Abstract
Summary
This study describes the impact of bicarbonate treatment for 3 months on net acid excretion (NAE), nitrogen excretion, and muscle performance in older men and women. Bicarbonate reduced NAE, and the decrement was associated with a decrease in nitrogen excretion. Treatment also improved muscle power and endurance in the women.
Introduction
Bicarbonate enhances muscle performance during strenuous exercise, but its effect on performance during normal activity in older subjects is unknown.
Methods
In this trial, healthy subjects age 50 and older were randomized to 67.5 mmol of bicarbonate or to no bicarbonate daily for 3 months. Changes in lower-extremity muscle power, endurance, urinary nitrogen, and NAE were compared across treatment groups in the 162 participants included in the analyses.
Results
In the men and the women, bicarbonate was well tolerated, and as expected, it significantly decreased NAE. The change in NAE correlated with change in nitrogen excretion in women (r=0.32, P=0.002) with a similar trend in men (r=0.23, P=0.052). In the women, bicarbonate increased double leg press power at 70% one repetition maximum by 13% (P=0.003) compared with no bicarbonate and improved other performance measures. Treatment with bicarbonate had no significant effect on muscle performance in the men.
Conclusions
Ingestion of bicarbonate decreased nitrogen excretion and improved muscle performance in healthy postmenopausal women. The bicarbonate-induced decline in NAE was associated with reduced nitrogen excretion in both men and women. These findings suggest that bicarbonate merits further evaluation as a safe, low-cost intervention that may attenuate age-related loss of muscle performance and mass in the elderly.
Keywords: Muscle endurance, Muscle power, Nitrogen excretion, Potassium bicarbonate
Introduction
Aging is associated with increased risk of falling, fractures, and other injuries [1]. Preserving muscle performance is an effective way to lower risk of falling [2]. Muscle performance has several components including strength, power, and endurance. Strength, the ability to generate force against a specific resistance, declines by 2.6% to 4.1% per year in older adults [3]. Muscle power, the product of muscle force and velocity, declines earlier and more dramatically than strength alone [4]. Impairment in peak muscle power is a strong predictor of functional limitation and disability in the elderly [5–7]. Muscle endurance is the ability to sustain repetitive muscular contractions for an extended period. As a group, these components of muscle performance influence the ability of older adults to carry out activities of daily living.
In young adults, the kidney responds to metabolic acidosis by increasing renal net acid excretion (NAE) to minimize perturbation of the blood pH [8]. The aging kidney, however, is less able to excrete excess hydrogen ions. Consequently, a mild but progressive metabolic acidosis occurs in elderly individuals who are exposed to a continuous challenge from acid-producing diets (e.g., diets relatively rich in meat and cereal grains relative to their content of fruits and vegetables) [9]. Oral administration of bicarbonate increases blood pH in a dose-related manner in healthy adults, both at rest and during exercise [10]; alkali ingestion also dramatically decreases net acid excretion [11]. Mild metabolic acidosis in the elderly is undesirable because it increases bone resorption [12]; it may also adversely affect muscle.
Metabolic acidosis has long been known also to promote protein degradation and nitrogen excretion [13, 14]. In several studies, daily administration of bicarbonate improved muscle power during intense exercise in healthy subjects [10, 15]. Whether the chronic ingestion of net acid-producing diets might adversely affect muscle performance in the elderly, however, has not been assessed, and no information is currently available on the effect of bicarbonate on muscle strength, power, or endurance in older subjects who are not engaged in strenuous exercise activities. This investigation tests the hypothesis that chronic treatment with bicarbonate will improve muscle performance and decrease nitrogen wasting in older men and postmenopausal women. We recently showed that treatment with bicarbonate reduced bone turnover and calcium excretion in healthy men and women age 50 and older [16]. We report here the impact of daily treatment with bicarbonate on muscle performance and nitrogen excretion in the same healthy older subjects.
Methods
Subjects
Healthy ambulatory men and women age 50 and older were recruited through direct mailings and advertisements. The women were menopausal for at least 6 months. Exclusion criteria, described in more detail elsewhere [16], included vegetarianism, diabetes mellitus, peptic ulcers or esophageal stricture, active malignancy, hyperparathyroidism, untreated thyroid disease, significant immune disorder, uncontrolled hypertension, heart disease, gastrointestinal reflux requiring treatment with sodium- or alkali-containing antacids, kidney stones in the last 5 years, creatinine clearance less than 50 ml/min/1.73×m2, 25-hydroxyvitamin D (25(OH)D) level below 40 nmol/L, use of gonadal hormones or other medications for osteoporosis in the last 2 years, and current use of NSAIDs more than three times per week or diuretics.
We prescreened 519 subjects with a telephone questionnaire and invited 316 for screening. Of these, 103 were found to be ineligible, 42 were potentially eligible but not enrolled, and 171 (76 men and 95 women) were enrolled. There were 148 whites, 14 blacks, and 8 Asians. The protocol was approved by the Tufts Medical Center-Tufts University Health Sciences Campus Institutional Review Board, and written informed consent was obtained from each subject.
Study design and supplements
In this 3-month, double-blind, placebo-controlled trial, subjects were randomized in blocks of four within sex and age (50–64 and 65 and older) strata to treatment with potassium bicarbonate, sodium bicarbonate, potassium chloride, or placebo. These groups were selected to determine the effects of potassium, bicarbonate, and the two combined on indices of bone metabolism. This design provided the opportunity to combine the bicarbonate or potassium groups in the event that only bicarbonate or potassium affected the endpoints of the trial. Bicarbonate alone affected the bone outcomes, and those results have been reported recently [16]. At study entry, we assessed the subjects’ medical history, physical activity level, usual diet and supplement use, muscle function, blood pressure, height and weight, and collected blood and urine. Subjects returned after 3 months for measurement of weight, muscle performance, biochemical assays, and other measures. Subjects came to the study site on study day 21 for a serum potassium safety measurement and for review of their pill compliance calendars.
The subjects were advised to maintain their usual diets and levels of physical activity to avoid taking their own calcium and vitamin D supplements and to avoid weight change during the study. Each subject took a supplement containing 600 mg of calcium as the triphosphate, 125 IU of vitamin D3 (Posture D® from US Rhodia), and a multivitamin containing 400 IU of vitamin D3 daily with breakfast. The active treatments were given in amounts of 67.5 mmol/day in nine gelatin capsules (containing 7.5 mmol each), and the placebo capsules contained an equal volume of microcrystalline cellulose. Subjects were asked to take one capsule three times daily during week 1, two capsules three times daily during week 2, and three capsules three times daily thereafter. All capsules were taken with an 8-oz glass of water immediately after meals. The capsules were made by the Medical Pharmacy and Supply, Stoughton, MA, USA.
Measurements
Leisure, household, and occupational activities were estimated with use of the Physical Activity Scale for the Elderly questionnaire [17].
Variation in the staff doing the muscle testing was minimized, and the tester was documented. Several of the tests required assessment of the one repetition maximum (1-RM), defined as the maximum load that can be lifted at one time. For each of the muscle groups, subjects performed five to eight repetitions at a resistance equivalent to 50% of their estimated 1-RM as an exercise-specific warm-up. They then performed an additional five to six sets of one repetition at increasingly heavier loads until they could no longer lift the weight. The highest load lifted was recorded as the 1-RM.
Muscle power and strength
Peak lower-extremity power output was assessed following the measurement of 1-RM as described above. Subjects performed five knee extensions and leg presses, as fast as possible through the full range of motion, at a resistance equivalent to 40% and 70% of the established 1-RM. The highest power output achieved for each of the two leg exercises at each percentage of 1-RM was recorded. The reliability of this measurement in older subjects in our laboratory is 0.901 [18]. Handgrip strength was assessed using a dynamometer, with the higher of two consecutive readings recorded as the maximum force produced with each hand. Mean strength of the two hands was used in the analysis.
Muscle endurance
Isokinetic muscle endurance of knee extensors and flexors was assessed on a Cybex II isokinetic dynamometer [19]. After a period of warm-up and familiarization, subjects performed 25 maximal contractions at 240°/s. The peak torque and its corresponding angle were recorded as the maximal effort. The right and left sides were measured separately and in random order, and the highest result taken as peak torque. Because two tests are necessary to achieve maximal results due to learning effect [20], the subjects were tested on both the screening and baseline visits, and the baseline values were used in the analyses.
Analytic methods
Blood was drawn between 7 am and 9:30 am after a 12-h fast. All samples from individual subjects except the safety blood on day 21 were batched for analyses. The 24-h urine measures are presented as creatinine ratios to correct for variation in the completeness of the collections. Serum 25(OH)D was measured with radioimmunoassay kits from Diasorin (Stillwater, MN, USA) with a coefficient of variation (CV) of 5.6% to 7.7%. Serum intact parathyroid hormone (PTH) and insulin-like growth factor 1(IGF-1) were measured by chemilumainescent immunoradiometric assays on an automated immunoassay system (IMMULITE® 1000, Diagnostic Product Corp., Los Angeles, CA, USA) with CVs of 3% to 9%. Serum calcium, potassium and creatinine, and urinary potassium and sodium were measured on an automated clinical chemistry analyzer (Olympus AU400, Olympus America Inc., Melville, NY, USA) with CVs of 3.0% to 6.0%. Twenty-four-hour urinary calcium was measured by direct-current plasma emission spectroscopy (Beckman Instruments, Fullerton, CA, USA) with a CV of 3–5%. Urinary nitrogen was measured with a model FP-2000 nitrogen/protein determinator (LECO, St. Joseph, MI, USA) with a precision of 15 ppm.
NAE was measured in 24-h urine collections by a modification of the Jorgensen titration method [21], as described by Chan [22]; NAE=titratable acid+NH4+−HCO3−. Briefly, titratable acid−HCO3− was assessed after addition of HCl, boiling the sample and then titrating the sample to neutral pH. To measure the NH4+, formol was added to the sample to release the H+ from NH4+, and the sample was again titrated to neutral pH. All titrations were carried out with a TIM 900 Titration Manager (Radiometer Analytical-Loveland, CO, USA). The precision of NAE measurements in our laboratory was determined by analyzing aliquots of a single 24-h urine collection on 15 different days. The aliquots were stored frozen at −20° and thawed only once. The CV of the NAE measurements was 10.1%.
Statistical analyses
Pearson correlation coefficients were used to describe simple linear associations between continuous variables. t tests were used to compare baseline characteristics across bicarbonate groups.
The effect of bicarbonate on 3-month changes in leg power, leg endurance, and grip strength and 24-h nitrogen/creatinine ratio were examined by analyses of covariance (ANCOVA). These analyses included the computation of means by treatment group adjusted for relevant factors and covariates. SPSS version 15.0 (SPSS Inc., Chicago, USA) was used for all statistical analyses, and P values<0.05 were considered to indicate statistical significance.
The ANCOVA analyses demonstrated a significant interaction of bicarbonate group with sex in the analyses of muscle performance; we, therefore, analyzed the data for men and women separately.
Status of subjects and adherence
Of the 171 enrolled, 7 subjects (4%) dropped out of the study (placebo group—one male with a cardiac event; sodium bicarbonate—one female with high blood pressure, one male with headaches, one male and one female lost interest; potassium chloride—one male with prostate cancer; potassium bicarbonate—one female lost interest). One additional male in the potassium bicarbonate group was excluded because of a suspected acid-base disorder (he had recently been observed to have a low urinary pH and NAE values that were tenfold higher than the group mean), and one female in the sodium bicarbonate group who had a high NAE/Cr value at baseline (18.85 mmol/mmol; the next highest value was 7.46, and the mean was 3.33 mmol/mmol). Thus, 162 subjects, 71 men and 91 women, are included in the analyses.
Results
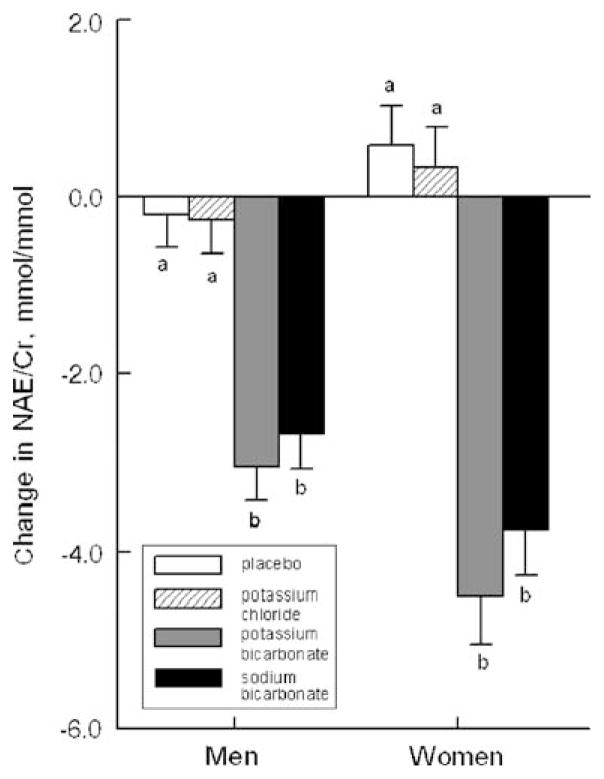
The 162 participants who completed this study had a mean age of 63.0±7.9 years and a weight of 73.6±13.6 kg. The four treatments were selected to allow us to determine whether the bicarbonate, the potassium, or both were effective in altering biochemical markers of bone turnover. As reported elsewhere, only the bicarbonate (i.e., not the potassium) was important [16]. NAE/Cr at baseline did not differ significantly among the four treatment groups. In Fig. 1, we show the effect of each of the four treatments on change in NAE/Cr. The two groups receiving bicarbonate salts had similar and significant decreases in NAE/Cr whereas the other two groups did not. Preliminary analyses indicated that the effects of treatment on our primary muscle outcomes did not differ significantly between the two bicarbonate groups or between the placebo and potassium chloride groups. Therefore, we pooled subjects into two groups, bicarbonate and no bicarbonate (control group) for subsequent analyses. To prevent any potential residual confounding from undetected effects of sodium or potassium, we adjusted for changes in urinary sodium and potassium excretion that occurred with treatment. Clinical characteristics of the bicarbonate and control groups are shown in Table 1. This table also shows the baseline muscle performance and laboratory values. There were no group differences in any of these factors in the men or the women. Both the men and the women consumed net acid-producing diets, as indicated by positive baseline mean NAE/Cr values in the range of 2.78 to 3.36 mmol/mmol.
Fig. 1.
Mean 3-month change in NAE by treatment group in the men and the women. Within sex, bars labeled a differ significantly from bars labeled b, P<0.001
Table 1.
Mean baseline (± standard deviation) clinical characteristics and muscle performance and biochemical measurements of the 162 study subjects by sex and treatment group
| Number | Men |
Women |
||
|---|---|---|---|---|
| Control | Bicarbonate | Control | Bicarbonate | |
| 35 | 36 | 49 | 42 | |
| Clinical characteristics | ||||
| Age (year) | 64.2±8.2 | 63.8±8.3 | 62.7±7.4 | 61.7±7.8 |
| Height (cm) | 174.4±7.2 | 175.8±7.9 | 161.7±5.3 | 162.4±6.7 |
| Weight (kg) | 79.1±12.0 | 83.9±12.1 | 67.2±12.5 | 67.6±9.4 |
| Creatinine clearance (ml/min/1.73×m2) | 96.2±21.7 | 96.5±26.7 | 88.6±22.8 | 88.4±20.1 |
| Activity scoreb | 176.0±88.3 | 147.4±93.2 | 161.9±81.0 | 162.8±71.9 |
| Lean tissue mass (%) | 71.0±6.0 | 68.6±6.3 | 58.4±7.4 | 58.7±6.7 |
| Leg power output | ||||
| Knee extension (40% 1-RM, W)c | 242.7±12.7 | 236.9±12.6 | 131.2±5.6 | 136.2±6.0 |
| Knee extension (70% 1-RM, W)c | 294.4±17.0 | 305.7±17.9 | 145.3±6.3 | 153.6±6.8 |
| Double leg press (40% 1-RM, W) | 1272.3±54.1 | 1199.0±57.3 | 744.8±25.6 | 741.7±31.1 |
| Double leg press (70% 1-RM, W) | 1286.1±67.1 | 1245.0±56.6 | 733.1±27.8 | 724.0±29.8 |
| Isokinetic leg endurancec | ||||
| Knee extension (240°/s, Nm) | 63.5±4.0 | 65.6±3.3 | 36.7±1.5 | 37.2±2.0 |
| Knee flexion (240°/s, Nm) | 53.8±2.5 | 54.5±2.9 | 34.2±1.5 | 35.9±1.7 |
| Grip strength (kgf)c | 43.2±1.1 | 43.0±1.2 | 27.2±0.7 | 27.0±0.07 |
| 24-h urine | ||||
| NAE (mmol) | 38.64±25.19 | 42.81±38.64 | 24.86±18.02 | 27.98±16.20 |
| Creatinine (Cr; mmol) | 13.31±3.01 | 13.11±3.72 | 8.68±1.98 | 8.71±2.65 |
| Nitrogen/Cr (mmol/mmol) | 66.8±14.3 | 73.4±17.3 | 79.6±18.2 | 83.5±17.4 |
| NAE/Cr (mmol/mmol) | 2.80±1.68 | 3.36±1.46 | 2.78±1.87 | 3.31±1.99 |
| Potassium/Cr (mmol/mmol) | 6.02±2.46 | 9.74±1.56 | 6.93±0.35 | 7.29±0.46 |
| Sodium/Cr (mmol/mmol) | 10.86±4.19 | 9.74±3.37 | 13.86±5.13 | 13.48±4.14 |
| Serum | ||||
| 25-OH vitamin D (nmol/L)b | 58.5±14.5 | 63.0±20.0 | 61.8±15.3 | 59.3±14.8 |
| PTH (pmol/L) | 5.60±1.93 | 6.22±2.43 | 5.73±1.84 | 6.07±1.91 |
| IGF-1 (nmol/L) | 975.4±256.0 | 902.6±262.6 | 894.2±245.7 | 970.6±273.8 |
Measured on the screening visit
There were up to two missing values in the physical activity score
Mean of both limbs
During the study, mean physical activity scores did not change significantly for the men or the women in either the bicarbonate or the control group. Similarly, weight did not change significantly in either group (weight changes in the bicarbonate and control groups were −0.11±2.0 and 0.09± 1.7 kg in the men and 0.44±1.9 and −0.13±1.4 kg in the women, respectively).
Muscle performance results
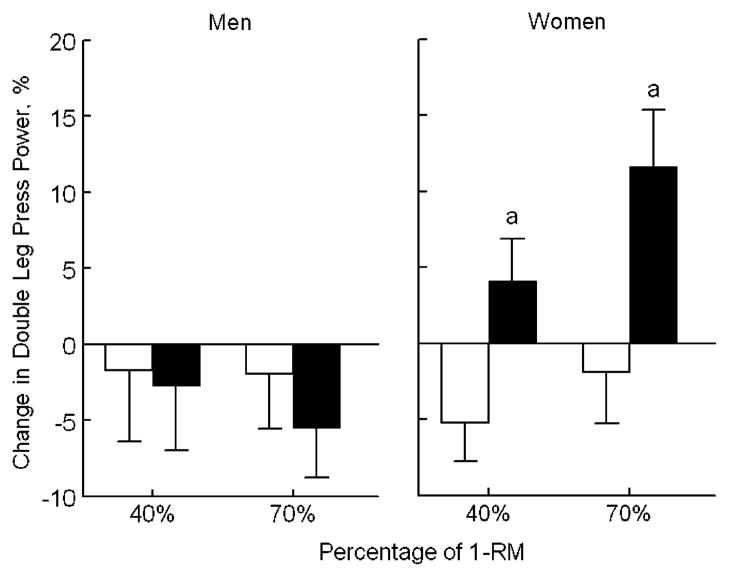
Mean percentage changes in the two groups, adjusted for baseline value, tester concordance, and for changes in K/Cr and Na/Cr are shown in Table 2. The women treated with bicarbonate had significantly improved lower-extremity power when compared with the women in the control group, as indicated by greater changes in the double leg press power output at both 40% and 70% of 1-RM (P=0.006 and P=0.003, respectively, Fig. 2). The women in the bicarbonate group also had more favorable adjusted mean changes in peak knee extension power at 70% 1-RM (P=0.014) and in isokinetic knee extension endurance at 240°/s (P=0.047) than the women in the control group (Table 2). Grip strength changed in a similar direction in the women, but the difference between groups was not statistically significant. Inclusion of age as a covariate in ANCOVA models did not significantly alter the effect of treatment on any of the muscle performance measures in the women. Treatment with bicarbonate caused no significant changes in muscle performance in the men.
Table 2.
Mean±SEM percent change in muscle strength and power by sex and bicarbonate treatment statusa
| Number | Men |
Women |
||||
|---|---|---|---|---|---|---|
| Control | Bicarbonate | P | Control | Bicarbonate | P | |
| 35 | 36 | 49 | 42 | |||
| Leg power output | ||||||
| Knee extension (40% 1-RM, W)b | 0.63±3.70 | 0.42±3.34 | 0.949 | 2.04±1.76 | 4.46±1.83 | 0.263 |
| Knee extension (70% 1-RM, W)† | 0.16±2.94 | −2.77±2.66 | 0.268 | 0.52±1.66 | 5.82±1.79 | 0.014 |
| Double leg press (40% 1-RM, W) | −1.72±4.68 | −2.71±4.25 | 0.812 | −5.20±2.55 | 4.08±2.82 | 0.006 |
| Double leg press (70% 1-RM, W) | −1.94±3.62 | −5.47±3.27 | 0.275 | −1.86±3.39 | 11.61±3.74 | 0.003 |
| Isokinetic leg enduranceb | ||||||
| Knee extension (240°/s, Nm) | 6.55±4.40 | 5.46±3.98 | 0.780 | −3.59±3.20 | 5.12±3.66 | 0.047 |
| Knee flexion (240°/s, Nm) | 7.48±4.58 | 5.66±4.15 | 0.658 | −7.30±3.09 | 0.76±3.54 | 0.057 |
| Grip strength (kgf)b | 4.42±2.05 | 2.83±1.85 | 0.382 | −1.13±1.21 | 1.07±1.38 | 0.178 |
Changes are adjusted for baseline strength, tester concordance, and for the changes in K/Cr and Na/Cr. There were up to six scattered missing values for individual tests in the control group and up to three in the bicarbonate group
Mean of both limbs
Fig. 2.
Effect of treatment with bicarbonate versus no bicarbonate on mean change in peak double leg press power output (adjusted for baseline value, tester concordance, and changes in urinary sodium/Cr and potassium/Cr). Open bars represent no bicarbonate and filled bars represent bicarbonate. a In peak double leg press power at both 40% and 70% of 1-RM, the treatment groups differed significantly in the women (P=0.006 and P=0.003, respectively) but not in the men
In the women, change in NAE/Cr was inversely correlated with change in most of the performance measures including double leg press power (at 40% 1-RM, r=−0.24, P=0.028; at 70% 1-RM, r=−0.20, P=0.059), knee extension at 240°/s (r=−0.24, P=0.021), knee flexion at 240° (r=−0.33, P=0.002), and grip strength (r=−0.28, P= 0.008). Change in NAE/Cr was not correlated with any of the performance measures in the men.
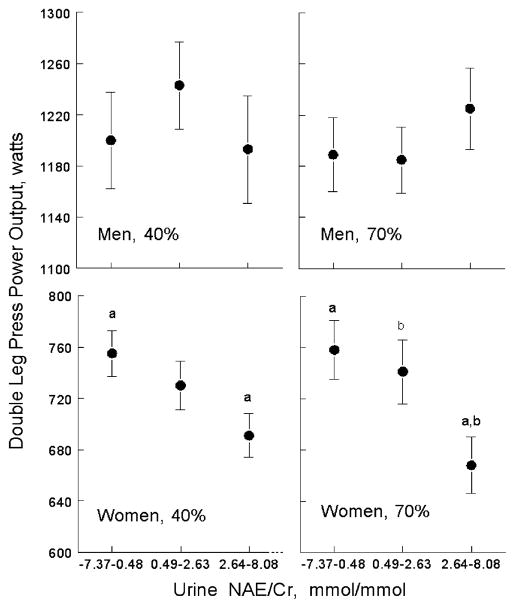
NAE/Cr on day 84 was associated with double leg press power at 40% and 70% (after adjustment for baseline double leg press values and for Na/Cr and K/Cr on day 84) in the women but not in the men. The associations are illustrated in Fig. 3 which displays the adjusted mean double leg press power values on day 84 by tertile of NAE/Cr in all men and all women.
Fig. 3.
Mean double leg press power output at 40% and 70% 1-RM by NAE/Cr tertile at the end of the study, adjusted for baseline value, and for sodium/Cr and potassium/Cr. a, b Like symbols indicate significant differences between tertiles at P<0.05
Biochemical results
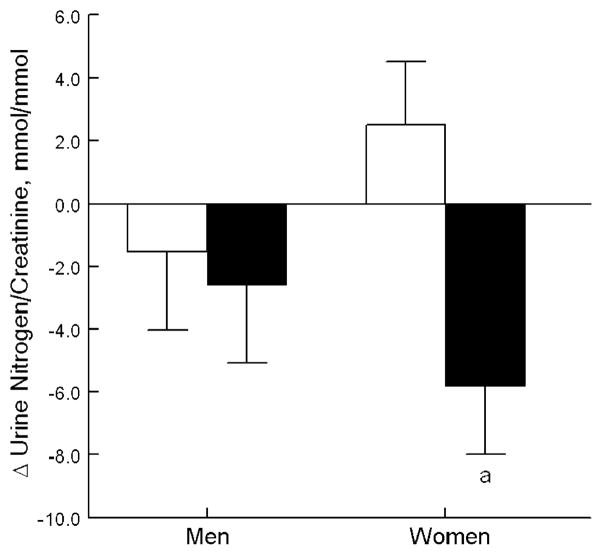
Mean±standard error of the mean (SEM) adjusted 3-month changes in the biochemical measures by sex and bicarbonate group are shown in Table 3. Women supplemented with bicarbonate had significantly greater changes in nitrogen/Cr excretion than those in the control group (P=0.004, after adjustment for baseline nitrogen/Cr and changes in K/Cr and Na/Cr) as shown in Fig. 4. Further adjustment for age did not alter these results. In the men, treatment with bicarbonate did not significantly change any measure except NAE/Cr. Changes in serum PTH and IGF-1 were minimal and not affected by treatment in the men or the women.
Table 3.
Mean±SEM change in laboratory values by sex and bicarbonate treatment statusa
| Number | Men |
Women |
||||
|---|---|---|---|---|---|---|
| Control | Bicarbonate | P | Control | Bicarbonate | P | |
| 35 | 36 | 49 | 42 | |||
| 24-h urine | ||||||
| Creatinine (Cr; mmol) | 0.67±0.54 | −0.27±0.53 | 0.225 | 0.55±0.24 | 0.71±0.26 | 0.671 |
| Nitrogen/Cr (mmol/mmol) | −1.53±2.50 | −2.58±2.50 | 0.767 | 2.50±2.02 | −6.14±2.18 | 0.004 |
| NAE/Cr (mmol/mmol) | −0.23±0.25 | −2.87±0.25 | <0.001 | 0.47±0.29 | −4.11±0.31 | <0.001 |
| Potassium/Cr (mmol/mmol) | 1.41±0.51 | 1.49±0.50 | 0.916 | 1.43±0.51 | 1.92±0.55 | 0.519 |
| Sodium/Cr (mmol/mmol) | 1.26±0.67 | 2.57±0.66 | 0.171 | 0.36±0.66 | 1.27±0.71 | 0.348 |
| Serum | ||||||
| PTH (pmol/L) | −0.01±0.27 | −0.36±0.27 | 0.377 | −0.25±0.20 | 0.19±0.21 | 0.139 |
| IGF-1 (nmol/L) | 17.59 ±18.43 | 21.57±18.21 | 0.880 | 7.50±20.04b | 9.10±21.49 | 0.959 |
Changes are adjusted for baseline laboratory value and changes in urinary sodium/Cr and urinary potassium/Cr.
N=48
Fig. 4.
Effect of treatment with bicarbonate versus no bicarbonate on mean change in urinary nitrogen/Cr and NAE/Cr (adjusted for baseline value and changes in urinary sodium/Cr and potassium/Cr). Open bars represent no bicarbonate and filled bars represent bicarbonate. a The treatment groups differ significantly in the women (P=0.004) but not the men
Change in NAE/Cr was significantly correlated with change in nitrogen/Cr excretion in the women (r=0.32, P=0.002), and there was a similar trend in the men (r= 0.23, P=0.052).
Safety/tolerability
After 3 weeks of treatment, three subjects not on potassium and five subjects on potassium had mildly elevated serum potassium levels in the range of 5.1–5.4 mmol/L. Twelve subjects discontinued treatment because of gastrointestinal symptoms: one on placebo, one on potassium bicarbonate, three on sodium bicarbonate, and seven on potassium chloride.
Discussion
Supplementation with bicarbonate for 3 months was well tolerated and had favorable effects on selected measures of muscle performance in the women but not the men. The greatest benefit of bicarbonate treatment was observed in lower body double leg press peak power output (at 70% 1-RM) which increased by 13% relative to the control group. The degree of improvement in double leg press peak power output is within the range seen in muscle performance measures with long-term use of added vitamin D [23–25], but is less than the 15% to 141% increases reported in power training exercise interventions of 12- to 16-week duration [18, 26, 27]. In the women, significant improvements while on bicarbonate were also noted in other power measurements, double leg press 40% and in knee extension 70%, and in one endurance measure, knee extension at 240°. The finding that only the bicarbonate altered NAE in combination with the findings that change in NAE was positively correlated with change in nitrogen excretion in men and women and inversely correlated with change in several measures of muscle power in the women suggests that the active component of our treatments was the bicarbonate. There is no indication that differences in vitamin D status confounded the bicarbonate results, since the bicarbonate and control groups had similar starting 25(OH)D levels and took similar doses of vitamin D3 daily throughout the study; however, we cannot exclude the possibility that the impact of vitamin D on muscle may be affected by the acid-base balance. There were no group differences in serum PTH, a hormone that has been associated with muscle weakness in an elderly population of fallers [28]. IGF-1 can increase lean tissue mass in adults [29], and there is evidence that IGF-1 levels are suppressed in metabolic acidosis [30]. However, there was no indication that IGF-1 levels were a mediator of the effects of bicarbonate on muscle performance in this study. We cannot exclude the possibility that a higher dose of bicarbonate would have increased serum IGF-1 levels.
This study tested only one relatively low dose of bicarbonate. Further work is needed to determine whether a higher dose would be more effective. As indicated in Fig. 3, fewer than one third of the subjects were excreting base during treatment. The inverse association of NAE with double leg press power in the women suggests that they may benefit more by ingesting more alkali, beyond the point of neutrality. Although alkali excretion is uncommon today, the studies of Sebastian et al. suggest the humans evolved consuming diets that achieved net base excretions close to 100 mmol/day on average [31].
The reason why we did not detect an effect of bicarbonate on muscle performance in the men is uncertain, but dose in relation to body size may have been a factor. In the women, the average daily dose of bicarbonate was 0.6 g/kg of body weight and 1.74 g/kg of lean tissue weight (that is, nonfat, nonbone tissue by dual energy X-ray absorptiometry) whereas in the men, it was only 0.48 g/kg and 1.20 g/kg, respectively. It is possible that the treatment effect differed according to lean tissue mass or another indicator of body size rather than sex, but there was too little overlap in weight and lean mass of the men and women to separate potential effects of sex and body size. Another possibility is that a true sex difference exists, and it is related to differences in circulating levels of testosterone, a hormone that is anabolic to muscle [32]. Higher testosterone levels presumably present (but not measured) in these men may have masked the more subtle effect of bicarbonate on performance. Higher serum testosterone levels have been associated with fewer falls in older men and women [33].
In both the men and the women, the change in NAE on treatment was positively correlated with change in nitrogen excretion. Although nitrogen excretion is not a specific indicator of muscle breakdown, in the setting of stable protein intake, exercise level, and body weight, a decrease in nitrogen excretion is consistent with decreased net muscle catabolism. Treatment with bicarbonate significantly lowered nitrogen excretion in the women in our study. This finding confirms the previous observation in 14 healthy postmenopausal women studied on metabolic diets that ingestion of a neutralizing dose of potassium bicarbonate significantly reduced nitrogen excretion [34]. In that study, Frassetto observed a 5.7% decrease in nitrogen excretion after 18 days of treatment with potassium bicarbonate. We observed a 6.9% decrease after 3 months of treatment. Longer term studies are needed to determine whether sustained treatment might reduce rates of loss of muscle mass in the elderly.
The mechanism(s) by which alkali affected muscle in this study are not certain. A possible mechanism may involve facilitating removal of hydrogen ions from muscle. During exercise, lactic acid is generated in muscle, and the accumulation of hydrogen ions in muscle is known to impair force generation and other aspects of muscle function [35, 36]. Extracellular acidosis inhibits hydrogen ion efflux from muscle in dogs [37]. Alkali is thought to promote the efflux of hydrogen ions from muscle cells by creating greater buffering capacity in the extracellular fluid [38]. We might speculate that use of bicarbonate to correct the chronic mild metabolic acidosis commonly present in older men and women may facilitate muscle performance in elders with usual levels of activity. Another possibility is that muscle wasting is an adaptive response to the mild metabolic acidosis that occurs with aging [13, 39, 40]. With muscle breakdown, the amino acids released into the blood stream provide substrate for the hepatic synthesis of glutamine. Glutamine is used by the kidney to synthesize ammonia [41]. Ammonia molecules spontaneously accept protons and are excreted as ammonium ions, thereby removing protons and mitigating the acidosis.
In conclusion, while it will never be a substitute for exercise, oral administration of bicarbonate modestly improved lower-extremity peak muscle power and endurance and reduced nitrogen excretion over a 3-month period in healthy older women. The decline in NAE during treatment was associated with reduced losses of nitrogen in the urine in both men and women. Based on these findings, bicarbonate appears to merit further consideration as a potential safe and low-cost intervention to reduce the declines in functional capacity and muscle mass that occur over time in the elderly.
Acknowledgments
This project was supported by Grant Number 1 RO1AR052322-01A1 from NIAMS and also received support from the US Department of Agriculture, Agricultural Research Service, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture.
We thank Dr. Catherine Gordon of Children’s Hospital in Boston for being the safety monitor of this study, the staffs of the Metabolic Research Unit and the Nutrition Evaluation Laboratory, and the study participants for their valuable contributions.
The author’s responsibilities were: B. Dawson-Hughes, C. Castaneda-Sceppa, S.S. Harris, and G.E. Dallal designed the study and B. Dawson-Hughes, C. Castaneda-Sceppa, S.S. Harris, N. Palermo, G. Cloutier, and L. Ceglia contributed to data collection and processing. All authors contributed to data interpretation and manuscript writing.
Footnotes
Conflicts of interest None.
References
- 1.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 2.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home-based exercise to prevent falls in elderly women. BMJ. 1997;315:1065–1069. doi: 10.1136/bmj.315.7115.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 5.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 6.Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983;24:670–680. doi: 10.1038/ki.1983.210. [DOI] [PubMed] [Google Scholar]
- 9.Frassetto LA, Morris RC, Jr, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271:t-22. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 10.Douroudos II, Fatouros IG, Gourgoulis V, Jamurtas AZ, Tsitsios T, Hatzinikolaou A, Margonis K, Mavromatidis K, Taxildaris K. Dose-related effects of prolonged NaHCO3 ingestion during high-intensity exercise. Med Sci Sports Exerc. 2006;38:1746–1753. doi: 10.1249/01.mss.0000230210.60957.67. [DOI] [PubMed] [Google Scholar]
- 11.Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol -Renal. 2003;284:F32–F40. doi: 10.1152/ajprenal.00212.2002. [DOI] [PubMed] [Google Scholar]
- 12.Green J, Kleeman CR. Role of bone in regulation of systemic acid-base balance. Kidney Int. 1991;39:9–26. doi: 10.1038/ki.1991.2. [DOI] [PubMed] [Google Scholar]
- 13.May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986;77:614–621. doi: 10.1172/JCI112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitch WE, Price SR, May RC, Jurkovitz C, England BK. Metabolic consequences of uremia: extending the concept of adaptive responses to protein metabolism. Am J Kidney Dis. 1994;23:224–228. doi: 10.1016/s0272-6386(12)80976-0. [DOI] [PubMed] [Google Scholar]
- 15.Edge J, Bishop D, Goodman C. The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol. 2006;96:97–105. doi: 10.1007/s00421-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B, Harris SS, Palermo NJ, Castaneda-Sceppa C, Rasmussen HM, Dallal GE. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab. 2009;94:96–102. doi: 10.1210/jc.2008-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 18.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50:655–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 19.Roitman J. American College of Sports M. Guidelines for exercise testing and prescription. Lippincott; Philadelphia: 2000. [Google Scholar]
- 20.Frontera WR, Hughes VA, Dallal GE, Evans WJ. Reliability of isokinetic muscle strength testing in 45- to 78-year-old men and women. Arch Phys Med Rehabil. 1993;74:1181–1185. [PubMed] [Google Scholar]
- 21.Jorgensen K. Titrimetric determination of the net excretion of acid/base in urine. Scand J Clin Lab Invest. 1957;9:281–291. doi: 10.3109/00365515709079972. [DOI] [PubMed] [Google Scholar]
- 22.Chan JC. The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin Biochem. 1972;5:94–98. doi: 10.1016/s0009-9120(72)80014-6. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Iwamoto J, Kanoko T, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial. J Bone Miner Res. 2005;20:1327–1333. doi: 10.1359/JBMR.050402. [DOI] [PubMed] [Google Scholar]
- 26.Reid KF, Callahan D, Carabello R, Phillips E, Frontera WR, Fielding RA. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res. 2008:20. doi: 10.1007/bf03324865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earles DR, Judge JO, Gunnarsson OT. Velocity training induces power-specific adaptations in highly functioning older adults. Arch Phys Med Rehabil. 2001;82:872–878. doi: 10.1053/apmr.2001.23838. [DOI] [PubMed] [Google Scholar]
- 28.Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, Allain TJ. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17:891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 29.Mauras N, Beaufrere B. Recombinant human insulin-like growth factor-I enhances whole body protein anabolism and significantly diminishes the protein catabolic effects of prednisone in humans without a diabetogenic effect. J Clin Endocrinol Metab. 1995;80:869–874. doi: 10.1210/jcem.80.3.7533772. [DOI] [PubMed] [Google Scholar]
- 30.Ballmer PE, McNurlan MA, Hulter HN, Anderson SE, Garlick PJ, Krapf R. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995;95:39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC., Jr Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002;76:1308–1316. doi: 10.1093/ajcn/76.6.1308. [DOI] [PubMed] [Google Scholar]
- 32.Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, McMillan CV, Bradley C, Martin FC. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–484. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Additive benefit of higher testosterone levels and vitamin D plus calcium supplementation in regard to fall risk reduction among older men and women. Osteoporos Int. 2008;19:1307–1314. doi: 10.1007/s00198-008-0573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frassetto L, Morris RC, Jr, Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metabol. 1997;82:254–259. doi: 10.1210/jcem.82.1.3663. [DOI] [PubMed] [Google Scholar]
- 35.Baker AJ, Brandes R, Weiner MW. Effects of intracellular acidosis on Ca2+ activation, contraction, and relaxation of frog skeletal muscle. Am J Physiol. 1995;268:C55–C63. doi: 10.1152/ajpcell.1995.268.1.C55. [DOI] [PubMed] [Google Scholar]
- 36.Sutton JR, Jones NL, Toews CJ. Effect of PH on muscle glycolysis during exercise. Clin Sci (Lond) 1981;61:331–338. doi: 10.1042/cs0610331. [DOI] [PubMed] [Google Scholar]
- 37.Hirche HJ, Hombach V, Langohr HD, Wacker U, Busse J. Lactic acid permeation rate in working gastrocnemii of dogs during metabolic alkalosis and acidosis. Pflugers Arch. 1975;356:209–222. doi: 10.1007/BF00583833. [DOI] [PubMed] [Google Scholar]
- 38.Mainwood GW, Worsley-Brown P. The effects of extracellular pH and buffer concentration on the efflux of lactate from frog sartorius muscle. J Physiol. 1975;250:1–22. doi: 10.1113/jphysiol.1975.sp011040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams B, Layward E, Walls J. Skeletal muscle degradation and nitrogen wasting in rats with chronic metabolic acidosis. Clin Sci. 1991;80:457–462. doi: 10.1042/cs0800457. [DOI] [PubMed] [Google Scholar]
- 40.Guder WG, Haussinger D, Gerok W. Renal and hepatic nitrogen metabolism in systemic acid base regulation. J Clin Chem Clin Biochem. 1987;25:457–466. doi: 10.1515/cclm.1987.25.8.457. [DOI] [PubMed] [Google Scholar]
- 41.Cersosimo E, Williams PE, Radosevich PM, Hoxworth BT, Lacy WW, Abumrad NN. Role of glutamine in adaptations in nitrogen metabolism during fasting. Am J Physiol. 1986;250:E622–E628. doi: 10.1152/ajpendo.1986.250.6.E622. [DOI] [PubMed] [Google Scholar]