Abstract
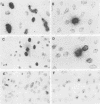
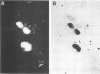
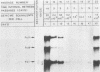
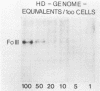
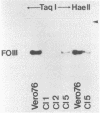
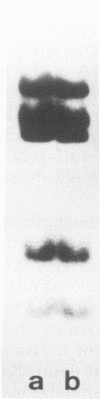
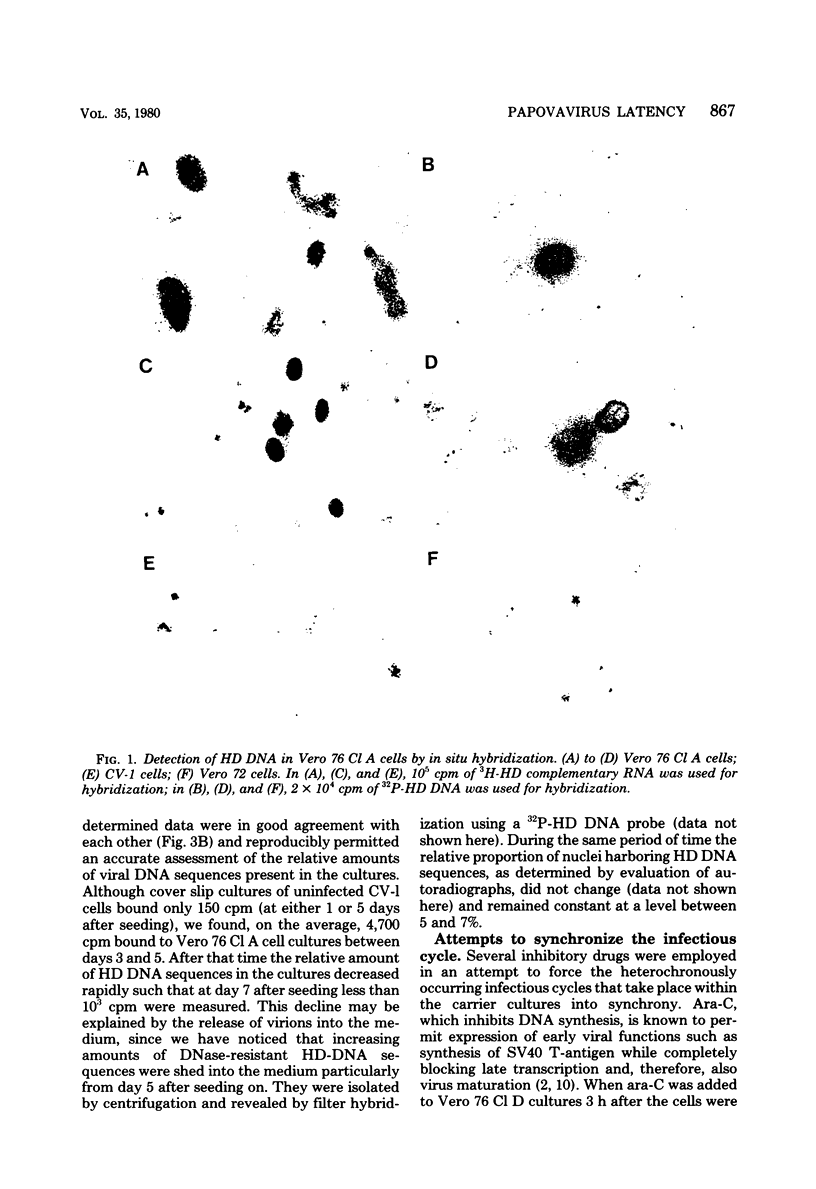
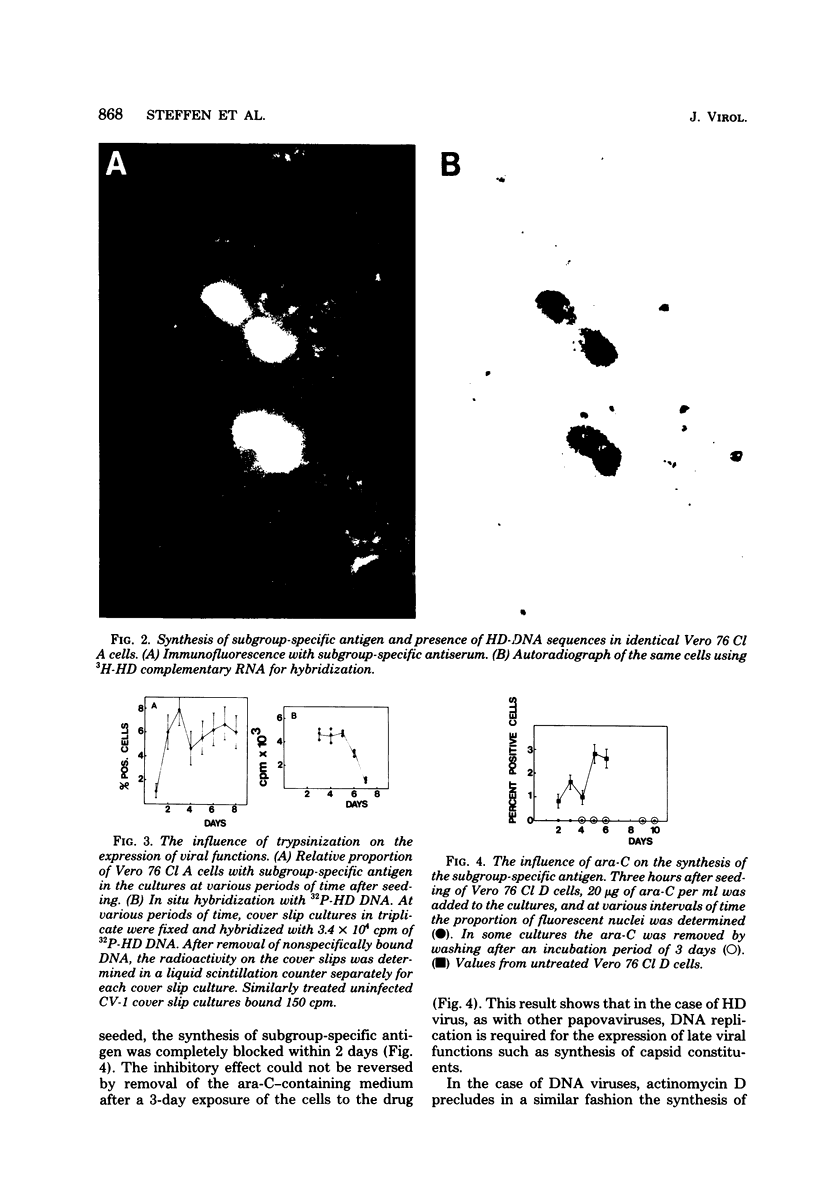
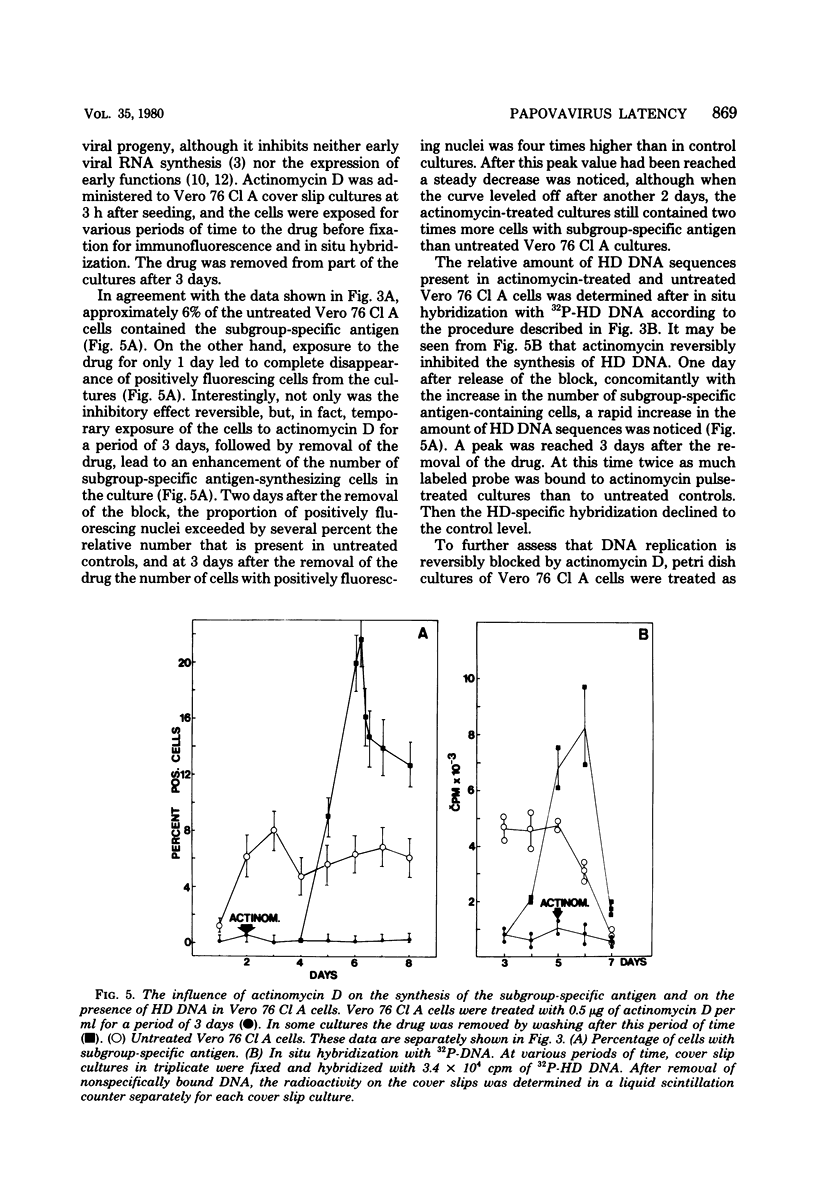
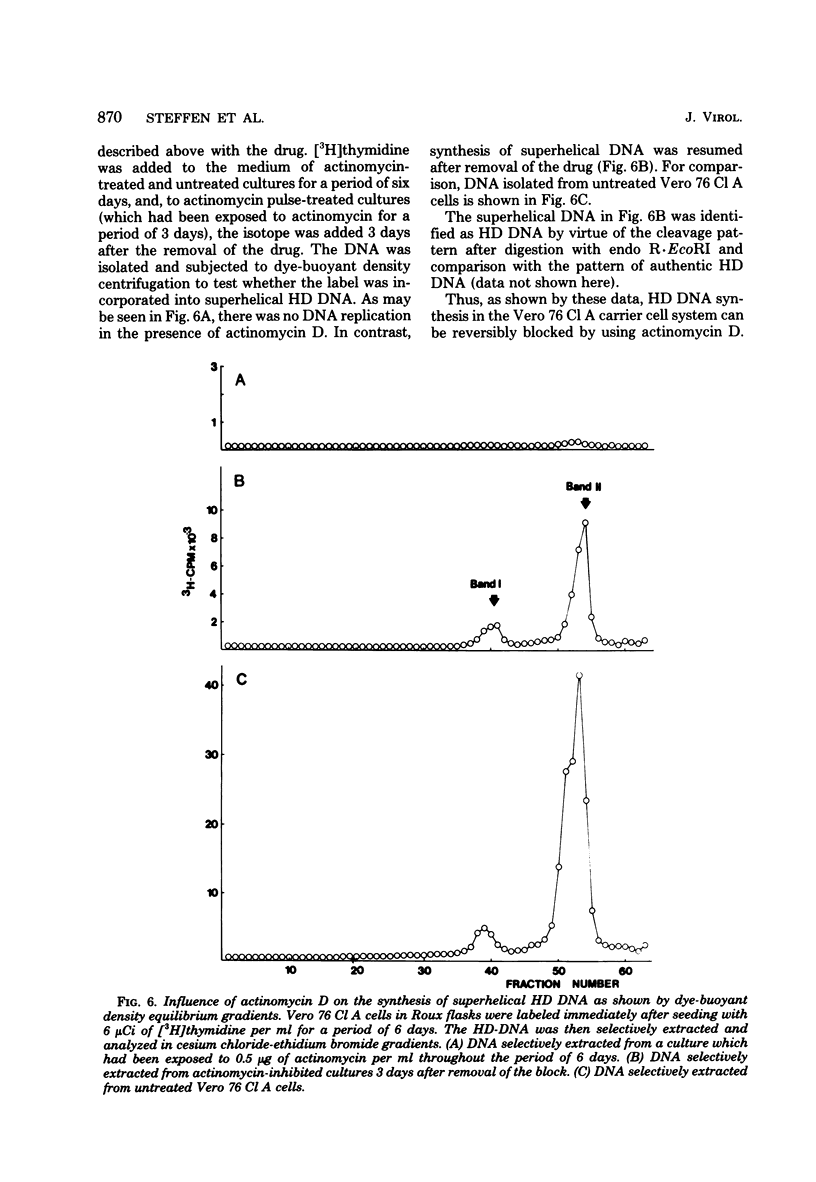
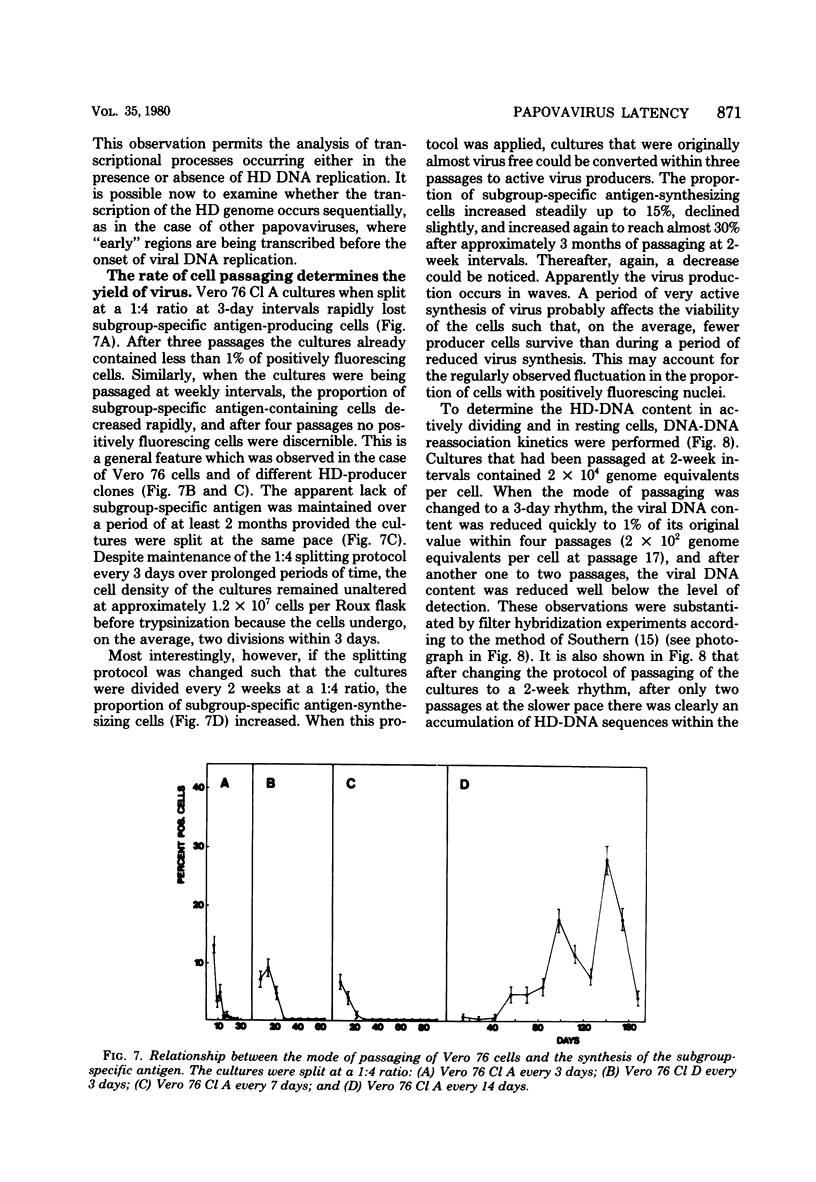
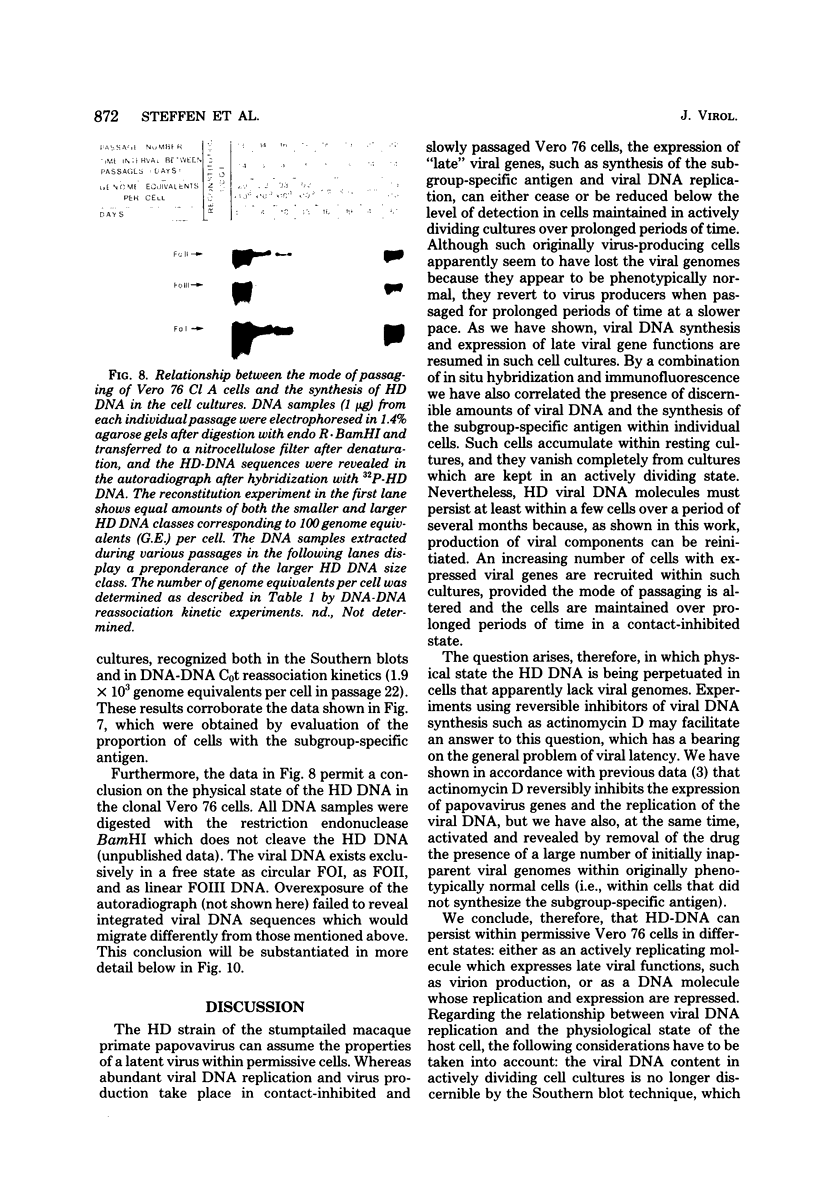
The stumptailed macaque papovavirus strain HD was discovered in a persistently infected cell line of primate origin designated Vero 76 (K. Bosslet and G. Sauer, J. Virol. 25:596--607, 1978; W. Waldeck and G. Sauer, Nature [London] 269:171--173, 1977). In clonal derivatives of Vero 76 cells a minor and variable proportion of cells is engaged in the productive synthesis of the HD virus strain. A combination of immunofluorescence using simian virus 40 polyoma subgroup-specific antiserum and in situ hybridization with HD complementary RNA revealed that only those cells which harbor discernible amounts of HD DNA also contain the subgroup-specific antigen. Treatment with arabinofuranosylcytosine caused irreversible disappearance of the antigen, whereas actinomycin D, in contrast, reversibly inhibited both HD DNA replication and synthesis of the subgroup-specific antigen. The proportion of HD DNA and subgroup-specific antigen-synthesizing cells in Vero 76 clonal lines could be either decreased or increased by the mode of passaging of the cell cultures. When cell cultures were split every 3 to 7 days at a 1:4 ratio, the amount of HD DNA sequences as revealed by DNA-DNA reassociation and by the Southern blotting technique fell below the level of detection after only a few passages. Furthermore, expression of the viral subgroup-specific antigen was no longer discernible. However, viral DNA persists in such latently infected cells, because a change in the splitting protocol to a 2-week passaging rhythm led to reinitiation of both viral DNA replication and expression of the subgroup-specific antigen. The HD DNA is perpetuated in a restricted state in latently infected cells in an episomal, unintegrated form as shown by Southern blot analysis. This finding complies with the fact that HD DNA-free subclones could be derived from persistently infected clonal Vero 76 cells. Such subclones have lost the viral genomes, probably owing to segregation during cell division.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosslet K., Sauer G. Biological properties and physical map of the genome of a new papovavirus, HD virus. J Virol. 1978 Feb;25(2):596–607. doi: 10.1128/jvi.25.2.596-607.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Rapp F. The effect of arabinofuranosylcytosine on the growth cycle of simian virus 40. Virology. 1965 Dec;27(4):490–495. doi: 10.1016/0042-6822(65)90174-1. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Sauer G., Sokol F. The effect of actinomycin D on the transcription and replication of simian virus 40 deoxyribonucleic acid. Virology. 1969 Feb;37(2):214–226. doi: 10.1016/0042-6822(69)90201-3. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G. Recent progress in Epstein-Barr virus research. Annu Rev Microbiol. 1977;31:421–445. doi: 10.1146/annurev.mi.31.100177.002225. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Newell N., Shah K. V., Law M. F., Gruss P., Sauer G., Kelly T. J., Jr Identification of the primate papovavirus HD as the stump-tailed macaque virus. J Virol. 1979 Apr;30(1):400–403. doi: 10.1128/jvi.30.1.400-403.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Stinski M. F. Persistence of the cytomegalovirus genome in human cells. J Virol. 1979 Sep;31(3):761–775. doi: 10.1128/jvi.31.3.761-775.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sauer G. Apparent differences in transcriptional control in cells productively infected and transformed by SV40. Nat New Biol. 1971 Jun 2;231(22):135–138. doi: 10.1038/newbio231135a0. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stuart W. D., Porter D. L. An improved in situ hybridization method. Exp Cell Res. 1978 Apr;113(1):218–222. doi: 10.1016/0014-4827(78)90105-2. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Linke H., Miyamura T., Fareed G. C. Persistent BK papovavirus infection of transformed human fetal brain cells. I. Episomal viral DNA in cloned lines deficient in T-antigen expression. J Virol. 1979 Mar;29(3):1177–1185. doi: 10.1128/jvi.29.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Sauer G. New oncogenic papova virus from primate cells. Nature. 1977 Sep 8;269(5624):171–173. doi: 10.1038/269171a0. [DOI] [PubMed] [Google Scholar]
- Westphal H. SV40 DNA strand selection by Escherichia coli RNA polymerase. J Mol Biol. 1970 Jun 14;50(2):407–420. doi: 10.1016/0022-2836(70)90201-9. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]