Abstract
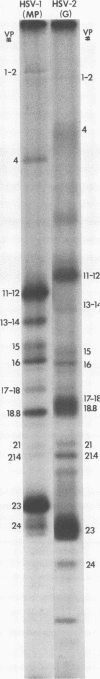
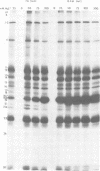
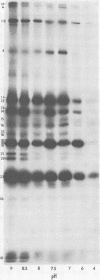
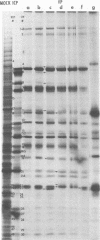
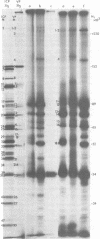
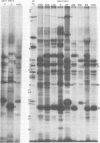
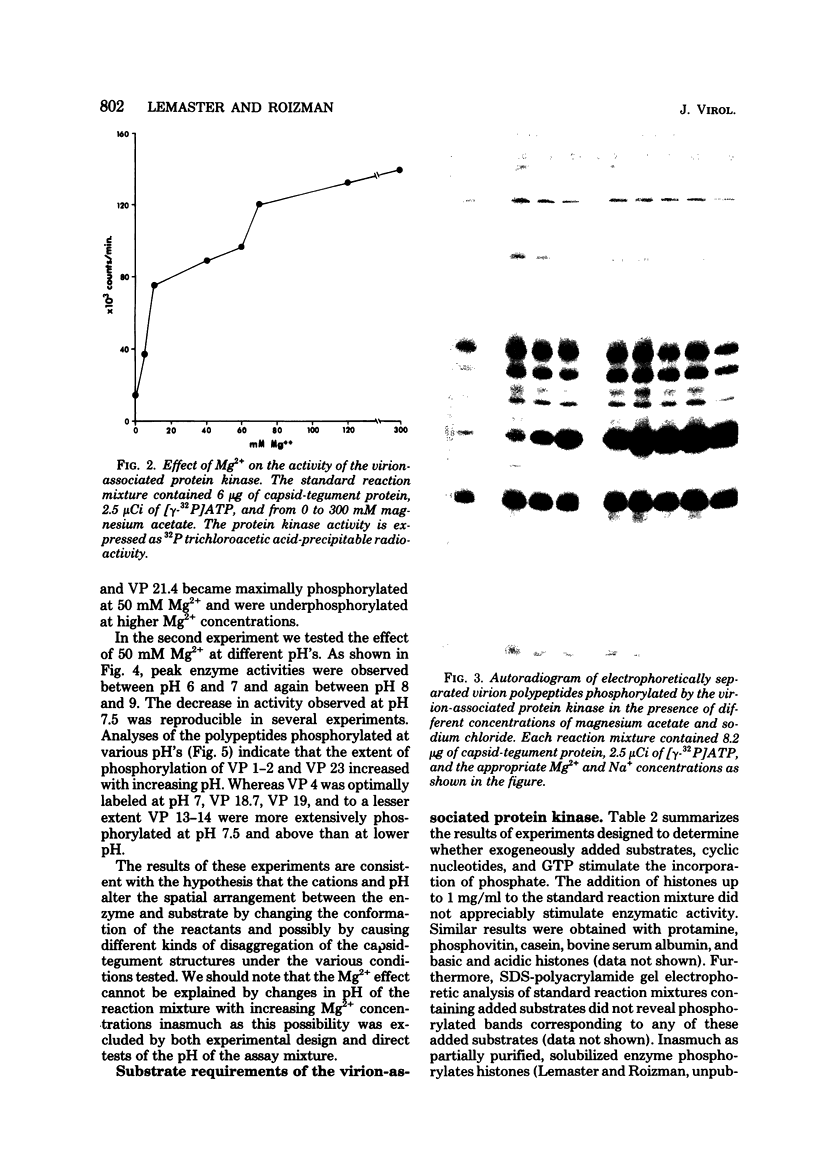
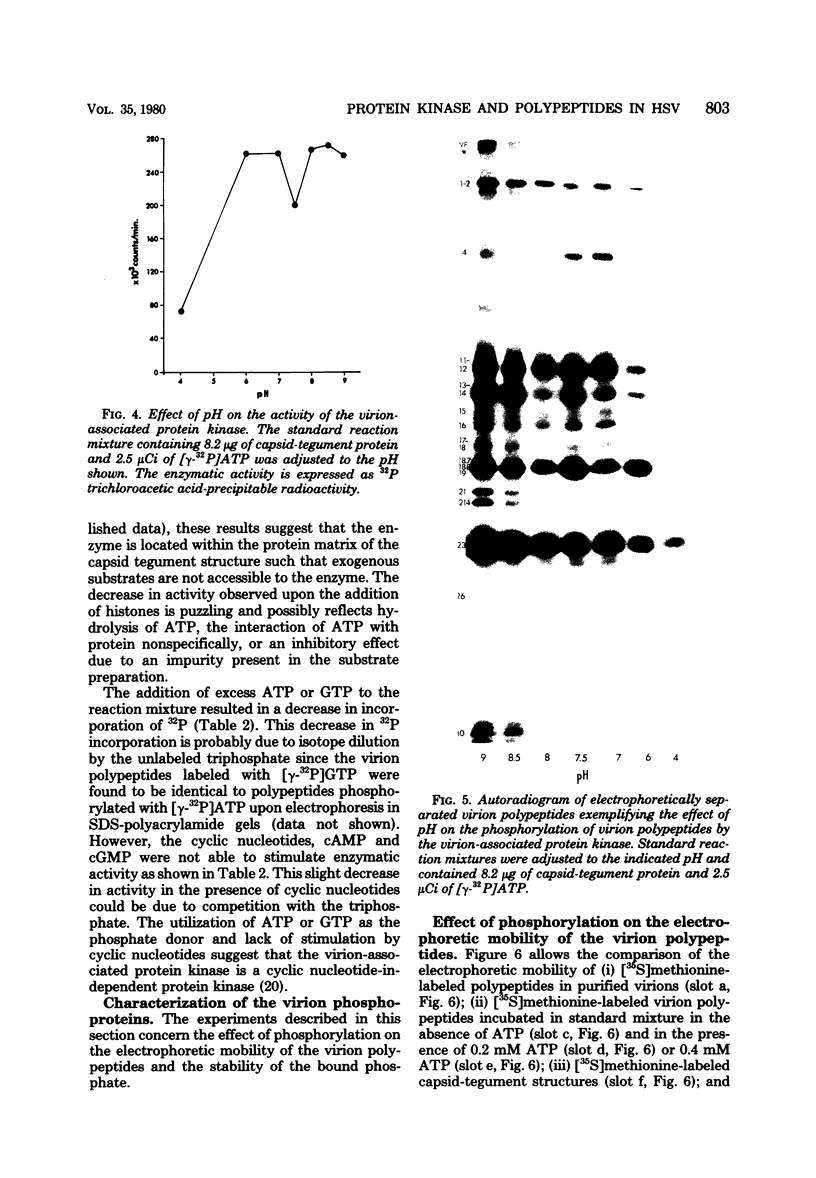
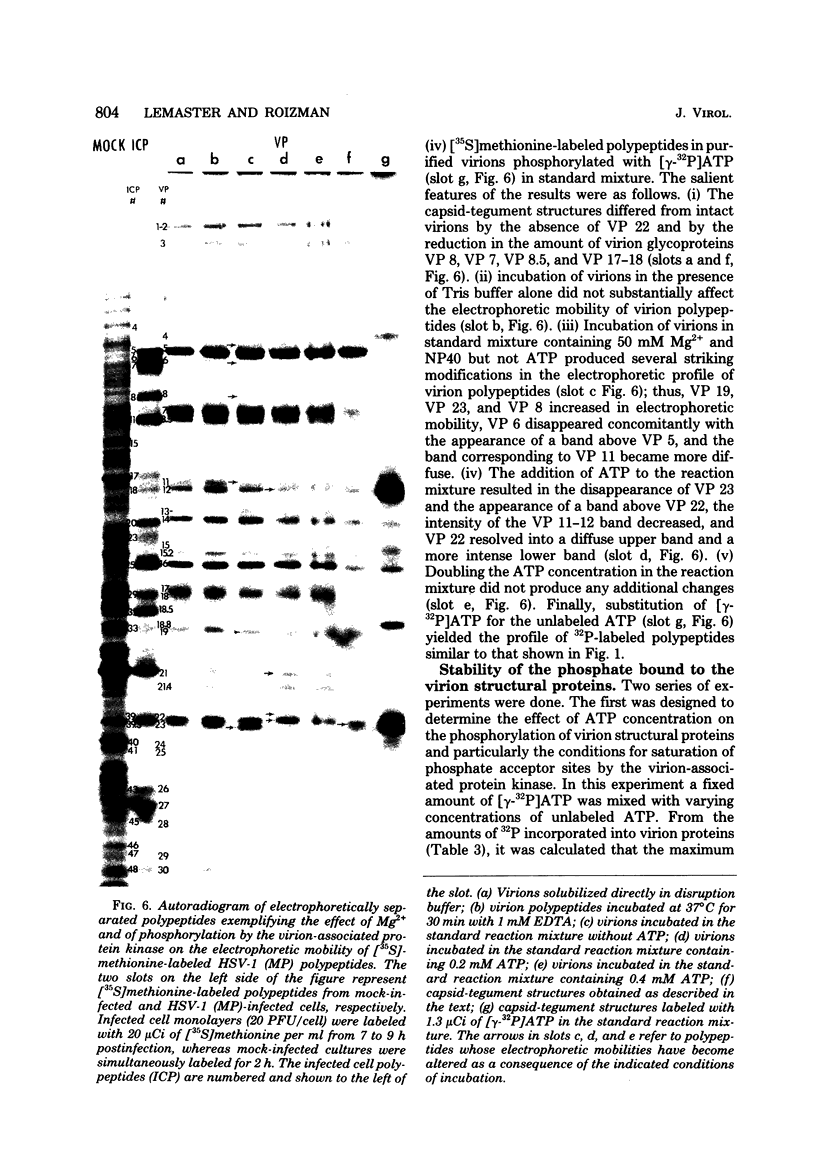
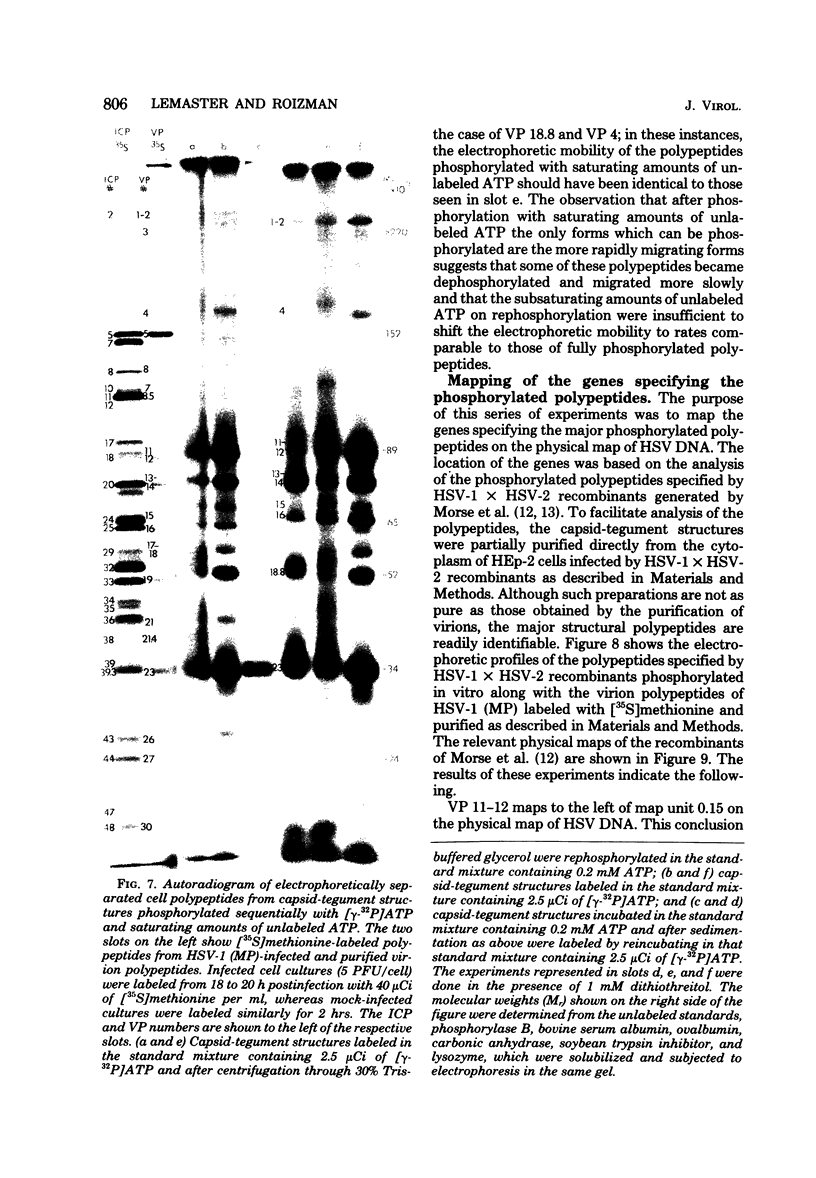
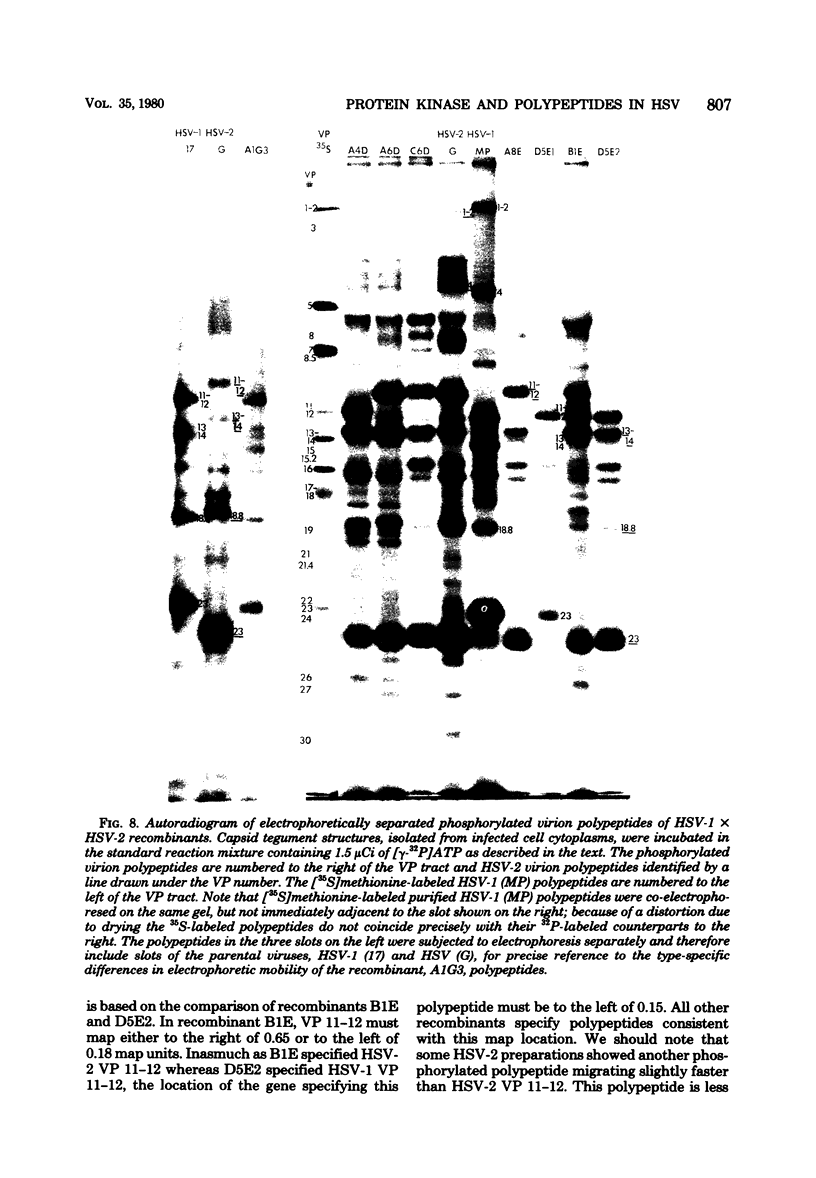
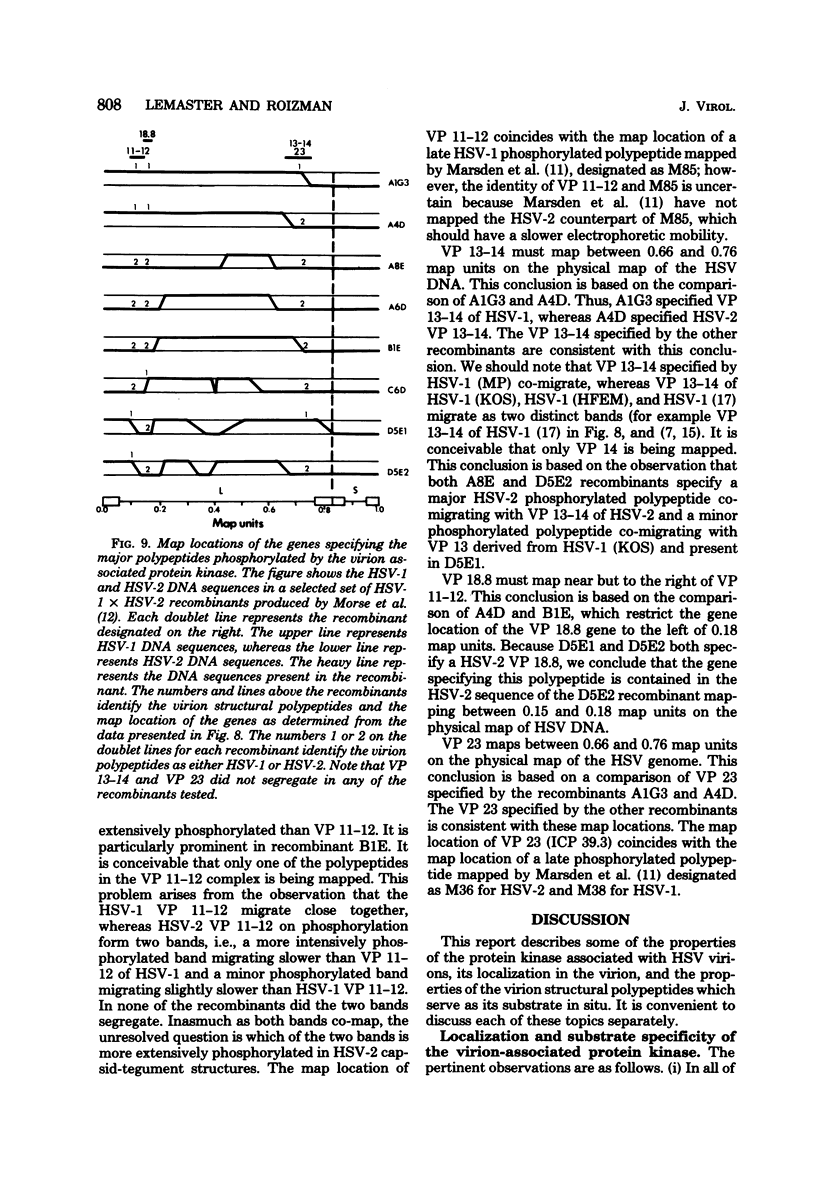
The protein kinase associated with purified herpes simplex virus 1 and 2 virions partitioned with the capsid-tegument structures and was not solubilized by non-ionic detergents and low, non-inhibitory concentrations of urea. The enzyme required Mg2+ or Mn2+ and utilized ATP or GTP. The activity was enhanced by non-ionic detergents and by Na+ even in the presence of high concentrations of of Mg2+, but not by cyclic nucleotides. The enzyme associated with capsid-tegument structures phosphorylated virion polypeptides only; exogenously added substrates (acidic and basic histones, casein, phosphovitin, protamine, and bovine serum albumin) were not phosphorylated. The major phosphorylated species were virion polypeptides (VP) 1-2, 4, 11-12, 13-14, 18.7, 18.8 and 23. VP 18.7 and VP 18.8 have not been previously detected, but may be phosphorylated forms of polypeptides co-migrating with VP 19. Of the remainder, only VP 23 has been previously identified as a capsid protein; the others are constituents of the tegument or of the under surface of the virion envelope. The distribution of the phosphate bound to viral polypeptides varied depending on the Mg2+ concentration and pH. In the absence of dithiothreitol, in vitro phosphate exchange was demonstrable in VP 23 and to a lesser extent in two other polypeptides on sequential phosphorylation frist with saturating amounts off unlabeled ATP and then with [gamma-32P]ATP. Analysis of the virion polypeptides specified by herpes simplex virus 1 X herpes simplex virus 2 recombinants indicates that the genes specifying the polypeptides which serve as a substrate for the protein kinase map in the unique sequences near the left and right reinterated DNA sequences of the L component.
Full text
PDF

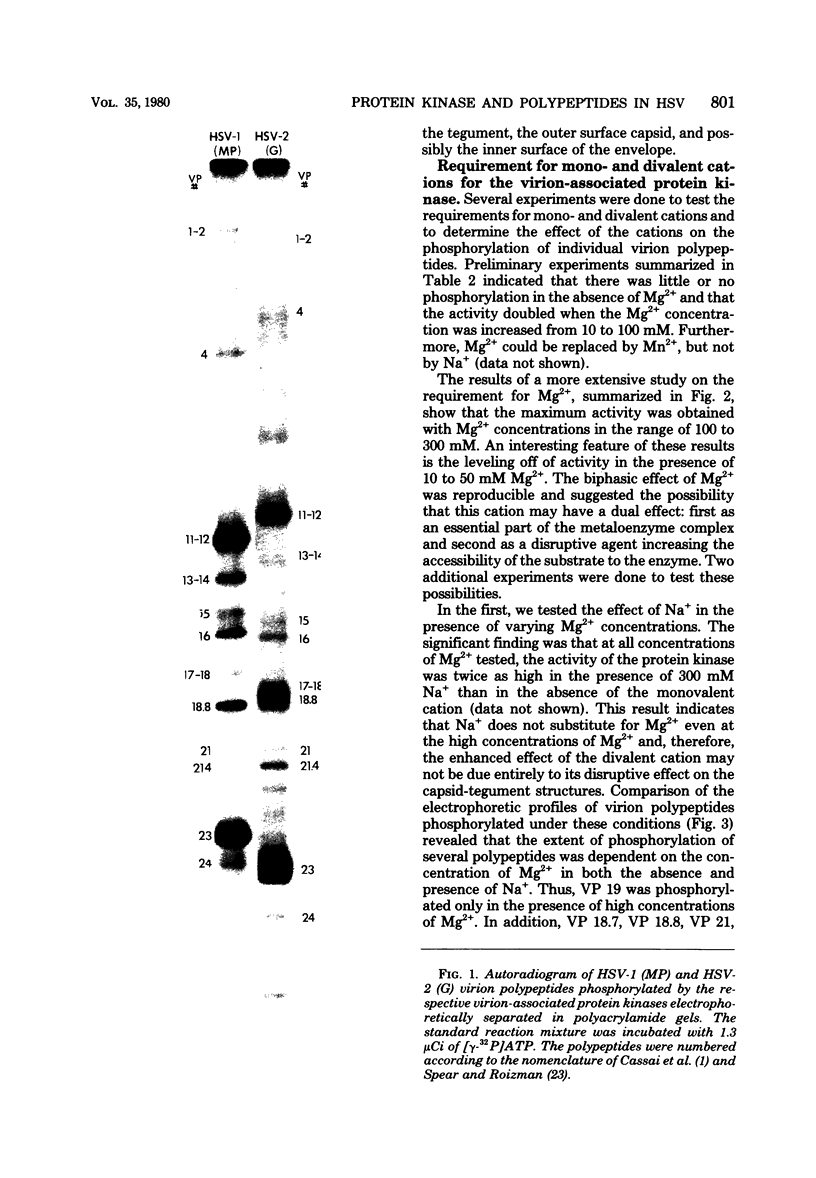




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Furlong D., Swift H., Roizman B. Arrangement of herpesvirus deoxyribonucleic acid in the core. J Virol. 1972 Nov;10(5):1071–1074. doi: 10.1128/jvi.10.5.1071-1074.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Twiddy E., Gilden R. V. Protein kinase associated with RNA tumor viruses and other budding RNA viruses. Virology. 1972 Feb;47(2):536–538. doi: 10.1016/0042-6822(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Heilman C. J., Jr, Zweig M., Stephenson J. R., Hampar B. Isolation of a nucleocapsid polypeptide of herpes simplex virus types 1 and 2 possessing immunologically type-specific and cross-reactive determinants. J Virol. 1979 Jan;29(1):34–42. doi: 10.1128/jvi.29.1.34-42.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Davison A. J., Marsden H. S., Timbury M. C., Subak-Sharpe J. H., Wilkie N. M. Recombinants between herpes simplex virus types 1 and 2: analyses of genome structures and expression of immediate early polypeptides. J Virol. 1978 Nov;28(2):499–517. doi: 10.1128/jvi.28.2.499-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C. C., Rogers H. W., Downer D. N., Gentry G. A. Protein kinase activity in equine herpesvirus. J Virol. 1972 Feb;9(2):216–222. doi: 10.1128/jvi.9.2.216-222.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Gravell M., Darlington R. Protein kinase in enveloped herpes simplex virions. Virology. 1972 Oct;50(1):287–290. doi: 10.1016/0042-6822(72)90374-1. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Roizman B., Schwartz J. Herpes simplex virus products in productive and abortive infection. II. Electron microscopic and immunological evidence for failure of virus envelopment as a cause of abortive infection. J Virol. 1968 Apr;2(4):384–392. doi: 10.1128/jvi.2.4.384-392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Virion-bound protein kinase in Semliki forest and Sindbis viruses. J Virol. 1974 Jun;13(6):1245–1253. doi: 10.1128/jvi.13.6.1245-1253.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- WILDY P., RUSSELL W. C., HORNE R. W. The morphology of herpes virus. Virology. 1960 Oct;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]