV infection models (Miller et al. 1990). Despite the fact that DA infection also causes progressive demyelinating disease, epitope spreading is not seen in mice infected with DA virus (Tsunoda and Fujinami 2005). Differences may also be due to mice receiving 9 × 107 PFU of BeAn versus 2 × 105 PFU of DA virus (more than a 400-fold difference) between some of the reports.
Dal Canto et al. (2000) tested whether PLP-specific immune responses in TMEV infection have functional significance, using spinal cord organotypic cultures (Rothstein et al. 1993). Lymph node cells were isolated from mice, 70 days after infection with BeAn virus, stimulated with PLP139–151 peptide or bovine MBP, and placed into the cultures. As a control, ovalbumin-specific lymphocytes were used. Lymphocytes stimulated with PLP139–151 had the ability to demyelinate the cultures, while MBP or ovalbumin-stimulated lymphocytes did not. This study suggests that the PLP-specific responses during the TMEV infection have a potential to induce demyelination.
CD8+ T cells
TMEV-specific CD8+ T cells are associated with disease resistance since: (1) resistance to TMEV infection is linked to the H-2D locus of the MHC class I genes (Clatch et al. 1985; Rodriguez et al. 1986); (2) transgenic expression of H-2D rendered susceptible strains of mice resistant to viral persistence (Azoulay et al. 1994; Rodriguez and David 1995); and (3) CD8+ CTLs recognize H-2D-restricted epitopes from the capsid proteins of TMEV. In addition, CD8+ T cells have been suggested to play an effector role in TMEV-induced demyelination since: (1) CD8+ T cells infiltrate the demyelinating lesions; (2) in vivo administration of CD8 antibody diminishes demyelination; (3) MHC class I molecules are upregulated in the CNS in TMEV-infected mice; and (4) CD4-deficient SJL mice show increased demyelination with severe neurologic deficits, while CD8-deficient SJL mice showed minimal deficits with no effect on the extent of demyelination (Murray et al. 1998). However, in mice that normally do not develop demyelination, elimination ofMHC class I through genetic knockout of β2-microglobulin makes them susceptible to demyelination. TMEV infection in β2-microglobulin-deficient mice on SJL (Aubagnac et al. 2001; Begolka et al. 2001) and B6 × 129 (Fiette et al. 1993) backgrounds results in exacerbation of neuropathology with an increase in viral persistence, while preservation of neurological function correlates with axonal preservation in mice with a B6 × 129 background (Ure and Rodriguez 2002). In addition, during the chronic phase, MHC class I-restricted CD8+ T cells have been suggested to play a role in the development of axonal damage (Rivera-Quiñones et al. 1998).
In DA infection, Tsunoda et al. (2002) detected CD8+ autoreactive CTLs from spleen cell cultures after stimulation with TMEV-infected antigen-presenting cells (APCs). These CTLs kill not only TMEV-infected but also uninfected syngeneic cells. Killing requires direct cell-to-cell contact, is mediated by the Fas-FasL pathway and is associated with IFN-γ production (Tsunoda et al. 2006). The CTL clones established from the CTL cultures also have autoreactivity, and intracerebral injection of the CTL clones induces degeneration of the white matter not only in the brain but also in the spinal cord with oligodendrocyte apoptosis (Tsunoda et al. 2005a). Furthermore, CD8+ T cell hybridomas that were generated from the T cell clones also produced IFN-γ when incubated with either infected or uninfected syngeneic target cells, and this IFN-γ production was blocked by CD8 or MHC class I antibody (Tsunoda et al. 2008). This suggests that CD8+ CTLs play a pathogenic role in CNS inflammatory demyelinating disease.
Viral epitope
TMEV is a non-enveloped positive-sense ssRNA virus (Libbey and Fujinami 2003). The viral genome is 8,100 nucleotides long. An open reading frame is first translated into a large polyprotein which is then cleaved into L (leader protein), P1 (capsid region), P2 (midsection), and P3 (right portion). The structural proteins consist of four capsid proteins, VP1, VP2, VP3, and VP4, which are encoded by P1; nonstructural proteins are encoded by P2 and P3 [reviewed in Tsunoda and Fujinami (1999)]. The GDVII and TO subgroups of TMEV are 90% identical at the nucleotide level and 95% identical at the amino acid level.
Immune responses against capsid proteins of TMEV seem to play protective as well as pathogenic roles. Mice immunized with DNA encoding VP1 showed exacerbation of demyelinating disease after a single plasmid injection, but not after two or three injections, while DNA immunizations against VP2 and VP3 showed a dose-dependent protection against demyelinating disease induced with DA virus (Tolley et al. 1999). In BeAn infection, immunization with VP1 or VP2, but not VP3, accelerates the onset of demyelination (Yauch et al. 1998).
Both T and B cell epitopes have been identified in capsid protein regions (Table 1). Interestingly, several linear antibody epitopes overlap with T cell epitopes (Inoue et al. 1994; Usherwood et al. 1995; Kim et al. 2000). Three predominant TMEV capsid epitopes (VP1233–250, VP274–86, and VP324–37) recognized by CD4+ T cells were identified for susceptible SJL/J (H-2s) mice (Yauch and Kim 1994; Gerety et al. 1994a; Yauch et al. 1995). Immunization with the epitope peptides indicated that VP1233–250 and VP274–86 epitopes exacerbate the disease while the VP324–37 epitope does not (Yauch et al. 1998). CD8+ T cells from susceptible SJL/J mice infected with BeAn virus recognize one dominant (VP3159–166) and two subdominant (VP111–20 and VP3173–181) epitopes (Kang et al. 2002b), while CD8+ T cells from SJL/J mice infected with DA virus recognize only VP111–20 (Kang et al. 2002a). CD4+ T cells from resistant B6 mice (H-2b) recognize VP2201–220 and VP421–40 (Kang et al. 2005), while B6 mice mount CTL responses toward VP2121–130, VP2165–173, and VP3110–120 (Dethlefs et al. 1997; Johnson et al. 1999, 2001; Lyman et al. 2002; Myoung et al. 2007).
Table 1.
TMEV epitopes
| Capsid | Amino acid | Virus | Mouse strain | Immune epitopes | References |
|---|---|---|---|---|---|
| VP2 | 2–16 | BeAn s.c. | SJL/J | Antibody epitope | (Inoue et al. 1994) |
| 74–86 | DA, BeAn | SJL/J | MHC class II T cell epitope | (Gerety et al. 1994a; Kang et al. 2002a) |
|
| 121–130 | DA | C57BL/6 | CNS CD8+ infiltrate (peptide-tetramer) | (Johnson et al. 1999) | |
| 121–130 | DA | IFN-γR−/− (129-Ifngrtm1) |
CTL, tetramer, administration of VP2121–130 inhibits CTL responses and results in less motor dysfunction |
(Johnson et al. 2001) | |
| 121–130 | BeAn, DA | C57BL/6 | Dominant CNS CTL epitope | (Borson et al. 1997; Kang et al. 2002a; Myoung et al. 2007) |
|
| 122–130 | DA i.p. | C57BL/6 | CTL epitope (in vitro stimulation) | (Dethlefs et al. 1997) | |
| 165–173 | BeAn i.c. | C57BL/6 | CTL epitope | (Lyman et al. 2002) | |
| 165–179 | BeAn s.c. | SJL/J, C57BL/6 | Antibody epitope | (Inoue et al. 1994) | |
| 201–220 (206–220) | BeAn? i.c. | C57BL/6 | CD4+ ELISPOT epitope | (Kang et al. 2005) | |
| VP3 | 24–37 | BeAn s.c. | SJL/J | Antibody epitope | (Inoue et al. 1994) |
| 24–37 | BeAn | SJL/J | T cell epitope | (Yauch et al. 1995) | |
| 24–37 | BeAn, DA | SJL/J | MHC class II T cell epitope | (Kang et al. 2002a) | |
| 110–120 | DA, BeAn i.c. | C57BL/6 | Subdominant CTL epitope | (Lyman et al. 2002, Kang et al. 2002a) |
|
| 159–166 | BeAn i.c. (not DA) |
SJL/J | Predominant CTL epitope | (Kang et al. 2002a,b) | |
| 173–181 | BeAn i.c. (not DA) |
SJL/J | Second CTL epitope | (Kang et al. 2002a,b) | |
| VP1 | 11–20 | DA, BeAn i.c. | SJL/J | Dominant (DA), 3rd (BeAn) CTL epitope | (Kang et al. 2002a,b) |
| 12–25 | BeAn s.c. | C57BL/6 | Antibody epitope | (Inoue et al. 1994) | |
| 33–47 | Inactivated BeAn |
CBA | MHC class II T cell epitope | (Usherwood et al. 1995) | |
| 101 | DA | BALB/c | Antibody neutralization site | (Zurbriggen et al. 1989) | |
| 146–160 | BeAn s.c. | SJL/J, C57BL/6 | Antibody epitope | (Inoue et al. 1994) | |
| 233–244 | BeAn | SJL/J | T cell epitope | (Yauch and Kim 1994) | |
| 233–250 | BeAn, DA | SJL/J | MHC class II T cell epitope | (Kang et al. 2002a) | |
| 233–255 | BeAn | CBA | Antibody epitope | (Usherwood et al. 1995) | |
| 262–276 | BeAn | CBA | Antibody epitope | (Usherwood et al. 1995) | |
| 262–276 | BeAn i.c., s.c | SJL/J | Antibody epitope | (Inoue et al. 1994) | |
| 268 | DA i.c. | SJL/J | Antibody neutralizing site | (Ohara et al. 1988) | |
| VP4 | 21–40 (25–38) | BeAn? ic | C57BL/6 | CD4+ ELISPOT epitope | (Kang et al. 2005) |
Abbreviation BeAn BeAn8386, CNS central nervous system, CTL cytotoxic T lymphocyte, DA Daniels strain, ELISPOT enzyme-linked immunospot assay, i.c. intracerebral injection, IFN interferon, i.p. intraperitoneal injection, MHC major histocompatibility complex, s.c. subcutaneous injection, TMEV Theiler’s murine encephalomyelitis virus, BeAn? BeAn strain was most likely used in the manuscript, since the research group usually uses BeAn strain in their other manuscripts, although the name of the viral strain was not described in the manuscript
Although the VP4 protein is buried in the virion, once processed intracellularly, peptides from VP4 can be presented by MHC class I or II molecules. Indeed, in TMEV-resistant B6 mice infected with the BeAn strain of TMEV, IFN-γ enzyme-linked immunospot assay and flow cytometry for intracellular IFN-γ production, using truncated peptides, indicated that one of the epitope regions recognized by CNS-infiltrating CD4+ T cells was VP425–38 (Kang et al. 2005). In addition, antibodies to VP4 of another picornavirus, poliovirus, are found to neutralize the virus at 37°C (Li et al. 1994). Thus, it could be possible that antibodies to VP4 are produced in TMEV infection, leading to virus neutralization.
T cell receptor usage
In MS and its animal models, the roles of specific T cell receptors (TCR) and the complementarity determining region 3 (CDR3) differ among models. A preferential usage of variable (V) β chains has been reported in some forms of EAE and in MS (Burns et al. 1989; Fritz et al. 2000; Matsumoto et al. 2003; Matsumoto 2005). Similarly, clonal expansion of T cells with certain CDR3 motifs has also been shown in EAE and MS (Matsumoto et al. 2003; Matsumoto 2005). However, predominant TCR clones derived from expanded Vβ8.2 spectratypes from different diseases frequently possess an identical CDR3 sequence, e.g., the DSSYEQYF sequence present in EAE, the early stage of experimental autoimmune carditis and cardiac allograft rejection (Matsumoto et al. 2000; Matsumoto 2000).
In TMEV infection, although susceptibility has been mapped to the Theiler’s murine encephalomyelitis virus demyelination-1 (Tmevd-1) gene on chromosome 6 near the TCRβ constant gene (Melvold et al. 1987; Kappel et al. 1991), it is controversial whether specific TCR Vβ usage or CDR3 motifs are important. Using PCR, Rodriguez et al. (1993) observed no difference in TCR usage in lymphocytes infiltrating the CNS between resistant B10.K and susceptible B10.Q or SJL/J mice during acute and chronic phases of DA infection. In addition, similar amplification of TCR Vβs was observed between the spleen and the CNS of both resistant and susceptible mice, indicating no preferential migration of specific T cells to the CNS. However, the same group demonstrated a correlation between an increase in susceptibility to demyelination with a deletion of the TCRVβ genes in RIIIS/J mice (Rodriguez et al. 1992). Bahk et al. (1997) suggested that the increased susceptibility to demyelination shown by Rodriguez’s group using RIIIS/J mice could be associated with TCR Jβ1-Cβ1 polymorphisms rather than the Vβ deletion. Musette et al. (1995) found no preferential Vβ family usage in spinal cords of TMEV-infected mice, while CDR3 spectratyping analysis suggested clonal expansion of T cells in the spinal cord.
In contrast, Kim et al. (1999) analyzed the T cell repertoire reactive to the major pathogenic VP1 epitope region (VP1233–250) using VP1233–250-specific T cell hybridomas. Interestingly, close to 50% of the hybridomas used Vβ16, while the CDR3 sequences of the hybridomas were markedly heterogeneous. No restriction was found in the Vα usage. The same group reported that Vβ usage was mainly restricted to Vβ1 and Vβ2 during the acute phase of BeAn infection (Kang et al. 2000). The diversity in Vβ usage increased gradually and peaked at 35 days after viral infection. Analysis of CDR3 sequences of Vβ1 and Vβ2 TCR amplified from the spinal cord suggested clonal expansion of T cells. The haplotype of the TCRα chain had no significant influence on TMEV-induced demyelination (Kappel et al. 1991; Bahk et al. 1997).
The mixed results in TMEV infection might be due to the “private specificity” seen in other viral infections, and the use of different TMEV strains. Anti-viral CD8+ T cell responses can be remarkably different even in genetically identical mice (Lin and Welsh 1998; Kim et al. 2005). Although the LCMV-specific CTL response is highly represented within the Vβ population in B6 mice, spectratypes and sequences of CDR3 regions of CD8+ T cells varied among individual B6 mice. In addition, if the TCR Vβ spectratype is dominated by the CD4+ T cell population, CD8+ T cell spectratypes will not be sufficient to influence the more dominant response associated with the CD4+ T cells in the total T cell population. The advantage of the TCR characterization is that it is applicable to the identification of the pathogenic TCR in human autoimmune diseases and their subsequent TCR-based immunotherapy without knowledge of putative autoantigens (Matsumoto et al. 2003).
Adhesion molecules
Adhesion molecules play important roles in many aspects of the immune response, including lymphocyte–endothelial cell adhesion, providing a co-stimulatory signal for antigen-specific T cell proliferation and fostering interactions between the APCs and T cells (Tsunoda et al. 2007d). Blocking the interaction between inflammatory cells and vascular endothelia can block cell entry into tissues, thus preventing harmful inflammatory responses, such as autoimmunity, but could also limit immunosurveillance by anti-viral T cells in sites of infection or latency.
In November 2004, the US Food and Drug Administration approved natalizumab (Tysabri®/Antegran®) for the treatment of MS. Natalizumab is a humanized monoclonal antibody raised against α4 integrin, which blocks the engagement of α4β1 integrin, also named very late antigen (VLA)-4 (Léger et al. 1997). From the clinical trial data, active therapy led to about a 66% reduction in relapse and a marked reduction in the annual rate of relapse (Miller et al. 2003). This represented a major advance in the treatment of MS patients, whose treatment options up until that point were limited to agents such as IFN-β (Avonex®, Betaseron®, Rebif®, and Betaferon®), glatiramer acetate (Copaxone®), and mitoxantron (Novantrone®; Verdun et al. 2002). In addition, treatment of patients with Crohn’s disease, an inflammatory bowel disease, with natalizumab increased rates of remission leading to marked clinical improvement (Ghosh et al. 2003). Despite this early enthusiasm, natalizumab was withdrawn from the market because two patients with MS (Kleinschmidt-DeMasters and Tyler 2005; Langer-Gould et al. 2005) and one patient with Crohn’s disease (Van Assche et al. 2005) who were treated with natalizumab developed progressive multifocal leukoencephalopathy (PML), a CNS demyelinating disease caused by JC virus (Finkelstein 1997; Koralnik 2004). Since VLA-4 is upregulated in memory T cells, treatment with VLA-4 antibody could block the interaction between JC virus-specific CD8+ T cells and endothelium in the CNS, leading to reactivation of JC virus (Du Pasquier et al. 2006). Experimentally, VLA-4 antibody treatment has been shown to increase viral titers in the CNS in Semliki Forest virus infection, another mouse model for MS (Smith et al. 2000).
In TMEV infection, intercellular adhesion molecule (ICAM)-1 and leukocyte function-associated antigen (LFA)-1 are upregulated in the CNS. ICAM-1 was upregulated on the cell surface of microglia, isolated from the brains of SJL mice, following in vitro infection with TMEV (Olson et al. 2001). Both ICAM-1 and vascular cell adhesion molecule (VCAM)-1 were upregulated on the cell surface of astrocytes, isolated from the brains of B6 and SJL mice, following in vitro infection with TMEV; however, the levels of the adhesion molecules were significantly higher in the B6 astrocytes compared to the SJL astrocytes (Carpentier et al. 2008). Suppression of ICAM-1 and VCAM-1 through administration of the cannabinoid agonist WIN55,212-2 at the time of TMEV infection in turn resulted in the suppression of demyelinating disease in SJL mice (Mestre et al. 2009). Targeted deletion of either L-selectin (L-sel−/−) or both ICAM-1 and P-selectin (ICAM-1/P-sel−/−) in genetically resistant mice (B6) affected the recruitment of immune cells to the CNS following intracerebral infection with TMEV as there was a 1.5- to twofold decrease in the numbers of CD4+ and CD8+ T cells in the brains of the adhesion-molecule-deleted mice compare to control mice (Njenga et al. 2004). This, however, did not hold true for TMEV-infected B6 mice carrying a deletion in only ICAM-1 (ICAM-1−/−); the numbers of virus-specific, IFN-γ-producing CD4+ and CD8+ T cells in the CNS of ICAM-1−/− mice were not changed from control mice (Kang et al. 2005). Treatment of SJL mice with ICAM-1 or LFA-1 antibodies during the acute phase (days 2 to 14) resulted in suppression of demyelinating disease (Inoue et al. 1997). However, treatment during the early chronic phase (3 to 4 weeks) showed no effect on demyelinating disease (Rose et al. 1999). This is in contrast to the results in EAE, where ICAM-1 or LFA-1 antibody treatment exacerbated EAE (Rose et al. 1999).
Direct viral infection
A direct role for virus in causing demyelination is supported by infection of athymic nude mice, which lack T cells. TMEV has been shown to induce demyelinating disease in nude mice as well as β2-microglobulin or MHC class II knockout mice (Roos and Wollmann 1984; Rosenthal et al. 1986).
In vitro infection of neuronal cell lines or organotypic cultures has also demonstrated that virus infection alone can induce neuropathology, including apoptosis of neurons (Tsunoda et al. 1997) and demyelination. The WW strain of TMEV induces degeneration of neurons and oligodendrocytes with demyelinating lesions 3 and 5 days after infection in both cerebellum and spinal cord organotypic cultures (Wroblewska et al. 1979). Thus, WW virus can infect oligodendrocytes and produce demyelination in “immune-free” CNS organotypic cultures. Similarly, demyelination and axonal degeneration were observed in a spinal cord slice culture, 17 h after WW or GDVII infection, while demyelination in the WW virus-infected cultures was more pronounced than in cultures infected with GDVII virus (Shahar et al. 1986). In vitro studies can mimic many aspects of TMEV infection, such as persistent infection in the mouse macrophage RAW264.7 cell line (Steurbaut et al. 2006). However, in the above studies TMEV can induce pathology in cerebellum cultures, while the cerebellum is generally preserved in vivo both during the acute and chronic phases of TMEV infection. In addition, TMEV can infect even insect cell lines in vitro (McCright and Fujinami 1997), while in vivo TMEV can induce disease only in mice.
In T and B lymphocyte-deficient RAG−/− mice, no demyelination was observed after intracerebral injection of TMEV, although only a 3-week survival of these mice may be insufficient for development of demyelination (Libbey and Fujinami 2003; Ure and Rodriguez 2005). In contrast, TMEV infection resulted in fatal encephalitis within 3 weeks of infection in SCID mice, which are deficient in both humoral and cellular immune responses (Ure and Rodriguez 2005). In this study, transfer of too few spleen cells did not protect against death and too many cells led to nearly complete viral clearance and little pathology. However, transfer of intermediate numbers of spleen cells resulted in viral persistence and demyelination, an effect independent of both CD4+ and CD8+ T cells.
Depleting lymphocytes in TMEV infection as a means of inhibiting demyelination or promoting remyelination can be problematic since it suppresses virus-specific immune responses and increases viral replication in the CNS, leading to a fatal infection or an increase in demyelination. Thus, manipulations designed to reduce immunopathology impact anti-viral immunity and alter viral pathogenesis. In TMEV infection in susceptible mice, a balance between viral replication and immune responses seems to be important in pathogenesis, since immune responses can have both protective and detrimental properties in demyelinating disease (Burt et al. 1999). Interestingly, in MS, treatment with lymphocyte-depleting antibodies reduces inflammation, but either has no effect on clinical disease or increases disability and worsens magnetic resonance imaging parameters of neuronal pathology.
Conclusion
Although TMEV-induced demyelination has similarities in pathogenesis with an autoimmune model for MS, EAE, these two models have several important differences, such as a requirement for viral persistence, immune responses, neuropathology, and clinical courses. Therefore, the TMEV model can be useful for the testing of new therapeutic strategies specifically in a viral model for MS, particularly for therapies targeting adhesion molecules, axonal degeneration, and immunosuppression, which can be beneficial for pure autoimmune CNS demyelinating diseases, such as EAE, but has been shown to be detrimental in virus-induced demyelinating diseases, including PML.
Acknowledgements
We thank Nikki J. Kirkman BS and Jane E. Libbey MS for many helpful discussions, and Daniel Doty, Faris Hasanovic BS, Krystal D. Porter BS, and Reina Yamaji MD for excellent technical assistance. We are grateful to Ms. Kathleen Borick for preparation of the manuscript.
This work was supported by NIH grants 1R21NS059724 (IT) and 1P01AI058105 (RSF).
Contributor Information
Ikuo Tsunoda, Email: itsuno@lsuhsc.edu, Department of Pathology, University of Utah School of Medicine, 30 North 1900 East, 3R330 SOM, Salt Lake City, UT 84132, USA.
Robert S. Fujinami, Email: Robert.Fujinami@hsc.utah.edu, Department of Pathology, University of Utah School of Medicine, 30 North 1900 East, 3R330 SOM, Salt Lake City, UT 84132, USA.
References
- Agol VI. Picornavirus genome: an overview. In: Semler BL, Wimmer E, editors. Molecular biology of picornaviruses. Washington, D.C: ASM; 2002. pp. 127–148. [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Aubagnac S, Brahic M, Bureau J-F. Viral load increases in SJL/J mice persistently infected by Theiler’s virus after inactivation of the β2m gene. J Virol. 2001;75:7723–7726. doi: 10.1128/JVI.75.16.7723-7726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay A, Brahic M, Bureau J-F. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler’s virus. J Virol. 1994;68:4049–4052. doi: 10.1128/jvi.68.6.4049-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahk YY, Kappel CA, Rasmussen G, Kim BS. Association between susceptibility to Theiler’s virus-induced demyelination and T-cell receptor Jβ1-Cβ1 polymorphism rather than Vβ deletion. J Virol. 1997;71:4181–4185. doi: 10.1128/jvi.71.5.4181-4185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano RL, Dal Canto MC. Serum and cells from Theiler’s virus-infected mice fail to injure myelinating cultures or to produce in vivo transfer of disease. The pathogenesis of Theiler’s virus-induced demyelination appears to differ from that of EAE. J Neurol Sci. 1984;66:283–293. doi: 10.1016/0022-510x(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Begolka WS, Haynes LM, Olson JK, Padilla J, Neville KL, Dal Canto MC, Palma J, Kim BS, Miller SD. CD8-deficient SJL mice display enhanced susceptibility to Theiler’s virus infection and increased demyelinating pathology. J NeuroVirol. 2001;7:409–420. doi: 10.1080/135502801753170264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Brück W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123:1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Kinkel RP, Kidd G, Rudick RA, Trapp BD. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology. 2001;57:1248–1252. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- Borson ND, Paul C, Lin X, Nevala WK, Strausbauch MA, Rodriguez M, Wettstein PJ. Brain-infiltrating cytolytic T lymphocytes specific for Theiler’s virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J Virol. 1997;71:5244–5250. doi: 10.1128/jvi.71.7.5244-5250.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenz EJ, Sauer BM, Lafrance-Corey RG, Deb C, Denic A, German CL, Howe CL. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol. 2009;175:668–684. doi: 10.2353/ajpath.2009.081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns FR, Li XB, Shen N, Offner H, Chou YK, Vandenbark AA, Heber-Katz E. Both rat and mouse T cell receptors specific for the encephalitogenic determinant of myelin basic protein use similar V alpha and V beta chain genes even though the major histocompatibility complex and encephalitogenic determinants being recognized are different. J Exp Med. 1989;169:27–39. doi: 10.1084/jem.169.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RK, Padilla J, Dal Canto MC, Miller SD. Viral hyperinfection of the central nervous system and high mortality after hematopoietic stem cell transplantation for treatment of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Blood. 1999;94:2915–2922. [PubMed] [Google Scholar]
- Carlson NG, Hill KE, Tsunoda I, Fujinami RS, Rose JW. The pathologic role for COX-2 in apoptotic oligodendrocytes in virus induced demyelinating disease: implications for multiple sclerosis. J Neuroimmunol. 2006;174:21–31. doi: 10.1016/j.jneuroim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Getts MT, Miller SD. Pro-inflammatory functions of astrocytes correlate with viral clearance and strain-dependent protection from TMEV-induced demyelinating disease. Virology. 2008;375:24–36. doi: 10.1016/j.virol.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash E, Chamorro M, Brahic M. Minus-strand RNA synthesis in the spinal cords of mice persistently infected with Theiler’s virus. J Virol. 1988;62:1824–1826. doi: 10.1128/jvi.62.5.1824-1826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Texada DE, Duggan C, Deng Y, Redens TB, Langford MP. Caspase-3 and -7 mediate apoptosis of human Chang’s conjunctival cells induced by enterovirus 70. Virology. 2006;347:307–322. doi: 10.1016/j.virol.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Clatch RJ, Melvold RW, Miller SD, Lipton HL. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TMEV-specific delayed-type hypersensitivity. J Neuroimmunol. 1985;135:1408–1414. [PubMed] [Google Scholar]
- Coffman RL. Origins of the TH1-TH2 model: a personal perspective. Nat Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, Paul WE. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986;136:4538–4541. [PubMed] [Google Scholar]
- Crozat K, Beutler B. TLR7: a new sensor of viral infection. Proc Natl Acad Sci USA. 2004;101:6835–6836. doi: 10.1073/pnas.0401347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Lipton HL. Primary demyelination in Theiler’s virus infection. An ultrastructural study. Lab Invest. 1975;33:626–637. [PubMed] [Google Scholar]
- Dal Canto MC, Lipton HL. Schwann cell remyelination and recurrent demyelination in the central nervous system of mice infected with attenuated Theiler’s virus. Am J Pathol. 1980;98:101–122. [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Barbano RL. Remyelination during remission in Theiler’s virus infection. Am J Pathol. 1984;116:30–45. [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Calenoff MA, Miller SD, Vanderlugt CL. Lymphocytes from mice chronically infected with Theiler’s murine encephalomyelitis virus produce demyelination of organotypic cultures after stimulation with the major encephalitogenic epitope of myelin proteolipid protein. Epitope spreading in TMEV infection has functional activity. J Neuroimmunol. 2000;104:79–84. doi: 10.1016/s0165-5728(99)00230-1. [DOI] [PubMed] [Google Scholar]
- Daniels JB, Pappenheimer AM, Richardson S. Observations on encephalomyelitis of mice (DA strain) J Exp Med. 1952;96:517–535. doi: 10.1084/jem.96.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefs S, Escriou N, Brahic M, van der Werf S, Larsson-Sciard E-L. Theiler’s virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J Virol. 1997;71:5361–5365. doi: 10.1128/jvi.71.7.5361-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier RA, Stein MC, Lima MA, Dang X, Jean-Jacques J, Zheng Y, Letvin NL, Koralnik IJ. JC virus induces a vigorous CD8+ cytotoxic T cell response in multiple sclerosis patients. J Neuroimmunol. 2006;176:181–186. doi: 10.1016/j.jneuroim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Brahic M, Pena-Rossi C. Theiler’s virus infection of β2-microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein SD. Polyomaviruses and progressive multifocal leukoencephalopathy. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, editors. Pathology of infectious disease. Stamford: Appleton & Lange; 1997. pp. 265–272. [Google Scholar]
- Fritz RB, Wang X, Zhao M-L. Alterations in the spinal cord T cell repertoire during relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164:6662–6668. doi: 10.4049/jimmunol.164.12.6662. [DOI] [PubMed] [Google Scholar]
- Fujinami RS, Zurbriggen A, Powell HC. Monoclonal antibody defines determinant between Theiler’s virus and lipid-like structures. J Neuroimmunol. 1988;20:25–32. doi: 10.1016/0165-5728(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Fujinami RS, Rosenthal A, Lampert PW, Zurbriggen A, Yamada M. Survival of athymic (nu/nu) mice after Theiler’s murine encephalomyelitis virus infection by passive administration of neutralizing monoclonal antibody. J Virol. 1989;63:2081–2087. doi: 10.1128/jvi.63.5.2081-2087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SJ, Karpus WJ, Cubbon AR, Goswami RG, Rundell MK, Peterson JD, Miller SD. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J Immunol. 1994a;152:908–918. [PubMed] [Google Scholar]
- Gerety SJ, Rundell MK, Dal Canto MC, Miller SD. Class IIrestricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994b;152:919–929. [PubMed] [Google Scholar]
- Geurts JJG, Stys PK, Minagar A, Amor S, Zivadinov R. Gray matter pathology in (chronic) MS: modern views on an early observation. J Neurol Sci. 2009;282:12–20. doi: 10.1016/j.jns.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Chir B, Palmer T, Donoghue S The Natalizumab Pan-European Study Group. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- Girard S, Gosselin AS, Pelletier I, Colbere-Garapin F, Couderc T, Blondel B. Restriction of poliovirus RNA replication in persistently infected nerve cells. J Gen Virol. 2002;83:1087–1093. doi: 10.1099/0022-1317-83-5-1087. [DOI] [PubMed] [Google Scholar]
- Inoue A, Choe YK, Kim BS. Analysis of antibody responses to predominant linear epitopes of Theiler’s murine encephalomyelitis virus. J Virol. 1994;68:3324–3333. doi: 10.1128/jvi.68.5.3324-3333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Koh C-S, Yamazaki M, Ichikawa M, Isobe M, Ishihara Y, Yagita H, Kim BS. Anti-adhesion molecule therapy in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Int Immunol. 1997;9:1837–1847. doi: 10.1093/intimm/9.12.1837. [DOI] [PubMed] [Google Scholar]
- Jelachich ML, Lipton HL. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelination: apoptosis in TMEV infection. In: Lavi E, Constantinescu CS, editors. Experimental models of multiple sclerosis. New York: Springer; 2005. pp. 697–708. [Google Scholar]
- Johnson AJ, Njenga MK, Hansen MJ, Kuhns ST, Chen L, Rodriguez M, Pease LR. Prevalent class I-restricted T-cell response to the Theiler’s virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J Virol. 1999;73:3702–3708. doi: 10.1128/jvi.73.5.3702-3708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Upshaw J, Pavelko KD, Rodriguez M, Pease LR. Preservation of motor function by inhibition of CD8+ virus peptide-specific T cells in Theiler’s virus infection. FASEB J. 2001;15:2760–2762. doi: 10.1096/fj.01-0373fje. [DOI] [PubMed] [Google Scholar]
- Kang J-A, Mohindru M, Kang B-S, Park SH, Kim BS. Clonal expansion of infiltrating T cells in the spinal cords of SJL/J mice infected with Theiler’s virus. J Immunol. 2000;165:583–590. doi: 10.4049/jimmunol.165.1.583. [DOI] [PubMed] [Google Scholar]
- Kang B-S, Lyman MA, Kim BS. Differences in avidity and epitope recognition of CD8+ T cells infiltrating the central nervous systems of SJL/J mice infected with BeAn and DA strains of Theiler’s murine encephalomyelitis virus. J Virol. 2002a;76:11780–11784. doi: 10.1128/JVI.76.22.11780-11784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B-S, Lyman MA, Kim BS. The majority of infiltrating CD8+ T cells in the central nervous system of susceptible SJL/J mice infected with Theiler’s virus are virus specific and fully functional. J Virol. 2002b;76:6577–6585. doi: 10.1128/JVI.76.13.6577-6585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B, Kang HK, Kim BS. Identification of capsid epitopes of Theiler’s virus recognized by CNS-infiltrating CD4+ T cells from virus-infected C57BL/6 mice. Virus Res. 2005;108:57–61. doi: 10.1016/j.virusres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kappel CA, Dal Canto MC, Melvold RW, Kim BS. Hierarchy of effects of the MHC and T cell receptor beta-chain genes in susceptibility to Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Immunol. 1991;147:4322–4326. [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Bahk YY, Kang HK, Yauch RL, Kang J-A, Park MJ, Ponzio NM. Diverse fine specificity and receptor repertoire of T cells reactive to the major VP1 epitope (VP1230-250) of Theiler’s virus: Vβ restriction correlates with T cell recognition of the c-terminal residue. J Immunol. 1999;162:7049–7057. [PubMed] [Google Scholar]
- Kim BS, Palma JP, Inoue A, Koh C-S. Pathogenic immunity in Theiler’s virus-induced demyelinating disease: a viral model for multiple sclerosis. Arch Immunol Ther Exp (Warsz ) 2000;48:373–379. [PubMed] [Google Scholar]
- Kim S-K, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med. 2005;201:523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ. New insights into progressive multifocal leukoencephalopathy. Curr Opin Neurol. 2004;17:365–370. doi: 10.1097/00019052-200406000-00019. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Brück W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Léger OJP, Yednock TA, Tanner L, Horner HC, Hines DK, Keen S, Saldanha J, Jones ST, Fritz LC, Bendig MM. Humanization of a mouse antibody against human alpha-4 integrin: a potential therapeutic for the treatment of multiple sclerosis. Hum Antibodies. 1997;8:3–16. [PubMed] [Google Scholar]
- Li Q, Yafal AG, Lee YM, Hogle J, Chow M. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J Virol. 1994;68:3965–3970. doi: 10.1128/jvi.68.6.3965-3970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Fujinami RS. Viral demyelinating disease in experimental animals. In: Herndon RM, editor. Multiple sclerosis: immunology, pathology and pathophysiology. New York: Demos; 2003. pp. 125–133. [Google Scholar]
- Lin MY, Welsh RM. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188:1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Collins J, Sharp E. The pathogenesis of Theiler’s GDVII encephalomyelitis virus infection in mice as studied by immunofluorescent technique and infectivity titrations. J Immunol. 1967;98:46–55. [PubMed] [Google Scholar]
- Lovas G, Szilágyi N, Majtényi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123:308–317. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- Lyman MA, Lee H-G, Kang B-S, Kang H-K, Kim BS. Capsid-specific cytotoxic T lymphocytes recognize three distinct H- 2Db-restricted regions of the BeAn strain of Theiler’s virus and exhibit different cytokine profiles. J Virol. 2002;76:3125–3134. doi: 10.1128/JVI.76.7.3125-3134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Ogunkolade BW, Brown MC, Atherton DJ, Perry VH. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc Natl Acad Sci USA. 1993;90:9717–9720. doi: 10.1073/pnas.90.20.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y. Characterization of T cell receptor (TCR) of organ-specific autoimmune disease-inducing T cells and TCR-based immunotherapy with DNA vaccines. J Neuroimmunol. 2000;110:1–12. doi: 10.1016/s0165-5728(00)00346-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y. New approach to immunotherapy against organ-specific autoimmune diseases with T cell receptor and chemokine receptor DNA vaccines. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:73–77. doi: 10.2174/1568008053174732. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Jee Y, Sugisaki M. Successful TCR-based immunotherapy for autoimmune myocarditis with DNA vaccines after rapid identification of pathogenic TCR. J Immunol. 2000;164:2248–2254. doi: 10.4049/jimmunol.164.4.2248. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Yoon WK, Jee Y, Fujihara K, Misu T, Sato S, Nakashima I, Itoyama Y. Complementarity-determining region 3 spectratyping analysis of the TCR repertoire in multiple sclerosis. J Immunol. 2003;170:4846–4853. doi: 10.4049/jimmunol.170.9.4846. [DOI] [PubMed] [Google Scholar]
- Matthews PM, De Stefano N, Narayanan S, Francis GS, Wolinsky JS, Antel JP, Arnold DL. Putting magnetic resonance spectroscopy studies in context: axonal damage and disability in multiple sclerosis. Semin Neurol. 1998;18:327–336. doi: 10.1055/s-2008-1040884. [DOI] [PubMed] [Google Scholar]
- McCright IJ, Fujinami RS. Lack of correlation of Theiler’s virus binding to cells with infection. J NeuroVirol. 1997;3 Suppl 1:S68–S70. [PubMed] [Google Scholar]
- Melvold RW, Jokinen DM, Knobler RL, Lipton HL. Variations in genetic control of susceptibility to Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell β-chain constant gene on chromosome 6. J Immunol. 1987;138:1429–1433. [PubMed] [Google Scholar]
- Mestre L, Docagne F, Correa F, Loría F, Hernangómez M, Borrell J, Guaza C. A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol Cell Neurosci. 2009;40:258–266. doi: 10.1016/j.mcn.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Miller SD, Gerety SJ, Kennedy MK, Peterson JD, Trotter JL, Tuohy VK, Waltenbaugh C, Dal Canto MC, Lipton HL. Class IIrestricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. III. Failure of neuroantigen-specific immune tolerance to affect the clinical course of demyelination. J Neuroimmunol. 1990;26:9–23. doi: 10.1016/0165-5728(90)90115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GPA, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW International Natalizumab Multiple Sclerosis Trial Group. A controlled trial of Natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- Murray PD, Pavelko KD, Leibowitz J, Lin X, Rodriguez M. CD4+ and CD8+ T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol. 1998;72:7320–7329. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musette P, Bureau J-F, Gachelin G, Kourilsky P, Brahic M. T lymphocyte repertoire in Theiler’s virus encephalomyelitis: the nonspecific infiltration of the central nervous system of infected SJL/J mice is associated with a selective local T cell expansion. Eur J Immunol. 1995;25:1589–1593. doi: 10.1002/eji.1830250618. [DOI] [PubMed] [Google Scholar]
- Myoung J, Hou W, Kang B, Lyman MA, Kang J-A, Kim BS. The immunodominant CD8+ T cell epitope region of Theiler’s virus in resistant C57BL/6 mice is critical for anti-viral immune responses, viral persistence, and binding to the host cells. Virology. 2007;360:159–171. doi: 10.1016/j.virol.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njenga MK, Marques C, Rodriguez M. The role of cellular immune response in Theiler’s virus-induced central nervous system demyelination. J Neuroimmunol. 2004;147:73–77. doi: 10.1016/j.jneuroim.2003.10.042. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. Immunology. After the toll rush. Science. 2004;303:1481–1482. doi: 10.1126/science.1096113. [DOI] [PubMed] [Google Scholar]
- Ohara Y, Senkowski A, Fu JL, Klaman L, Goodall J, Toth M, Roos RP. Trypsin-sensitive neutralization site on VP1 of Theiler’s murine encephalomyelitis viruses. J Virol. 1988;62:3527–3529. doi: 10.1128/jvi.62.9.3527-3529.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olitsky PK. Viral effect produced by intestinal contents of normal mice and of those having spontaneous encephalomyelitis. Proc Soc Exp Biol Med. 1939;41:434–437. [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler’s virus. J Virol. 2001;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. Animal models for multiple sclerosis. Adv Neurol. 2006;98:77–89. [PubMed] [Google Scholar]
- Palma JP, Yauch RL, Lang S, Kim BS. Potential role of CD4+ T cell-mediated apoptosis of activated astrocytes in Theiler’s virus-induced demyelination. J Immunol. 1999;162:6543–6551. [PubMed] [Google Scholar]
- Penberthy WT, Tsunoda I. The importance of NAD in multiple sclerosis. Curr Pharm Des. 2009;15:64–99. doi: 10.2174/138161209787185751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MBA. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramió-Torrentà L, Sastre-Garriga J, Ingle GT, Davies GR, Ameen V, Miller DH, Thompson AJ. Abnormalities in normal appearing tissues in early primary progressive multiple sclerosis and their relation to disability: a tissue specific magnetisation transfer study. J Neurol Neurosurg Psychiatry. 2006;77:40–45. doi: 10.1136/jnnp.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt JA, Stitz L, Wekerle H, Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Quiñones C, McGavern D, Schmelzer JD, Hunter SF, Low PA, Rodriguez M. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998;4:187–193. doi: 10.1038/nm0298-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, David CS. H-2 Dd transgene suppresses Theiler’s virus-induced demyelination in susceptible strains of mice. J NeuroVirol. 1995;1:111–117. doi: 10.3109/13550289509111015. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz J, David CS. usceptibility to Theiler’s virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Patick AK, Pease LR, David CS. Role of T cell receptor Vβ genes in Theiler’s virus-induced demyelination of mice. J Immunol. 1992;148:921–927. [PubMed] [Google Scholar]
- Rodriguez M, Prayoonwiwat N, Zhou P, David C. Expression of T cell receptor Vβ transcripts in central nervous system of mice susceptible and resistant to Theiler’s virus-induced demyelination. J Neuroimmunol. 1993;47:95–100. doi: 10.1016/0165-5728(93)90288-a. [DOI] [PubMed] [Google Scholar]
- Romanova LI, Lidsky PV, Kolesnikova MS, Fominykh KV, Gmyl AP, Sheval EV, Hato SV, van Kuppeveld FJM, Agol VI. Antiapoptotic activity of the cardiovirus leader protein, a viral “security” protein. J Virol. 2009;83:7273–7284. doi: 10.1128/JVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos RP, Wollmann R. DA strain of Theiler’s murine encephalomyelitis virus induces demyelination in nude mice. Ann Neurol. 1984;15:494–499. doi: 10.1002/ana.410150516. [DOI] [PubMed] [Google Scholar]
- Rose JW, Hill KE, Wada Y, Kurtz CIB, Tsunoda I, Fujinami RS, Cross AH. Nitric oxide synthase inhibitor, aminoguanidine, reduces inflammation and demyelination produced by Theiler’s virus infection. J Neuroimmunol. 1998;81:82–89. doi: 10.1016/s0165-5728(97)00162-8. [DOI] [PubMed] [Google Scholar]
- Rose JW, Welsh CT, Hill KE, Houtchens MK, Fujinami RS, Townsend JJ. Contrasting effects of anti-adhesion molecule therapy in experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis. J Neuroimmunol. 1999;97:110–118. doi: 10.1016/s0165-5728(99)00064-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Fujinami RS, Lampert PW. Mechanism of Theiler’s virus-induced demyelination in nude mice. Lab Invest. 1986;54:515–522. [PubMed] [Google Scholar]
- Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci USA. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Nakanishi M, Koike F, Miyake S, Yamamoto T, Kawai M, Kikuchi S, Nomura K, Yokoyama K, Ota K, Kanda T, Fukazawa T, Yamamura T. Microarray analysis identifies an aberrant expression of apoptosis and DNA damage-regulatory genes in multiple sclerosis. Neurobiol Dis. 2005;18:537–550. doi: 10.1016/j.nbd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Schlitt BP, Felrice M, Jelachich ML, Lipton HL. Apoptotic cells, including macrophages, are prominent in Theiler’s virus-induced inflammatory, demyelinating lesions. J Virol. 2003;77:4383–4388. doi: 10.1128/JVI.77.7.4383-4388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar A, Frankel G, David Y, Friedmann A. In vitro cytotoxicity and demyelination induced by Theiler viruses in cultures of spinal cord slices. J Neurosci Res. 1986;16:671–681. doi: 10.1002/jnr.490160408. [DOI] [PubMed] [Google Scholar]
- Sharma R, Narayana PA, Wolinsky JS. Grey matter abnormalities in multiple sclerosis: proton magnetic resonance spectroscopic imaging. Mult Scler. 2001;7:221–226. doi: 10.1177/135245850100700402. [DOI] [PubMed] [Google Scholar]
- Shriver LP, Dittel BN. T-cell-mediated disruption of the neuronal microtubule network: correlation with early reversible axonal dysfunction in acute experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:999–1011. doi: 10.2353/ajpath.2006.050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel BV. Identification of virus isolated from Hodgkin’s disease lymph nodes serially passaged in mouse brain. Virology. 1961;14:378–379. [Google Scholar]
- Smith JP, Morris-Downes M, Brennan FR, Wallace GJ, Amor S. A role for α4-integrin in the pathology following Semliki Forest virus infection. J Neuroimmunol. 2000;106:60–68. doi: 10.1016/s0165-5728(99)00235-0. [DOI] [PubMed] [Google Scholar]
- So EY, Kang MH, Kim BS. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler’s murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia. 2006;53:858–867. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- Son K-N, Pugazhenthi S, Lipton HL. Activation of tumor suppressor protein p53 is required for Theiler’s murine encephalomyelitis virus-induced apoptosis in M1-D macrophages. J Virol. 2009;83:10770–10777. doi: 10.1128/JVI.01030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steurbaut S, Rombaut B, Vrijsen R. Persistent infection of RAW264.7 macrophages with the DA strain of Theiler’s murine encephalomyelitis virus: an in vitro model to study viral persistence. J NeuroVirol. 2006;12:108–115. doi: 10.1080/13550280600714120. [DOI] [PubMed] [Google Scholar]
- Stroop WG, Baringer JR, Brahic M. Detection of Theiler’s virus RNA in mouse central nervous system by in situ hybridization. Lab Invest. 1981;45:504–509. [PubMed] [Google Scholar]
- Stroop WG, Brahic M, Baringer JR. Detection of tissue culture-adapted Theiler’s virus RNA in spinal cord white matter cells throughout infection. Infect Immun. 1982;37:763–770. doi: 10.1128/iai.37.2.763-770.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M. Spontaneous encephalomyelitis of mice—a new virus disease. Science. 1934;80:122. doi: 10.1126/science.80.2066.122-a. [DOI] [PubMed] [Google Scholar]
- Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolley ND, Tsunoda I, Fujinami RS. DNA vaccination against Theiler’s murine encephalomyelitis virus leads to alterations in demyelinating disease. J Virol. 1999;73:993–1000. doi: 10.1128/jvi.73.2.993-1000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S, Chapman NM, Drescher KM, Kono K, Tapprich W. Evolution of virulence in picornaviruses. Curr Top Microbiol Immunol. 2006;299:193–209. doi: 10.1007/3-540-26397-7_7. [DOI] [PubMed] [Google Scholar]
- Trottier M, Kallio P, Wang W, Lipton HL. High numbers of viral RNA copies in the central nervous system of mice during persistent infection with Theiler’s virus. J Virol. 2001;75:7420–7428. doi: 10.1128/JVI.75.16.7420-7428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I. Axonal degeneration as a self-destructive defense mechanism against neurotropic virus infection. Future Virol. 2008;3:579–593. doi: 10.2217/17460794.3.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Fujinami RS. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Fujinami RS. Theiler’s murine encephalomyelitis virus. In: Ahmed R, Chen ISY, editors. Persistent viral infections. Chichester: Wiley; 1999. pp. 517–536. [Google Scholar]
- Tsunoda I, Fujinami RS. Inside-out versus outside-in models for virus induced demyelination: axonal damage triggering demyelination. Springer Semin Immunopathol. 2002;24:105–125. doi: 10.1007/s00281-002-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Fujinami RS. TMEV and neuroantigens: Myelin genes and proteins, molecular mimicry, epitope spreading, and autoantibody-mediated remyelination. In: Lavi E, Constantinescu CS, editors. Experimental models of multiple sclerosis. New York: Springer; 2005. pp. 593–616. [Google Scholar]
- Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler’s murine encephalomyelitis virus. Acta Neuropathol (Berl) 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kurtz CIB, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Tolley ND, Theil DJ, Whitton JL, Kobayashi H, Fujinami RS. Exacerbation of viral and autoimmune animal models for multiple sclerosis by bacterial DNA. Brain Pathol. 1999;9:481–493. doi: 10.1111/j.1750-3639.1999.tb00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Theil DJ, Fujinami RS. Antibody association with a novel model for primary progressive multiple sclerosis: induction of relapsing-remitting and progressive forms of EAE in H2S mouse strains. Brain Pathol. 2000;10:402–418. doi: 10.1111/j.1750-3639.2000.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Fujinami RS. Induction of autoreactive CD8+ cytotoxic cells during Theiler’s murine encephalomyelitis virus infection: implications for autoimmunity. J Virol. 2002;76:12834–12844. doi: 10.1128/JVI.76.24.12834-12844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Libbey JE, Fujinami RS. Axonal injury heralds virus-induced demyelination. Am J Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Lane TE, Blackett J, Fujinami RS. Distinct roles for IP-10/CXCL10 in three animal models, Theiler’s virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult Scler. 2004;10:26–34. doi: 10.1191/1352458504ms982oa. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Kobayashi-Warren M, Fujinami RS. Central nervous system pathology caused by autoreactive CD8+ T cell clones following virus infection. J Virol. 2005a;79:14640–14646. doi: 10.1128/JVI.79.23.14640-14646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Libbey JE, Kuang L-Q, Terry EJ, Fujinami RS. Massive apoptosis in lymphoid organs in animal models for primary and secondary progressive multiple sclerosis. Am J Pathol. 2005b;167:1631–1646. doi: 10.1016/S0002-9440(10)61247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Libbey JE, Kobayashi-Warren M, Fujinami RS. IFN-γ production and astrocyte recognition by autoreactive T cells induced by Theiler’s virus infection: role of viral strains and capsid proteins. J Neuroimmunol. 2006;172:85–93. doi: 10.1016/j.jneuroim.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Libbey JE, Fujinami RS. TGF-β1 suppresses T cell infiltration and VP2 puff B mutation enhances apoptosis in acute polioencephalitis induced by Theiler’s virus. J Neuroimmunol. 2007a;190:80–89. doi: 10.1016/j.jneuroim.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am J Pathol. 2007b;171:1563–1575. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Terry EJ, Fujinami RS. Contrasting roles for axonal degeneration in an autoimmune versus viral model for multiple sclerosis: when can axonal injury be beneficial? Am J Pathol. 2007c;170:214–226. doi: 10.2353/ajpath.2007.060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Terry EJ, Marble BJ, Lazarides E, Woods CM, Fujinami RS. Modulation of experimental autoimmune encephalomyelitis by VLA-2 blockade. Brain Pathol. 2007d;17:45–55. doi: 10.1111/j.1750-3639.2006.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Kobayashi-Warren M, Libbey JE, Fujinami RS. Central nervous system degeneration caused by autoimmune cytotoxic CD8+ T cell clones and hybridomas. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of neuroscience. New York: Springer; 2008. pp. 619–625. [Google Scholar]
- Turrin NP. Central nervous system Toll-like receptor expression in response to Theiler’s murine encephalomyelitis virus-induced demyelination disease in resistant and susceptible mouse strains. Virol J. 2008;5:154. doi: 10.1186/1743-422X-5-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R, Kalkuhl A, Deschl U, Baumgärtner W. Machine learning approach identifies new pathways associated with demyelination in a viral model of multiple sclerosis. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2008.00646.x. doi: 10.1111/j.1582-4934.2008.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ure DR, Rodriguez M. Preservation of neurologic function during inflammatory demyelination correlates with axon sparing in a mouse model of multiple sclerosis. Neuroscience. 2002;111:399–411. doi: 10.1016/s0306-4522(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Ure DR, Rodriguez M. Histopathology in the Theiler’s virus model of demyelination. In: Lavi E, Constantinescu CS, editors. Experimental models of multiple sclerosis. New York: Springer; 2005. pp. 579–591. [Google Scholar]
- Ure DR, McGavern DB, Sathornsumetee S, Rodriguez M. The contribution of axonal injury to neurologic dysfunction in Theiler’s virus-induced inflammatory demyelinating disease. In: Hommes OR, Clanet M, Wekerle H, editors. Genes and viruses in multiple sclerosis. Amsterdam: Elsevier; 2001. pp. 79–88. [Google Scholar]
- Usherwood EJ, Johnston IC, Lovelidge LJ, Tonks P, Nash AA. Lymphocyte recognition elements on the VP1 protein of Theiler’s virus. Immunology. 1995;85:190–197. [PMC free article] [PubMed] [Google Scholar]
- Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- Verdun E, Isoardo G, Oggero A, Ferrero B, Ghezzi A, Montanari E, Zaffaroni M, Durelli L. Autoantibodies in multiple sclerosis patients before and during IFN-beta 1b treatment: are they correlated with the occurrence of autoimmune diseases? J Interferon Cytokine Res. 2002;22:245–255. doi: 10.1089/107999002753536220. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- Wroblewska Z, Gilden DH, Wellish M, Rorke LB, Warren KG, Wolinsky JS. Virus-specific intracytoplasmic inclusions in mouse brain produced by a newly isolated strain of Theiler virus. I. Virologic and morphologic studies. Lab Invest. 1977;37:595–602. [PubMed] [Google Scholar]
- Wroblewska Z, Kim SU, Sheffield WD, Gilden DH. Growth of the WW strain of Theiler virus in mouse central nervous system organotypic culture. Acta Neuropathol (Berl) 1979;47:13–19. doi: 10.1007/BF00698267. [DOI] [PubMed] [Google Scholar]
- Yamada M, Zurbriggen A, Fujinami RS. Monoclonal antibody to Theiler’s murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J Exp Med. 1990;171:1893–1907. doi: 10.1084/jem.171.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Kim BS. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler’s virus is located within VP1233-244. J Immunol. 1994;153:4508–4519. [PubMed] [Google Scholar]
- Yauch RL, Kerekes K, Saujani K, Kim BS. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler’s virus in demyelination-susceptible SJL/J mice. J Virol. 1995;69:7315–7318. doi: 10.1128/jvi.69.11.7315-7318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Palma JP, Yahikozawa H, Koh C-S, Kim BS. Role of individual T-cell epitopes of Theiler’s virus in the pathogenesis of demyelination correlates with the ability to induce a Th1 response. J Virol. 1998;72:6169–6174. doi: 10.1128/jvi.72.7.6169-6174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Luo L, Moore GRW, Paty DW, Cynader MS. Dendritic and synaptic pathology in experimental autoimmune encephalomyelitis. Am J Pathol. 2003;162:1639–1650. doi: 10.1016/S0002-9440(10)64298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoecklein LJ, Pavelko KD, Gamez J, Papke L, McGavern DB, Ure DR, Njenga MK, Johnson AJ, Nakane S, Rodriguez M. Direct comparison of demyelinating disease induced by the Daniels strain and BeAn strain of Theiler’s murine encephalomyelitis virus. Brain Pathol. 2003;13:291–308. doi: 10.1111/j.1750-3639.2003.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A, Hogle JM, Fujinami RS. Alteration of amino acid 101 within capsid protein VP-1 changes the pathogenicity of Theiler’s murine encephalomyelitis virus. J Exp Med. 1989;170:2037–2049. doi: 10.1084/jem.170.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]